Abstract
Introduction
The aim of this study was to evaluate oral single/multiple doses of Fosfomycin Trometamol with clinical and microbiological efficacy in:
Asymptomatic bacteriuria in pregnancy.
Endourological procedures.
Lower urinary tract infections.
Material and methods
This prospective, uncontrolled, open label study was conducted in two tertiary hospitals over a period of three years. A total of 400 patients were included in the study. Group A (200 patients) with asymptomatic bacteriuria in pregnancy and Group B (200 Patients) with symptomatic lower urinary tract infections and with any day care endourological procedures were enrolled in our study. Efficacy end points like post- antibiotic urinalysis, microbiological efficacy and clinical improvement with adverse effects of the drug were evaluated.
Results
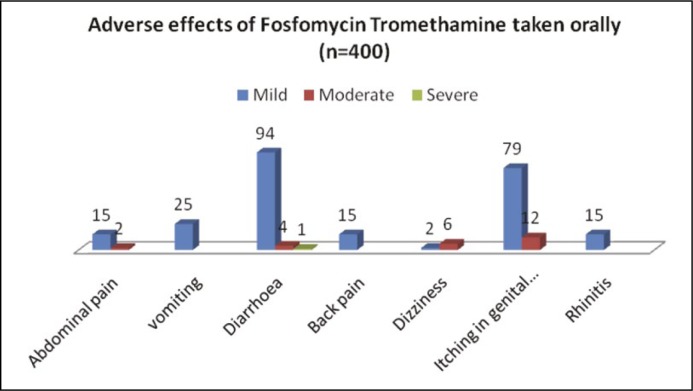
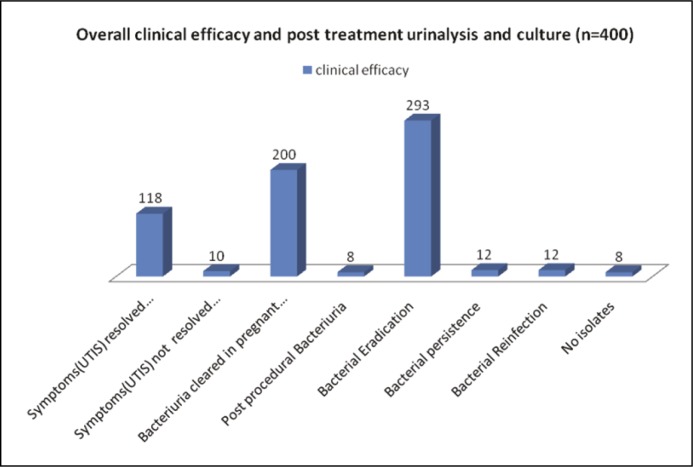
Of the 400 patients studied, 98% returned for follow-up. Out of the 304 urinary isolates in Table 2 (ASB and symptomatic LUTS) grown on urinary culture, majority of the isolates were Gram-negative Enterobacteriacae family. After oral single/multiple doses of fosfomycin, bacterial eradication, bacterial persistence, bacterial reinfection were 96.3%, 3.9%, 3.9% respectively (Figure 2). No isolates were grown in 8 cases (Table 2). However, on administration of the drug 23.5% patients noticed diarrhea (loose stools) followed by itching (19.7%) in genital area (Figure 1).
Conclusions
Fosfomycin Trometamol is a bactericidal antibiotic with a broad spectrum activity against Gram-positive also Gram-negative bacteriae. It has an advantage of oral single /multiple doses, higher eradication rate of bacteria after 48 hours, excellent tolerability and safety in pregnancy and other female age groups. We recommend Fosfomycin Trometamol as the drug of choice particularly in patients with poor drug compliance and for minor day care endourological procedures.
Keywords: asymptomatic bacteriuria (ASB), fosfomycin trometamol, lower urinary tract symptoms, urinary tract infections
INTRODUCTION
Urinary tract infections (UTIs) are common, affect woman of all ages and vary dramatically in their presentation. They are a common cause of morbidity and can lead to significant mortality. Clinical manifestations can vary from asymptomatic bacterial colonization of the bladder to irritative symptoms. While upper tract infections are associated with fever, chills and flank pain. In pregnant woman, the incidence of a UTI can be as high as 8% [1, 2]. Bacteriuria is defined as the presence of >105 colonies of a single pathogen per milliliter of urine. It may be either an asymptomatic bacteriuria in pregnancy (ASB) or a symptomatic acute cystitis and acute pyelonephritis [3]. However, a spontaneous resolution of bacteriuria in pregnant women is unlikely unless treated. Non-pregnant patients often clear their asymptomatic bacteriuria, while pregnant women become symptomatic and tend to have an associated subsequent acute pyelonephritis in 20-50% of cases [4]. Escherichia coli accounts for 80 to 90% of infections. Other Gram-negative rods such as Proteus mirabilis and Klebsiella pneumoniae are also common. Group B Streptococci and Staphylococcus saprophyticus are less common causes of UTI.
MATERIAL AND METHODS
This was a prospective study conducted in two medical centers from January 2011 to January 2014. A total of 400 patients were enrolled in our study. 200 patients (Group A) with asymptomatic bacteriuria (ASB) on antenatal visit were supervised by a team of Obstetricians and Gynecologists on an outpatient basis and 200 patients (Group B) with symptoms of lower urinary tract infections/urethral instrumentation were supervised by a team of urologists in the Sher-i-Kashmir Institute of Medical Sciences. For all the patients the study was explained and written with an informed consent obtained.
Exclusion criteria:
History of allergy to fosfomycin
Irritable bowel syndrome
Current antibiotic therapy
Known anatomic urinary abnormality were excluded.
Protocol
First Visit
Group A. Patients were instructed to fill out the questionnaire form which included – demographic data, medical history, current antibiotic therapy, duration of amenorrhea and past obstetric history.
Group B. Patients were instructed to fill out the questionnaire form which included – demographic data, medical history, duration of lower urinary tract symptoms (irritative/obstructive symptoms) any fever with chills and rigors, unexplained hematuria and indications for cystoscopy and urethral instrumentation, if present.
After collecting information from both groups, routine antenatal laboratory tests were done in Group A. In Group B serum chemistry, liver function tests with a complete hemogram was done. Both groups were instructed to collect midstream urine samples for urinalysis and culture/sensitivity and within the same day a single dose of fosfomycin trometamol 3 gram sachet was given and advised to be taken in the evening before bed time with one glass of water. However, in Group B two more doses of the 3 gram sachet was advised to take on the 3rd and 5th day with obstructive and irritative LUTS associated with chills and rigors. On referral to our centers in both groups (ASB in pregnancy [200 pts]) and symptomatic lower urinary tract symptoms (72 pts) had urinalysis with bacteriuria done earlier before starting the oral fosfomycin trometamol. For patients with planned endourological day care procedures an oral single dose was given four hours before the procedure. Both groups were given a helpline number to call if any adverse events occurred.
Follow-up
Second visit. On Day 4 after the first visit, in both groups, a second midstream urine samples were sent for post-treatment microscopy and culture sensitivity. Any adverse effects and clinical improvements in the symptomology in symptomatic patients (Earlier LUTS) was noted.
Third visit. On Day 7, Group B completed a hemogram with urinalysis and culture sensitivity tests. Clinical improvement in the initial symptomatology (earlier LUTS) and adverse effects was noted.
Fourth visit. On Day 12, Group B patients again had a urinalysis and culture sensitivity test completed.
Evaluation of clinical and microbiological efficacy and post treatment urinalysis with adverse effects was assessed as:
- Clinical efficacy
- Resolution of symptoms completely (Earlier LUTS)
- Resolution of symptoms incompletely (Earlier LUTS)
- Microbiological efficacy
- Eradication of pathogenic bacteria
- Bacterial persistence
- Reinfection
- Adverse effects
- Mild discomfort
- Moderate discomfort
- Severe discomfort
- Post Treatment Urinalysis
- Complete Clearance of Bacteriuria
- Incomplete Clerance of Bacteriuria
RESULTS
In Group A, the majority of the patients were in the average socioeconomic status and were in the age group of 30–40 yrs. While in Group B, the majority of the patients were in the poor socioeconomic status and were in the age group <30 years (Table 1). Most of the urinary isolates grown on the culture were in the Enterobacteriaceae family (Table 2). Total E.Coli in both groups was 178 followed by ESBL (E.Coli) 25 in both groups. Gram-positive cocci were in 17 cases and polymicrobial infections were seen in 9 cases. No isolates were seen in 8 cases. However, adverse effects (Figure 1) were mild in the form of loose stools (diarrhea) (23.5%), itching in the genital area (19.75%), abdominal pain (3.75%) followed by back pain (3.75%). Side effects were transient and self-limited. Out of the 304 urinary isolates, the majority of isolates were sensitive to fosfomycin with bacterial eradication, bacterial persistence and bacterial reinfection being 96.3%, 3.9%, 3.9% respectively (Figure 2). In our study there were 25 isolates of the extended spectrum beta lactamases (ESBL) with bacterial persistence in 4 cases and bacterial reinfection in 1 case (Table 2). Out of the 25 isolates (ESBL), 20 (80%) were sensitive to fosfomycin.
Table 1.
Demographic characteristics and urinary isolates grown in both Groups (n = 400)
| Group A (n = 200) | Group B (n = 200) | p-value | |
|---|---|---|---|
| Socioeconomic status High Average Poor |
36 (18.0) 100 (50.0) 64 (32.0) |
27 (13.5) 77 (38.5) 96 (48.0) |
0.005* |
| Age group (years) ≤ 30 30-40 40-50 > 50 |
90 (45.0) 95 (47.5) 7 (3.5) 0 (0.0) |
73 (36.5) 45 (22.5) 47 (23.5) 10 (5.0) |
≤0.0001* |
| Gravida Primi Multi |
145 (72.5) 55 (27.5) |
– – |
|
| Microorganism E.Coli E.Coli (ESBL) Klebsiella pneumonia Pseudomonas aeurginosa Enterobacter species Coliform Bacteria Staphylococcus aeurus Group B streptococci Polymicrobial infections Enterobacter No isolates |
133 10 15 2 20 8 0 7 0 20 5 |
45 15 10 5 4 2 6 4 9 4 3 |
Significant at 5% level of significance values within parenthesis are percentages
Table 2.
Success rate of fosfomycin tromethamine in eradicating uropathogenic bacteria (Group A and Group B)
| Microrganism (No. of cases) | Groups | n | Eradication | persistence | Reinfection | p-value |
|---|---|---|---|---|---|---|
| E.coli (178) | A | 133 | 132 | 0 | 01 | 0.003* |
| B | 45 | 40 | 02 | 03 | ||
| E.coli (ESBL) (25) | A | 10 | 08 | 01 | 01 | 0.392 |
| B | 15 | 12 | 03 | 0 | ||
| Pseudomonas aeurginosa (7) | A | 02 | 02 | – | – | |
| B | 05 | 05 | – | – | ||
| Proteus (9) | A | 0 | – | – | – | |
| B | 09 | 05 | 02 | 02 | ||
| Coliform Bacteria (10) | A | 08 | 08 | – | – | |
| B | 02 | 02 | – | – | ||
| Klebsiella pneumonia (25) | A | 15 | 14 | – | 01 | |
| B | 10 | 06 | 02 | 02 | ||
| Staphylococcus aeurus (6) | A | 0 | 0 | 0 | – | |
| B | 06 | 05 | 01 | – | ||
| Group B streptococci (11) | A | 07 | 07 | – | – | |
| B | 04 | 04 | – | – | ||
| Polymicrobials (9) | A | 0 | – | – | – | |
| B | 09 | 08 | 01 | – | ||
| Enterobacter (24) | A | 20 | 18 | – | 02 | |
| B | 04 | 04 | – | – | ||
| No isolates (8) | A | 05 | – | – | – | |
| B | 03 | – | – | – | ||
| Total (304) (culture positive) |
Significant at 5% level of significance
Figure 1.
Adverse effects of Fosfomycin Tromethamine taken orally (n = 400).
Figure 2.
Overall clinical efficacy and post treatment urinalysis and culture (n = 400).
DISCUSSION
Fosfomycin, originally named phosphonomycin was discovered in Spain in 1969 [5]. It is a phosphonic acid derivative, with an extremely low molecular weight and shows no binding to proteins. Fosfomycin tromethamine has been extensively used in Europe for many years. In the USA, the FDA has approved oral fosfomycin only for uncomplicated UTIs. Fosfomycin is available in two oral formulations – fosfomycin tromethamine (synonym trometamol), a soluble salt with improved bioavailability over fosfomycin, which is synthetically prepared and fosfomycin calcium. There is also an intravenous formulation-fosfomycin disodium. However, fosfomycin tromethamine is the preferred formulation for oral administration because it is more readily absorbed into the blood compared to fosfomycin calcium [6, 7]. Fosfomycin is a bactericidal antibiotic in both Gram-negative and Gram-positive bacteria. Its unique mechanism of action may provide a synergistic effect to other antibiotics including beta-lactams, aminoglycosides and fluoroquinolones [8, 9, 10]. A single dose of fosfomycin tromethamine produces a therapeutic concentration in the urine for the 1-3 days reaching a peak concentration in the urine with 1053-4415 mg/l and the fosfomycin concentration in urine is maintained at levels greater than 12 mg/l for 24-48 hrs, which is sufficient to suppress a variety of pathogenic bacteria in the urinary tract [11]. Comparative clinical trials suggest that a single 3-g dose of fosfomycin tromethamine is as clinically effective as the 7-to 10-day treatment regimens of standard agents such as nitrofurantoin, norfloxacin, and trimethoprim/sulfamethoxazole used to treat UTIs. In pregnant woman, the incidence of a UTI can be as high as 8% [1]. It may be either an asymptomatic bacteriuria in pregnancy(ASB) or symptomatic acute cystitis and acute pyelonephritis [3]. However, a spontaneous resolution of bacteriuria in pregnant women is unlikely unless treated. In our prospective study patients received a single dose of 3 grams in ASB in pregnancy, 3 doses of fosfomycin on alternate days (day 1, 3rd and fifth day) in symptomatic lower urinary tract infections with documented bacteriuria and a single dose of 3 grams prophylactically for endourological intervention. In our study in Group A, patients with the ASB majority had an E.coli isolate on culture which was consistent with previous studies [12, 13, 14]. We observed an overall sensitivity in both Groups 94.95% for E.coli, ESBL E.coli 84% and for Klebsiella pneumonia 80%. Similair results were obtained by Falagas et al. [15]. The systemically reviewed 17 studies accounting for 5057 clinical isolates of Enterobacteriaceae evaluating antimicrobial activity of fosfomycin for infections caused by multidrug – resistant (MDR) Enterobacteriaceae using a provisional MIC Susceptibility breakpoint of 64 mg/l or less, the majority of E.Coli and Klebsiella pneumonia isolates producing ESBL were susceptible to fosfomycin (96.8% and 81.3% respectively). In our study the total Gram-positive bacteria were in 17 cases with 6 cases being Staphylococcus aureus with a sensitivity of 83.8% and 11 cases being Group B Streptococci with 100% sensitivity. Similar results were documented in other studies [16, 17]. These studies also concluded that fosfomycin is very effective against MDR pathogens such as methicillin resistant Staphylococcus (MRSA) and Vancomycin Resistant Enterococci. In our study 8 patients had no isolates grown on culture sensitivity even after the 48 hrs and required a trained laboratory staff in initial reporting of urinalysis of the 5 cases in Group A and the 3 cases in Group B to avoid unnecessary antibiotic usage. We also evaluated its role as a prophylactically single dose in endourological day care procedures. No published series is found in the literature of a single dose used in the day care of endourological procedures. In our study a total of 72 patients (Group B) underwent endourological procedures with a single oral dose of fosfomycin 3 grams given orally 4 hrs before the procedure. Only 8 patients had bacteriuria on Day 5 and were not symptomatic and so additional antibiotic was given, which subsequently got cleared on Day 7. Fosfomycin attains its peak plasma urinary concentrations within 4 hrs of dosing. After a single dose, urine fosfomycin levels >128 mg/l are maintained for at least 36–48 hrs. These levels are suffici ent to inhibit most urinary pathogens. Urinary fosfomycin levels are not compromised by mild degrees of renal insufficiency. With oral single/multiple doses it avoids injection related pain, need of paramedic personnel for injection and avoids post injection related complications (abscess). In our study the adverse effect was mainly diarrhea (2-3 loose stools) and genital itching which were transient and subsided without any medication. We also advocate in the absence of urine culture sensitivity data that oral fosfomycin tromethamine be given because of low resistance, compared with other commonly prescribed Quinolones and Penicillin derivatives.
CONCLUSIONS
Fosfomycin Trometamol is a bactericidal antibiotic with a broad-spectrum activity against Gram-positive and Gram-negative bacteriae. It has an advantage of oral single /multiple doses, higher eradication rate of bacteria after 48 hrs, excellent tolerability and safety in pregnancy and other female age groups. We recommend Fosfomycin Trometamol as the drug of choice particularly in patients with poor drug compliance and in addition for minor day care endourological procedures.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Patterson TF, Andriole VT. Bacteriuria in pregnanacy. Infect Dis Clin North Am. 1987;1:807–822. [PubMed] [Google Scholar]
- 2.Mikhail MS, Anyaegbunam A. Lower Urinary Tract dysfunction in pregnancy: a review. Obstet Gynecol Surv. 1995;50:675–683. doi: 10.1097/00006254-199509000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Kass EH, Finland M. Asymptomatic infections of the urinary tract. J Urol. 2002;168:420–424. [PubMed] [Google Scholar]
- 4.Stein G, Funfstuck R. Asymptomatic bacteriuria-what to do. Nephrol Dial Transplant. 1999;14:1618–1621. doi: 10.1093/ndt/14.7.1618. [DOI] [PubMed] [Google Scholar]
- 5.Hendlin D, Stapley EO, Jackson M, et al. Phosphonomycin. A new antibiotic produced by strains of streptomyces. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;46:1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 7.Woodruff HB, Mata JM, Hernandez S, et al. Fosfomycin: laboratory studies. Chemotherapy. 1977;23:1–22. doi: 10.1159/000222020. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Kanno H. Synergistic activities of combination of beta lactams, fosfomycin, and tobramycin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;26:789–791. doi: 10.1128/aac.26.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin NX, Neu NM, Neu HC. Synergy of fosfomycin with beta-lactam antibiotics against staphylococci and aerobic Gram-negative bacilli. Drugs Exp Clin Res. 1986;12:943–947. [PubMed] [Google Scholar]
- 10.Okazaki M, Suzuki K, Asano N, et al. Effectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assay. J Infect Chemother. 2002;8:37–42. doi: 10.1007/s101560200004. [DOI] [PubMed] [Google Scholar]
- 11.Patel SS, Balfour JA, Bryson HM. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs. 1997;53:637–656. doi: 10.2165/00003495-199753040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kutlay S, Kutlay B, Karaahmetoglu O, Ak C, Erkaya S. Prevalance, detection and treatment of asymptomatic bacteriuria in a Turkish obstetric population. J Reprod Med. 2003;48:627–630. [PubMed] [Google Scholar]
- 13.Teppa RJ, Roberts JM. The uriscreen test to detect significant asymptomatic bacteriuria during pregnancy. J Soc Gynecol Investig. 2005;12:50–53. doi: 10.1016/j.jsgi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Enayat K, Fariba F, Bahram N. Asymptomatic bacteriuria among pregnant woman referred to outpatient clinics in Sanandaj, Iran. Int Braz J Urol. 2008;34:699–704. doi: 10.1590/s1677-55382008000600004. [DOI] [PubMed] [Google Scholar]
- 15.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010;10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 16.Maraki S, Samonis G, Rafailidis PI, Vouloumanou EK, Mavromanolakis E, Falagas ME. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob Agents Chemother. 2009;53:4508–4510. doi: 10.1128/AAC.00721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu CL, Liu CY, Huang YT, et al. Antimicrobial susceptibilities of commonly encountered bacterial isolates to fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother. 2011;55:4295–4301. doi: 10.1128/AAC.00349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]