Abstract
Introduction
Long-term outcomes of patients treated for invasive bladder cancer in Poland are poorly documented in the literature. Impact of various clinical parameters on their survival is even less well studied. Radical cystectomy is a major surgery, so the patients’ condition can be equally important as cancer stage. The aim of the study was to assess 5-year overall survival (OS) after cystectomy and impact of comorbidity on OS in a single Polish academic centre.
Material and methods
Clinical data of all patients who underwent cystectomy in years 2004-2006 for urothelial cancer were retrospectively reviewed. Survival status was determined at least 5 years after surgery. Pathological variables, comorbidities, surgery delay and complications were evaluated as potential predictors of OS. Kaplan-Meier estimates of the survival function as well as Cox proportional hazards models were utilized.
Results
Thirty-day, 1-year and 5-year OS for 63 patients was 98.4%, 58.7% and 31.7%, respectively. None of the investigated parameters were significantly related to five-year OS. However, a composite parameter consisting of stage, diabetes status and postoperative course was found as a significant predictor. Five-year OS in 16 patients with pT1-2 and without diabetes and without post-operative complications was higher than in the remaining 47 patients (56% vs. 23%; P = 0.02).
Conclusions
Five-year OS in our group was lower than in most published international series but concordant with a previous Polish report. Improvement in survival after radical cystectomy may be expected when early diagnosis will be accompanied by optimal care of patients with diabetes mellitus and avoidance of postoperative complications.
Keywords: bladder cancer, lymphadenectomy, cystectomy, survival, diabetes mellitus, postoperative complications
INTRODUCTION
Around 15–20% of primary bladder tumours are initially diagnosed as invasive urothelial carcinoma [1]. It is considered the second most common cause of death among urological malignancies. Radical cystectomy with bilateral pelvic lymphadenectomy is the most effective treatment with 5-year overall survival (OS) reported between 50 and 70% according to large, recently published series [2–9]. For organ confined, lymph node negative disease, 5-year OS is estimated to be even as high as 80%. However, in patients with extravesicular disease and lymph node involvement this drops to 25% [8].
Stage, grade and nodal status are proved as the most significant predictors of OS with hazard ratio exceeding 2 [9]. Other potential factors include: neoadjuvant chemotherapy [9], smoking habits [9], general condition status [10], age [11], American Society of Anesthesiologists score [12], Eastern Cooperative Oncology Group score, Elixhauser index [13] or delay time from first symptoms to surgery [14].
In Poland, the mortality rate among patients after cystectomy has not been investigated thoroughly. Age-standardized mortality rates for bladder cancer in Poland and in other European countries are around 4.0/100000 [15, 16]. This fundamental epidemiological parameter may reflect various processes at a high scale, being unsuitable to evaluate precisely treatment effectiveness.
With only one report from a Polish department published in recent years [17] we decided to investigate 5-year OS and to identify preoperative factors related to OS among patients treated in our department.
MATERIAL AND METHODS
This retrospective study involved all patients who underwent radical cystectomy for urothelial carcinoma in years 2004–2006 in a single Polish academic centre. Patients who were diagnosed with bladder malignancy other than urothelial cancer, as well as patients with no relevant clinical information in the medical records, were excluded from the analysis.
Preoperative staging included transurethral resection of the tumour, bimanual examination, intravenous urography, chest X-ray, abdominal CT and ultrasound of the urinary tract. Patients with distant metastases were referred to a regional oncological centre. A decision to implement lymphadenectomy and its extent was left to the discretion of a surgeon. Neoadjuvant or adjuvant radio- or chemotherapy was not used. Chemotherapy was given only to patients with distant metastases found in follow-up.
All specimens obtained during surgery were examined by a single pathologist. Staging was performed according to the 2002 TNM classification [18]. The primary endpoint was a 5-year OS. Data considering mortality were acquired from the Republic of Poland National Registry of Residents, which gathers information on all citizens of Poland including dates of death. This information was available for all patients.
Medical data was extracted from hospital records retrospectively. The following variables were tested for significance in the survival analysis: stage and grade of the tumour, lymphadenectomy status, lymph node invasion, time from first episode of haematuria to cystectomy, time from staging resection (or the last resection in case of recurrent tumours) to cystectomy, age, comorbidities, age-adjusted Charlson Comorbidity Index, perioperative and postoperative complications.
OS was assessed as the time from cystectomy to the date of death. Patients who were still alive were censored at the date of December 31st, 2011. Differences in OS between the subgroups stratified according to values of clinical parameters were compared with the log-rank test. A multivariate survival model was constructed using the Cox proportional hazards regression with P < 0.05 being considered statistically significant.
Distribution of values for analysed variables was checked for normality with a Shapiro-Wilk test. When distribution was proved to be normal, mean and standard deviation values were presented. Descriptive statistics of other variables were presented as median values and quartiles in brackets.
Statistical calculations were performed with STATISTICA 10 software (StatSoft Inc., Tulsa, USA)
RESULTS
In the defined period of time, 89 radical cystectomies were performed. For 26 patients diagnosis was other than invasive urothelial cancer or relevant clinical information was missing. They were excluded from the analysis. Median age of the 63 enrolled patients was 67 (59; 75). Median time from the first episode of haematuria to cystectomy was 7 (2.5; 34) months and median time from the last resection to cystectomy was 1.5 (1; 2.5) months. Information about smoking was incomplete and therefore this parameter was not tested. Median value of age-adjusted Charlson Comorbidity Index was 2 (0; 4). Other clinical and pathological characteristics of the group are summarized in Table 1. Significant comorbidities were present in 33 (52.4%) patients (Table 2).
Table 1.
Patients’ clinical and pathological characteristics
| Variable | N (%) |
|---|---|
| Gender | |
| Male | 46 (73%) |
| Female | 17 (27%) |
| Pathological stage | |
| pT0 | 2 (3.2%) |
| pT1 | 2 (3.2%) |
| pT2 | 22 (34.9%) |
| pT3 | 30 (47.6%) |
| pT4 | 7 (11.1%) |
| Grade | |
| G0 | 2 (3.2%) |
| G2 | 31 (49.2%) |
| G3 | 30 (47.6%) |
| Lymph node status | |
| Nx | 17 (27%) |
| pN0 | 29 (46%) |
| pN+ | 17 (27%) |
| Urinary diversion | |
| Ileal conduit | 60 (95.2%) |
| Neobladder | 1 (1.6%) |
| Ureterosigmoidostomy | 2 (3.2%) |
Table 2.
Incidence and significance of clinical variables for overall survival. Univariate analysis for the whole group (n = 63)
| Variable | N | 5-year OS (present vs. absent) | P for log-rank test |
|---|---|---|---|
| Significant comorbidity | 33 (52%) | 24% vs. 40% | 0.42 |
| Coronary artery disease | 19 (30%) | 32% vs. 32% | 0.63 |
| Myocardial infarction | 7 (11%) | 29% vs. 31% | 0.78 |
| Diabetes mellitus | 10 (16%) | 10% vs. 36% | 0.08 |
| Chronic kidney disease | 8 (13%) | 37% vs. 31% | 0.99 |
| Chronic obstructive pulmonary disease or asthma | 5 (8%) | 20% vs. 33% | 0.91 |
| Cerebrovascular event | 3 (5%) | 33% vs. 32% | 0.83 |
| Time from TUR to cystectomy ≤3 vs. >3 months | 10 (16%) | 40% vs. 30% | 0.71 |
| Time from haematuria ≤6 vs. >6 months | 24 (38%) | 42% vs. 30% | 0.91 |
| Early postop. complication | 16 (25%) | 25% vs. 34% | 0.099 |
| Stage T3-T4 | 37 (59%) | 24% vs. 42% | 0.16 |
| Grade G3 | 30 (48%) | 33% vs. 29% | 0.73 |
| Nodes N+ | 17 (37%)* | 23% vs. 38% | 0.14 |
OS – overall survival; Log-rank test was calculated for all available follow-up, not censored at 5 years.
Calculated for 46 patients with known node status
Radical cystectomy was accompanied by lymphadenectomy in 46 (73%) patients. Postoperative complications occurred in 16 patients (Table 3).
Table 3.
Type and severity of early postoperative complications according to Clavien-Dindo classification
| Grade | Patients | Specific complication | Events |
|---|---|---|---|
| II | 5 | Transient cardiac ischemia | 2 |
| Monoparesis | 1 | ||
| Epididymitis | 1 | ||
| Pneumonia | 1 | ||
| IIIb | 9* | Intestinal anastomosis leakage | 2 |
| Ileus | 3 | ||
| Ureteric stricture | 1 | ||
| Eventration | 3 | ||
| Urinary leakage | 3 | ||
| IV | 0 | ||
| V | 2 | Gastrointestinal bleeding | |
| Septic shock | 1 |
Three patients experienced more than one complication.
Clavien-Dindo Grade I complications are not reported.
Five-year OS status was established for all patients. Median time of follow-up for patients with censored data was 81 (72; 89) months. Thirty-day mortality was 1.6%. Thirty-seven patients (58.7%) were alive after one year. Two-year and 5-year OS was 41.3% and 31.7% respectively. Median survival time was 16.5 months. Logistic regression analysis showed that death from any reason within 5 years from surgery was not significantly related to any of the potential clinical and pathological variables. Only for coexistence of DM, a trend was found with p = 0.08 in a univariate analysis. Results of the log-rank test for dichotomized qualitative variables are presented in Table 2. Graphic analysis of Kaplan-Meier curves indicated that high stage (T3-4), postoperative complications and diabetes mellitus (DM) influenced survival the most.
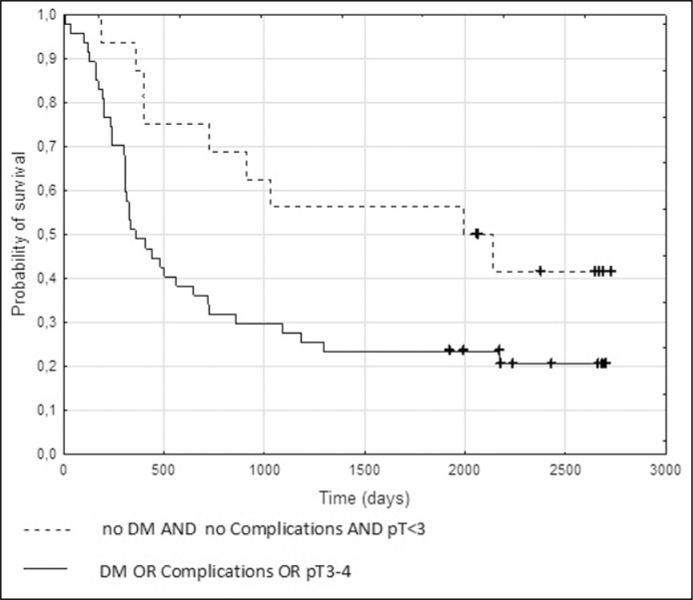
Using Cox proportional hazards regression, a trend towards significance (P = 0.054) was found for the model consisting of the three variables with the lowest P values in univariate analysis: stage, diabetes mellitus status and postoperative complications. N-status was excluded for its lack of complete data. Nine of sixteen (56%) patients with stage pT1-2, without diabetes mellitus and without postoperative complications survived 5 years, while among 47 people with at least one of the adverse factors 5-yr survival was 23% (P = 0.02 in the log rank test; odds ratio 4.2, 95% confidence interval 1.3 – 13.9; Figure 1).
Figure 1.
Kaplan-Meier curves depicting overall survival after radical cystectomy in patients with: pT1-2 tumours AND no diabetes mellitus (DM) AND no complications (n = 16) versus patients with: pT3-4 tumours OR DM OR complications (n = 47). The difference in survival is statistically significant (P = 0.02 in the log-rank test).
DISCUSSION
Five-year OS in this study (31.7%) was slightly higher than previously reported by another Polish academic centre (5-year OS: 25% [19]), but substantially inferior to large series from recognized centres reported in recent years [3–9]. However, in smaller groups from less well-known institutions, survival rates are also close to ours [20, 21]. Many factors may contribute to such an outcome, such as advanced stage at presentation, delay of treatment, improper operative technique, perioperative complication rate or health status of patients.
Since delay in treatment is considered to be one of the factors affecting stage and prognosis, we paid special attention to this issue. Reputable centres showed median delay from last resection of 2-3 months [4, 9]. With median delay of 1.5 month and median time from the first episode of haematuria to surgery of 7 months it seems that our patients did not wait longer and it should not have affected treatment outcome. Despite that, the rate of locally advanced disease was much higher (58.7%) than in most published studies (19–52% [3–9]), although it was also noticeably lower than reported by Lemiński et al. (70% [19]).
Apart from disease stage, two other issues may explain poor outcome of RC in our hands: extent of lymphadenectomy and use of chemotherapy. In years 2004-2006 extended bilateral lymphadenectomy was not performed routinely in our department. Although rate of positive nodes (27%) was not much different from other studies (18–25%), in a quarter of patients status of nodes was deficient, while in the others extent of LND did not always meet current standards. There is a lot of evidence for positive effect of extended LND on survival, thus we believe that this issue could seriously impact the outcome. The effect of neoadjuvant and adjuvant therapy is not very obvious. Adjuvant therapy was used in 20% of patients from previously cited series. Neoadjuvant chemotherapy was used less frequently or was not used at all. In our group neither neoadjuvant nor immediate adjuvant chemotherapy was given unless distant metastases were diagnosed. Regarding adjuvant treatment, our approach was consistent with international recommendations [22]. At that time only few studies, all with significant drawbacks, addressed that topic. Recently, recommendations have been changed and now adjuvant chemotherapy in case of pT3/4 or pN+ disease has been accepted [23]. Our current approach follows these recommendations.
Both ours and Lemiński's studies show results inferior to those available in international literature. Another explanation of this phenomenon is worse health status of Polish patients. Life expectancy for men in Poland is 73.5 years, which is 4-6 years shorter than for most Western European countries. Also, tobacco consumption is more widespread [24] and cardiovascular status is worse [25]. With over 30% incidence of coronary artery disease and 15% incidence of DM our group is representative of the Polish population and stands for high risk of perioperative complications.
Effect of DM on survival is of special interest. With P = 0.08 in univariate survival analysis this factor showed the strongest trend towards significance. There are very few studies that discuss impact of specific comorbidities on OS after cystectomy [26]. However, during the last decade some authors reported on the relationship between DM and urothelial carcinoma [27, 28]. Recently, the association of DM with OS after radical treatment of urothelial carcinoma has also been found [29, 30]. This relation has yet to be explained. Possible mechanisms include micro- and macroangiopathy and nephropathy leading to increased risk of death from non-oncological reasons.
Our data suggest that early postoperative complications are also related to poor outcome. Complications result either from surgical quality or from patient health status. However, in our analysis early complications were stronger predictors of poor outcome than nodal status or Charlson Comorbidity Index.
Higher stage, grade and positive lymph nodes are established predictors of survival. Lack of significance in differences between OS for pT2 vs. pT3-4 tumours and for pN vs. pN+ is mainly caused by the inadequate size of our group. No difference between G2 and G3 tumours can be assigned to inconsistence in grade evaluation, although one experienced uropathologist was responsible for this process in all cases. Also, with longer follow-up, predictive value of oncological variables is decreasing and factors related to general health status become more important [6].
It is quite likely that the presented 5-year OS for patients treated 10 years ago does not correspond to our contemporary series of patients, as we have significantly modified our management. Selection according to general health status and extended LND has become a standard, while ureterosigmoidostomy was abandoned. Another study will clarify the role of parameters discussed here for RC results.
CONCLUSIONS
The effect of cystectomy on survival of patients with muscle-invasive urothelial bladder cancer is not homogenous. Mortality rate in Polish centres is much higher than in most published series. The explanation for this is multifactorial. Worse performance status of patients and less extensive lymphadenectomy are possible reasons. The role of adjuvant therapy is less clear. While extended lymphadenectomy has become a standard, our attention must be focused on optimal management of vascular and metabolic comorbidities, as well as on the prevention of early postoperative complications.
CONFLICTS OF INTEREST
Bartosz Dybowski is the deputy editor-in-chief of the Central European Journal of Urology.
References
- 1.Heney NM. Natural history of superficial bladder cancer. Prognostic features and long-term disease course. Urol Clin North Am. 1992;19:429–433. [PubMed] [Google Scholar]
- 2.Bachir BG, Aprikian AG, Fradet Y, et al. Regional differences in practice patterns and outcomes in patients treated with radical cystectomy in a universal healthcare system. Can Urol Assoc J. 2013;7:E667–672. doi: 10.5489/cuaj.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruins HM, Arends TJ, Pelkman M, Hulsbergen-van de Kaa CA, van der Heijden AG, Witjes JA. Radical cystectomy in a Dutch University hospital: long-term outcomes and prognostic factors in a homogeneous surgery-only series. Clin Genitourin Cancer. 2014;12:190–195. doi: 10.1016/j.clgc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. 2012;61:1039–1047. doi: 10.1016/j.eururo.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–696. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 6.Ploussard G, Shariat SF, Dragomir A, et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol. 2014;66:361–370. doi: 10.1016/j.eururo.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Sonpavde G, Khan MM, Lerner SP, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol. 2011;185:456–461. doi: 10.1016/j.juro.2010.09.110. [DOI] [PubMed] [Google Scholar]
- 8.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 9.Yafi FA, Aprikian AG, Chin JL, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a Canadian multicentre experience. BJU Int. 2011;108:539–545. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 10.Psutka SP, Carrasco A, Schmit GD, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer. 2014;120:2910–2918. doi: 10.1002/cncr.28798. [DOI] [PubMed] [Google Scholar]
- 11.Comploj E, West J, Mian M, et al. Comparison of complications from radical cystectomy between old-old versus oldest-old patients. Urol Int. 2015;94:25–30. doi: 10.1159/000358731. [DOI] [PubMed] [Google Scholar]
- 12.Breyer J, Denzinger S, Otto W, et al. Outcome of patients with pathological tumor stage T3 urothelial carcinoma of the bladder following radical cystectomy in a single-center series with 116 patients. Urol Int. 2014;93:311–319. doi: 10.1159/000360483. [DOI] [PubMed] [Google Scholar]
- 13.Boorjian SA, Kim SP, Tollefson MK, et al. Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol. 2013;190:55–60. doi: 10.1016/j.juro.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Poletajew S, Lisiński J, Moskal K, et al. The time from diagnosis of bladder cancer to radical cystectomy in Polish urological centres – results of CysTiming Poland study. Cent European J Urol. 2014;67:329–332. doi: 10.5173/ceju.2014.04.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Wojciechowska U, Didkowska J, Zatoński W. Warsaw: Polish National Cancer Registry Department of Epidemiology and Cancer Prevention, Maria Sklodowska-Curie Memorial Cancer Centre; 2012. Cancer in Poland in 2010. [Google Scholar]
- 17.Lemiński A, Słojewski M, Sikorski A. Przeżycie chorych z naciekającym rakiem pęcherza moczowego poddanych cystektomii. Urol Pol. 2006;59:182–185. [Google Scholar]
- 18.Sobin L H, Wittekind C. TNM classification of malignant tumours. New York: Wiley-Liss; 2002. p. 239. [Google Scholar]
- 19.Lemiński A, Puszynski M, Kups M, Slojewski M, Sikorski A. C27 Results of surgical treatment of invasive bladder tumors in Poland–Single center observation of 402 patients after radical cystectomy. Eur Urol Suppl. 2012;11:88. [Google Scholar]
- 20.Basiri A, Pakmanesh H, Tabibi A, et al. Overall survival and functional results of prostate-sparing cystectomy: a matched case-control study. Urol J. 2012;9:678–684. [PubMed] [Google Scholar]
- 21.Kucuk U, Pala EE, Cakir E, et al. Clinical, demographical and histopathological prognostic factors for urothelial carcinoma of the bladder. Cent European J Urol. 2015;68:30–36. doi: 10.5173/ceju.2015.01.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenzl A, De Santis M, Jakse G. Guidelines on muscle-invasive and metastatic bladder cancer; Arnhem; Stockholm, Sweden: EAU Guidelines Edition presented at the 24th EAU Annual Congress; 2009. pp. 38–39. [Google Scholar]
- 23.Witjes JA CE, Cowan NC, De Santis M. Guidelines on muscle-invasive and metastatic bladder cancer. EAU Guidelines Edition presented at the 30th EAU Annual Congress; Madrid. 2015. p. 31. [Google Scholar]
- 24.Kaleta D, Usidame B, Dziankowska-Zaborszczyk E, Makowiec-Dabrowska T, Leinsalu M. Prevalence and factors associated with hardcore smoking in Poland: findings from the Global Adult Tobacco Survey (2009-2010) BMC Public Health. 2014;14:583. doi: 10.1186/1471-2458-14-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35:2929. doi: 10.1093/eurheartj/ehu378. [DOI] [PubMed] [Google Scholar]
- 26.Novotny V, Zastrow S, Koch R, Wirth MP. Radical cystectomy in patients over 70 years of age: impact of comorbidity on perioperative morbidity and mortality. World J Urol. 2012;30:769–776. doi: 10.1007/s00345-011-0782-0. [DOI] [PubMed] [Google Scholar]
- 27.Kravchick S, Gal R, Cytron S, et al. Increased incidence of diabetes mellitus in the patients with transitional cell carcinoma of urinary bladder. Pathol Oncol Res. 2001;7:56–59. doi: 10.1007/BF03032606. [DOI] [PubMed] [Google Scholar]
- 28.Woolcott CG, Maskarinec G, Haiman CA, Henderson BE, Kolonel LN. Diabetes and urothelial cancer risk: the Multiethnic Cohort study. Cancer Epidemiol. 2011;35:551–554. doi: 10.1016/j.canep.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang I, Jung SI, Nam DH, et al. Preoperative hydronephrosis and diabetes mellitus predict poor prognosis in upper urinary tract urothelial carcinoma. Can Urol Assoc J. 2013;7:e215–220. doi: 10.5489/cuaj.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieken M, Xylinas E, Kluth L, et al. Effect of diabetes mellitus and metformin use on oncologic outcomes of patients treated with radical cystectomy for urothelial carcinoma. Urol Oncol. 2014;32:49 e7–14. doi: 10.1016/j.urolonc.2013.07.006. [DOI] [PubMed] [Google Scholar]