Abstract
To establish the extent of varicocele as the cause of infertility in men and compare the various techniques of treatment.
We searched PubMed and the Cochrane Library database using varicocele, male infertility, varicocelectomy as keywords.
Varicocele seems to be a growing problem considered to be one of the most common causes of male infertility in recent times. Nevertheless, its role remains unclear. The best treatment option seems to be microscopic surgery – the most effective and linked to rare surgical complications. But the greatest clinical problem remains the selection of patients to treat – recently it is believed that varicocelectomy is a possibly advisable option in patients with clinical varicocele and seminal parameter impairment [1].
More high-quality, multicenter, long-term randomized controlled trials (RCT's) are required to verify the findings.
Keywords: varicocele, varicocelectomy, male infertility, infertile, pregnancy rate
INTRODUCTION
The World Health Organization (WHO) defines infertility as the inability to achieve pregnancy within 12 months of regular intercourse for couples in conception [2]. Male infertility is a growing problem in developed countries all around the world. It is estimated that one in seven couples in the UK experience some difficulty conceiving at some point in their reproductive study. Sixteen percent of couples will fail to conceive after 1 year of attempts. Infertility affects 13–20% of couples in Poland [3]. It is estimated that the male factor of couple infertility is between 25–50% [2] with a mean value around 30% [3]. The pattern of infertility in Poland shows an increase in the rate of the male factor (57.8%) and a decrease in the rate of the female factor (7.72%). The cumulative male factor frequency was calculated as 76%, while the female was 26% [4]. Demographic data on UK fertility rates provides evidence to suggest that male infertility is increasing. This, however, may be the result of increasing publicity of available treatments and thus may reflect an increasing willingness of couples to seek advice and treatment [3].
Causes of male infertility are usually classified into three groups [3]:
- Pretesticular
- Hypothalamic disease – Kallmann syndrome
- Pituitary disease – tumors, radiation, surgery, hyperprolactinemia, anabolic steroids, hyper / hypothyroidism
- Testicular
- Congenital – Klinefelter syndrome, Noonan syndrome, cryptorchidism
- Acquired – injury, surgery, varicocele, systemic disease (renal failure) chemotherapy, testicular tumors
- Post – testicular
- Congenital – CF (Cystic Fibrosis)
- Acquired – vasectomy, infections (Chlamydia)
- Immunological
- Sexual dysfunction – erectile, ejaculatory function, diabetes, multiple sclerosis,
Epidemiology
The most common cause of male infertility is varicocele – a surgically correctable or at least improvable form of infertility. They are present in 15% of the normal male population and in up to 40% of patients with male infertility. It is believed to be the cause of up to 35% of primary infertility and 69-81% of secondary infertility [5]. The causes of varicocele are multifactorial, but at the end the result is a pathological dilatation of the veins draining the testicles [6].
Harrison after inducing varicocele in monkeys confirmed that increased pressure on the venous side of testicular capillary beds decreases blood flow and might be the cause of testicular damage. The most significant changes were observed in the left testis (with induced varicocele), but the contralateral testis was also affected [7]. Elevated temperature due to the induced varicocele decreased sperm concentration, motility grade, motile and living spermatozoa count leading to infertility in rabbits [8]. Bilateral intratesticular decreases in testosterone concentrations within weeks after the varicocele induction was proved in rat models [9]. A similar phenomenon with an accompanying normal testosterone concentration in peripheral plasma is observed in humans. Wang studying the mechanism of varicocele induced infertility investigated the expression of the α subunit of hypoxia – inducible factor (HIF-1α) willing to determine the apoptosis index (AI) in varicocelised rat testes. Bilateral testicular hypoxia and increased germ cell apoptosis can be caused by left- sided varicocele leading to testicular dysfunction [10].
With a regard to the ethical aspects, invasiveness and population, the variable status of the varicocele, age and fertility, it is difficult to design similar studies in humans. Because of that the precise mechanism through which varicocele impairs male fertility still remains uncertain. Hypotheses implicating the reflux of renal/adrenal toxic metabolites, testicular hypoxia due to venous stasis, hormonal dysfunction, hypertension in the internal spermatic veins, and increase in the testicular temperature delineates the current understanding of the pathophysiology. The effect of varicocele is induced by the elevation of temperature due to the reflux of warm abdominal blood, which leads to the reduction of testosterone synthesis in Leydig cells, as well as the reduction in the function of Sertoli cells [11]. The scrotal temperature reduces in infertile men after varicocelectomy [12].
Recently, several studies suggested an association between oxidative stress and impaired sperm function in patients with varicocele, as evidenced by the increased levels of reactive oxygen species (ROS) and reduced antioxidant capacity [2, 13]. These parameters may lead to increased DNA damage such as DNA fragmentation connected to reduced fertilization in both normal fertilization and assisted reproductive techniques [14]. In a meta-analysis by Agarwal it was suggested that oxidative stress-induced injury was one of the main causes of varicocele related infertility [15, 16].
Although a Cochrane database suggested no benefit of varicocele treatment in relation to a couple's chances of conception compared with control subjects, this meta-analysis included patients with subclinical varicocele or normal semen analyses [17, 18]. Ficarra in 2006 reviews recent (in 2006) available RCT's giving critical opinion about the studies – emphasizing heterogeneity and poor methodological quality [1].
Abdel-Meguid A. et al. [19] was aware of the contraindicatory findings in multiple trials and so designed a prospective, non-masked parallel-group RCT measuring pregnancy rate (PR), changes of semen parameters and adverse events. Outcomes of PR achieved 13.9% at control arm and 32.9% of varicocele treated patients. This trial reached the number needed to treat (NNT) 5.27 patients.
Finally in the Marmar et al. [20] study, after identification of the 101 studies relevant in the subject, only 5 were included due to meeting the requirements. In the meta- analysis, a 33% pregnancy rate was reported in patients who underwent the surgical treatment of varicocele in comparison to the 15.5% PR in the control groups receiving no treatment [21]. NNT was 5.7. These studies prove superiority of varicocelectomy over observation at level 1b evidence. Due to this fact varicocele is nowadays recognized as the most surgically correctable cause of male infertility.
Treatment
There are several surgical techniques for the varicocele treatment. That includes embolization (interventional radiology), open non – microsurgical, laparoscopic and microsurgical techniques. Each of these techniques has been well described in the literature, including the advantages and disadvantages and so because of that it is from the clinical point of view worth establishing which of these seems to be the best. When comparing all these techniques it is important to take under consideration parameters such as: pregnancy rates, postoperative complications such as hydrocele formation, recurrence or persistence (in recent meta-analysis semen quality improvement is rarely taken under consideration).
Before surgical intervention may be considered basic diagnostic rules should be obeyed. Routine evaluation of infertile men with varicocele should include a medical and reproductive history, physical examination (incl. digital rectal examination – prostatitis) and a minimum of two semen analyses [6]. Indications for varicocele treatment are: Grade II (palpable varicocele), couple known infertility, females correct fertility (or treatable cause of infertility), abnormal semen quality. Some authors suggest that all of the four criteria must be met to qualify a patient for surgical treatment [6].
The best treatment option for varicocele should be to include higher seminal improvement and PR with low complication rates (recurrence, persistence hydrocele formation, atrophy of the testis). Therefore, the ideal technique should aim for the ligation of all internal and external spermatic veins with preservation of spermatic arteries and lymphatics [22].
Cayan reports the highest spontaneous pregnancy rate after microsurgical techniques – 41.97%, in comparison to the Palomo technique (open non-micro technique) – 36% and 30.07% with laparoscopic approach [22]. Overall recurrence rates is 14.97% in the Palomo technique (2.63% – Ivanissevich), 1.5% in microsurgery and 4.3% in laparoscopy [22]. Hydrocele – the most common long time surgical complication – forms most commonly after the Palomo techniques – ca 8%, 2.8% after laparoscopy and only in 0.4% after microsurgical intervention. Diegidio reports similar data on PR 44.75% after subinguinal microsurgical intervention, 34.21% – after Palomo, 31% after embolization and 27% after laparoscopy, respectively [6].
Studies suggest that varicocele repair significantly increases sperm parameters – sperm concentration, motility and total motile sperm count. What is more is that varicocelectomy is considered to have a positive effect on Leydig cell function, improving serum testosterone levels [19, 23, 24]. This effect is rarely included in recent meta-analyses because of the lack of the studies on this subject and no universal comparison techniques. In addition, spontaneous pregnancy rate is considered the best indicator to assess fertility status. Only a few recent studies report pre- and postoperative hormone levels, especially the testosterone level. All of these [24, 25, 26] report a significant increase after surgery. Campbell and Walsh (Urology 10th edition) suggested that varicocelectomy might improve semen analysis in 60-80% of men [27]. Microsurgical varicocelectomy results in the return of sperm in the ejaculate in up to 60% of azoospermic men with palpable varicocele. The results in varicocelectomy are also related to the size of the varicocele. The repair of large varicocele results in a significantly greater improvement in the semen quality than repair of small varicocele.
Surgical options
Apart from statistics each of the highlighted techniques has its own advantages and disadvantages (Table 1).
Table 1.
Outcomes of different surgical treatment options
| Pregnancy rate | Recurrence | Hydrocele formation | |
|---|---|---|---|
| Open surgery | 33.57-37.6% | 5.51-14.97% (Palomo) 2.63% (Ivanissevich) |
8.24-9.09% (Palomo) 7.3% (Ivanissevich) |
| Laparoscopy | 30.07-40.4% | 4.3–6.1% | 2.84% |
| Microsurgical | 41.97-50.9% | 0-1.05% | 0.44% |
| Radiological embolization | 31.93-33.2% | 4.29-12.7% | Not available – not typically seen |
Palomo – probably the least challenging, low cost technique does not allow the ligation of the external gonadal vessels and external spermatic vein (secondary cause) which seems to be the cause of recurrence and/or persistence of varicocele after surgical treatment (estimated to be between 11% and 15%) [21]. There are two modifications of this technique – the inguinal (Ivanissevich) and subinguinal approaches. The subinguinal approach benefits with preserving the muscle layers and inguinal canal; however, it is also technically challenging due to the greater number of spermatic veins and arteries below the ring. Modifications of these techniques consist of the injection of dye into the lymphatics and in addition the Doppler test in order to improve the recognition of vessels.
Laparoscopy enables higher magnification (than other non- micro techniques) – this allows for the identification and preservation of the internal spermatic artery and lymphatic vessels which is considered to prevent testicular atrophy and minimize hydrocele formation. On the other hand, this method does not allow for the approach to the external spermatic veins which are considered to be the second cause of varicocele. What is more is that this technique is still considered to be fairly invasive (due to the general anesthesia with complete control over the respiratory system). Besides it requires some laparoscopic experience from the surgeon (learning curve) – due to the technique itself and the most challenging part – differing vessels. It also has a risk of intestinal and major vascular injuries during needle or trocar insertion. In present review, 7.6% of the patients had major complications with laparoscopic varicocelectomy [22].
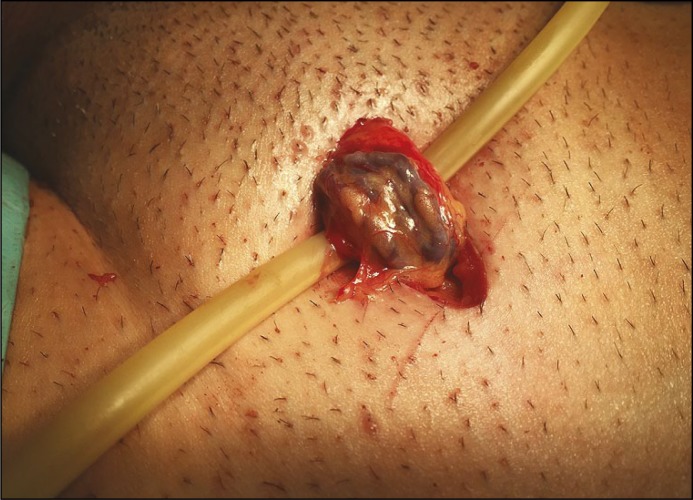
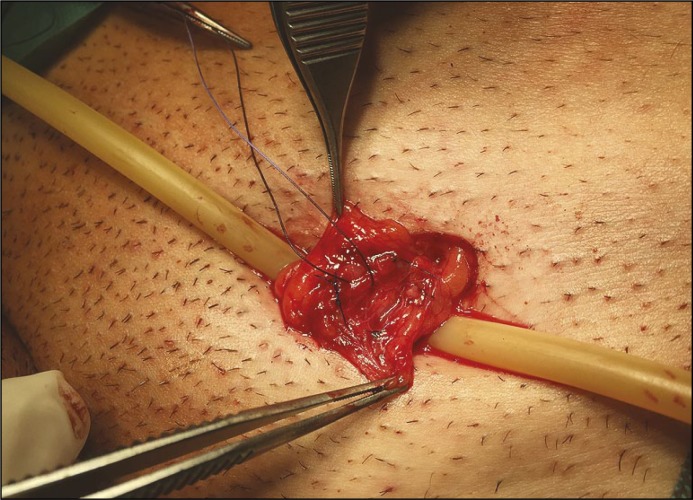
Microsurgical varicocelectomy seems to have only one disadvantage – operation duration which is significantly longer than other surgical techniques that require training. What is more is that the microscope is now brought into the operating field in order to improve the ability in differing arteries, veins and lymphatic vessels (Figure 1) which is considered to significantly decrease the incidence of hydrocele formation, testicular artery injury (and testicular atrophy) and recurrence (Figure 2).
Figure 1.
Microsurgical varicocelectomy. Spermatic cord delivered to the operative field just before introducing microscope (own material).
Figure 2.
Microsurgical varicocelectomy. Veins after isolation and suture ligation. Identified spermatic artery – marked with a different colour suture (own material).
Lately, the discussion over the role played by artery sparing during surgical approaches has returned. Fast et al. (2013) [28] reports that after 41 laparoscopic artery sparing varicocelectomies there was no testicular atrophy, but surprisingly there was an increase in the rate of persistency/recurrence of varicocele (12.2% vs. 5.4%). Due to the small number of patients and lack of other RCTs, more investigation is needed on this subject [28].
Guidelines
The most up to date 2014 EAU guidelines have been published. According to this, the varicocele treatment should be considered in cases of a clinical varicocele, oligospermia, infertility duration of ≥2 years and otherwise unexplained infertility in the couple (level of evidence: A). On the other hand, there is no evidence indicating any benefit from varicocelectomy in infertile men who have normal semen analysis or in men with subclinical varicocele – in such situations treatment is not recommended (level of evidence: A) [29].
Varicocele treatments in different age groups
Infertility problems are nowadays more often seen in older patients due to social changes. A worthy discussion seems to be the cut- off line of age when the varicocele should be offered as an infertility treatment option. El-Shazly [30] compares two age groups of patients with clinically detectable bilateral varicocele and accompanying infertility – 83 patients; Group I (55 patients) with the age ranging from 25 to 40 years, and Group II (28 patients) with the age >40 years (range 41–53 years). In all cases laparoscopic varicocelectomy was performed. The only parameter compared was semen quality. After 3 months, 26 cases (47.2%) of Group I and 11 cases (39.2%) of Group II had improvement in semen quality. After 6 months, there was an improvement in the semen quality in 32 cases (58.2%) in Group I and in 15 cases in Group II (53.5%). According to this data laparoscopic varicocelectomy in older men can achieve improvement in semen quality comparable to relatively younger patients. Zini [31] compared 115 men aged 40 years and older and 466 men younger than 40 years with clinical varicocele and infertility. All patients underwent microscopic varicocelectomy done by the same surgeon. There were no significant differences in the baseline sperm parameters and in spontaneous pregnancy rates after varicocelectomy in couples with advanced paternal age (40 years or older) compared with the younger couples (49% vs. 39%, respectively). Hsiao [32] included in his retrospective study 272 patients with varicocele – 85 over 40 years old. Data confirms that subinguinal microsurgical varicocelectomy is associated not only with significant increases in sperm concentration and total sperm count, but in the serum testosterone level as well, across all age groups studied.
DISCUSSION
Male infertility becomes a more often seen clinical problem not only by urologists, but gynecologists as well, due to the fact it is a couple problem. Varicocele is the most common male infertility cause which is surgically treatable. While multiple surgical approaches exist, microsurgical treatment has been shown to be the most efficacious and cost-effective. Higher pregnancy rates, lower varicocele recurrence and less hydrocele formation are the most important advantages of this technique [6] especially with the help of a microscope.
The role of varicocelectomy still remains unclear. Available RCT are heterogeneous and due to this it is hard to draw conclusions leading to the creation of clearer guidelines. Ficarra et al. [1] suggests that varicocele repair remains an advisable option in patients with clinical varicocele and seminal parameter impairment, whose female partners do not show any obvious gynecological causes of infertility [1].
Prospective randomized studies with a large number of patients, uniform semen and clinical test are needed to clarify the role of varicocele, the surgical treatment and what is most important – to the ability to draw out the group of patients to whom such a treatment could bring benefits.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Ficarra V, Cerruto MA, Liguori G, et al. Treatment of varicocele in subfertile men: The Cochrane Review-a contrary opinion. Eur Urol. 2006;49:258–263. doi: 10.1016/j.eururo.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–67. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karavolos S, Stewart J, Evbuomwan I, McEleny K, Aird I. Assessment of the infertile male. The Obstetrician & Gynaecologist. 2013;15:1–9. [Google Scholar]
- 4.Bablok L, Dziadecki W, Szymusik I, et al. Patterns of infertility in Poland - multicenter study. Neuro Endocrinol Lett. 2011;32:799–804. [PubMed] [Google Scholar]
- 5.Choi WS, Kim SW. Current issues in varicocele management: a review. World J Mens Health. 2013;31:12–20. doi: 10.5534/wjmh.2013.31.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diegidio P, Jhaveri JK, Ghannam S, Pinkhasov R, Shabsigh R, Fishc H. Review of current varicocelectomy techniques and their outcomes. BJU Int. 2011;108:1157–1172. doi: 10.1111/j.1464-410X.2010.09959.x. [DOI] [PubMed] [Google Scholar]
- 7.Harrison RM, Lewis RW, Roberts JA. Testicular blood flow and fluid dynamics in monkeys with surgically induced varicoceles. J Androl. 1983;4:256–260. doi: 10.1002/j.1939-4640.1983.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 8.Sofikitis NV, Miyagawa I, Incze P, Andrighetti S. Detrimental effect of left varicocele on the reproductive capacity of the early haploid male gamete. J Urol. 1996;156:267–270. [PubMed] [Google Scholar]
- 9.Rajfer J, Turner TT, Rivera F, Howards SS, Sikka SC. Inhibition of testicular testosterone biosynthesis following experimental varicocele in rats. Biol Reprod. 1987;36:933–937. doi: 10.1095/biolreprod36.4.933. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Sun Y, Wang L, et al. Hypoxia-induced apoptosis in the bilateral testes of rats with left-sided varicocele: a new way to think about the varicocele. J Androl. 2010;31:299–305. doi: 10.2164/jandrol.108.007153. [DOI] [PubMed] [Google Scholar]
- 11.Khera M, Lipshultz LI. Evolving approach to the varicocele. Urol Clin North Am. 2008;35:183–189. doi: 10.1016/j.ucl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Wright EJ, Young GP, Goldstein M. Reduction in testicular temperature after varicocelectomy in infertile men. Urology. 1997;50:257–259. doi: 10.1016/s0090-4295(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 13.Blumer CG, Restelli AE, Giudice PT, et al. Effect of varicocele on sperm function and semen oxidative stress. BJU Int. 2012;109:259–265. doi: 10.1111/j.1464-410X.2011.10240.x. [DOI] [PubMed] [Google Scholar]
- 14.Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril. 2011;96:1283–1287. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology. 2009;73:461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006;12:630–633. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 17.Evers JL, Collins JA. Surgery or embolisation for varicocele in subfertile men. Cochrane Database Syst Rev. 2004:CD000479. doi: 10.1002/14651858.CD000479.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Evers JL, Collins JA. Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003;361:1849–1852. doi: 10.1016/S0140-6736(03)13503-9. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–461. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Marmar JL, Agarwal A, Prabakaran S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88:639–648. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Ding H, Tian J, Du W, Zhang L, Wang H, Wang Z. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int. 2012;110:1536–1542. doi: 10.1111/j.1464-410X.2012.11093.x. [DOI] [PubMed] [Google Scholar]
- 22.Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. 2009;30:33–40. doi: 10.2164/jandrol.108.005967. [DOI] [PubMed] [Google Scholar]
- 23.Su LM, Goldstein M, Schlegel PN. The effect of varicocelectomy on serum testosterone levels in infertile men with varicoceles. J Urol. 1995;154:1752–1755. [PubMed] [Google Scholar]
- 24.Cayan S, Kadioglu A, Orhan I, Kandirali E, Tefekli A, Tellaloglu S. The effect of microsurgical varicocelectomy on serum follicle stimulating hormone, testosterone and free testosterone levels in infertile men with varicocele. BJU Int. 1999;84:1046–1049. doi: 10.1046/j.1464-410x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 25.Grober ED, Chan PT, Zini A, Goldstein M. Microsurgical treatment of persistent or recurrent varicocele. Fertil Steril. 2004;82:718–722. doi: 10.1016/j.fertnstert.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Cayan S, Acar D, Ulger S, Akbay E. Adolescent varicocele repair: long-term results and comparison of surgical techniques according to optical magnification use in 100 cases at a single university hospital. J Urol. 2005;174:2003–2006. doi: 10.1097/01.ju.0000176488.44895.7b. [DOI] [PubMed] [Google Scholar]
- 27.Campbell-Walsh, Urology. 10th ed. 2012. pp. 648–687. [Google Scholar]
- 28.Fast AM, Deibert CM, Van Batavia JP, Nees SN, Glassberg KI. Adolescent varicocelectomy: does artery sparing influence recurrence rate and/or catch-up growth? Andrology. 2014;2:159–164. doi: 10.1111/j.2047-2927.2013.00142.x. [DOI] [PubMed] [Google Scholar]
- 29.Jungwirth A, Diemer T, Dohle GR, Giwercman A, Kopa Z, Krausz C, Tournaye H. Guidelines on Male Infertility, European Association of Urology. 2014. p. 31. [DOI] [PubMed] [Google Scholar]
- 30.El-Shazly M, Eissa B. Laparoscopic varicocelectomy in Infertile Men: Does Age Matter? Urol Int. 2013;91:192–196. doi: 10.1159/000350860. [DOI] [PubMed] [Google Scholar]
- 31.Zini A, Boman J, Jarvi K, Baazeem A. Varicocelectomy for infertile couples with advanced paternal age. Urology. 2008;72:109–113. doi: 10.1016/j.urology.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao W, Rosoff JS, Pale JR, Greenwood EA, Goldstein M. Older age is associated with similar improvements in semen parameters and testosterone after subinguinal microsurgical varicocelectomy. J Urol. 2011;185:620–625. doi: 10.1016/j.juro.2010.09.114. [DOI] [PubMed] [Google Scholar]