Abstract
A macrocyclic tetralactam host is threaded by a highly fluorescent squaraine dye that is flanked by two polyethyleneglycol (PEG) chains with nanomolar dissociation constants in water. Furthermore, the rates of bimolecular association are very fast with kon ~106–107 M−1s−1. The association is effective under cell culture conditions and produces large changes in dye optical properties including turn-on near-infrared fluorescence that can be imaged using cell microscopy. Association constants in water are ~1000 times higher than in organic solvents and strongly enthalpically favored at 27 °C. The threading rate is hardly affected by the length of the PEG chains that flank the squaraine dye. For example, macrocyle threading by a dye conjugate with two appended PEG2000 chains is only three times slower than threading by a conjugate with triethyleneglycol chains that are twenty times shorter. The results are a promising advance towards synthetic mimics of streptavidin/biotin.
The binding pockets within biological receptors usually contain a mixture of hydrophobic and polar residues that act synergistically to drive shape-selective binding of target guest molecules.1 A classic example is the remarkably strong binding of biotin by the streptavidin/avidin protein (Ka ~ 1013–15 M−1) which is achieved primarily by a mixture of hydrophobic interactions and a large number of hydrogen bonds.2 One of the classic goals of supramolecular chemistry is to produce synthetic mimics of these remarkable binding systems; however, there are presently very few uncharged organic host-guest binding partners that associate in water with very high affinity. A 2003 literature survey by Houk and coworkers determined that the average Ka was 103.4±1.6 M−1.3 Since then there have been several impressive demonstrations of high affinity binding of small molecule guests by cyclodextrin,4 cucurbituril,5 or cyclophane hosts.6 These container molecules share a common barrel-like molecular architecture with a hydrophobic interior and a series of polar groups around the periphery of each portal. While these hosts have undoubted practical and scientific value, the spatial separation of hydrophobic and polar regions restricts the structural range of host-guest pairs that can be designed for selective recognition.7 In 1991, Diederich articulated the molecular design concept of a rigid cyclophane host with a mixed cavity comprised of hydrophobic surfaces and inward directed hydrogen bonding residues.8 He suggested that this type of biomimetic host architecture would bind complementary guests with high affinity and shape selectivity due to favorable enthalpic changes (Scheme 1).9
Scheme 1.
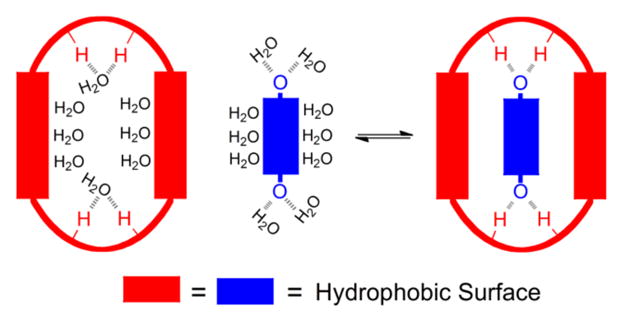
Binding in water by an amphiphilic cavity. Formation of host-guest hydrogen bonds compensates for the desolvation of polar groups and combines with desolvation of hydrophobic surfaces to produce strong complexation.8
Water-soluble hosts with amphiphilic cavities are relatively rare, with previous studies of calixpyrroles and oligolactam hosts in water reporting dissociation constants that are micromolar or above.10 Here we describe a new water-soluble organic host-guest pair in which polar and hydrophobic interactions combine to give nanomolar dissociation constants. The supramolecular design is based on two previous discoveries. In 2007, we described the organic-soluble macrocyclic tetralactam host M1 with two anthracene side walls (Scheme 2) and showed that it was able to encapsulate a deep-red fluorescent squaraine dye.11 Each squaraine oxygen atom formed bifurcated hydrogen bonds with the proximal host amide NH residues and there was complementary stacking of the host and guest aromatic surfaces. Binding constants were moderate at ~106 M−1. In 2012, the water-soluble analogue M2 was prepared and evaluated as a fluorescent host for monosaccharides.12 The study also showed that the structure of empty M2 is highly preorganized in aqueous solution - the four NH residues are forced by the adjacent peri hydrogens to point into the binding cavity. This observation prompted us to investigate if M2 could bind water-soluble squaraine guests.
Scheme 2.
Compounds studied
The squaraine dyes used in this study have chemical structures containing two electron-donating 2-aminothiophene units which diminish the electrophilicity of the central C4O2 core such that dyes S1–S4 resist nucleophilic attack by the aqueous solvent. The improved chemical stability was a significant breakthrough that enabled accurate squaraine binding studies to be conducted in water. As expected for squaraine dyes, compounds S1–S4 exhibit intense and narrow deep-red absorption and emission bands that make them very attractive for many types of optical imaging, sensing and light harvesting applications.13 Furthermore, the squaraine absorption and emission bands are red-shifted by 20–40 nm when the dye is encapsulated inside the tetralactam macrocycle, a diagnostic optical change that greatly facilitates association measurements.11 The organic-soluble host M1 and water-soluble M2 have identical binding cavities and differ only in the peripheral appendages. The organic-soluble bisalkyne squaraine S1 was prepared in a straightforward manner and polyethyleneglycol (PEG) chains of two different lengths were covalently attached to create structures S2 and S3 which were soluble in various organic solvents and water.
Host/guest complexation was studied in three solvents, chloroform, methanol, and water. In each case, macrocycle threading and dye encapsulation was indicated by diagnostic changes in NMR chemical shifts and absorption/fluorescence maxima bands, and fluorescence energy transfer from the macrocycle anthracene units to the encapsulated squaraine. In Figure 1, are partial 1H NMR spectra showing the changes in chemical shift due to complexation of S3 by M2 to form M2⊃S3 in D2O. A ROESY spectrum confirmed the threaded structure with a through-space correlation between the macrocycle anthracene protons E and E′ and the thiophene protons 1 and 2. Analogous NMR experiments to form M1⊃S1 in CDCl3 and M2⊃S1 in CD3OD are described in the Supporting Information. In each case, the changes in chemical shift are consistent with the aromatic stacking and hydrogen bonding effects caused by dye encapsulation inside the macrocycle. For example, the squaraine thiophene protons 1 and 2 move upfield whereas the macrocycle proton B moves downfield. The squaraine dyes exist as conformational isomers, based on the relative orientation of the thiophene units (cis or trans), and the NMR studies revealed that the trans squaraine isomer was the major isomer encapsulated by the macrocycle (see Supporting Information for a computational model of the host/guest complex and further discussion of the squaraine cis-trans isomerization).
Figure 1.
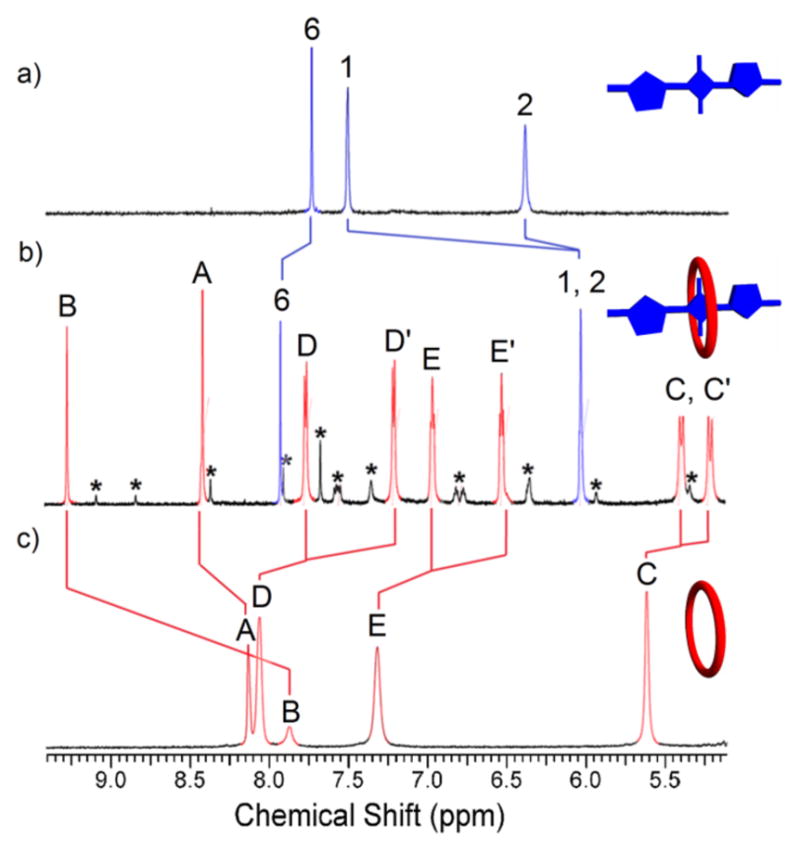
Partial 1H NMR (600MHz, D2O, 25 °C) of: a) squaraine dye S3 (2.0 mM); b) complex M2⊃S3 (2.0 mM); and c) empty macrocycle M2 (2.0 mM). *designates NMR signals for the minor M2⊃S3 complex with encapsulated S3 in a cis conformation (trans:cis ratio for encapsulated S3 ~10:1).
The red shifted squaraine absorption and emission bands made it straightforward to monitor macrocycle threading and dye encapsulation (Figure 2). For example, encapsulation of S3 by M2 moved the squaraine absorbance maxima to 678 nm and the sample solution exhibited a distinct color change from blue to green. There was also an increase in fluorescence quantum yield, and both factors combined to produce a very large switch-on fluorescence effect at the red-shifted emission wavelength of 712 nm (Figure 2d). Additional evidence for squaraine complexation was the observation of efficient energy transfer from an excited anthracene unit in M2 (ex: 390 nm) to the encapsulated squaraine dye (em: 712 nm) (Figure 2e).11 In contrast, these substantial optical changes did not occur when M2 was mixed with the control dye S4 because macrocycle threading was blocked by the pair of split triethyleneglycol chains that flanked the dye structure.14 A final piece of evidence for complete encapsulation of S3 by M2 was increased resistance to chemical bleaching of the squaraine color by highly nucleophilic sulfide dianion.15 In agreement with previous observations, addition of excess Na2S to free squaraine dye S3 produced a 55% decrease in squaraine absorbance over 20 minutes due to nucleophilic attack, whereas there was no decrease in squaraine color intensity when Na2S was added to a sample of S3 that had been premixed with M2 (forming M2⊃S3) (Figure S11).
Figure 2.
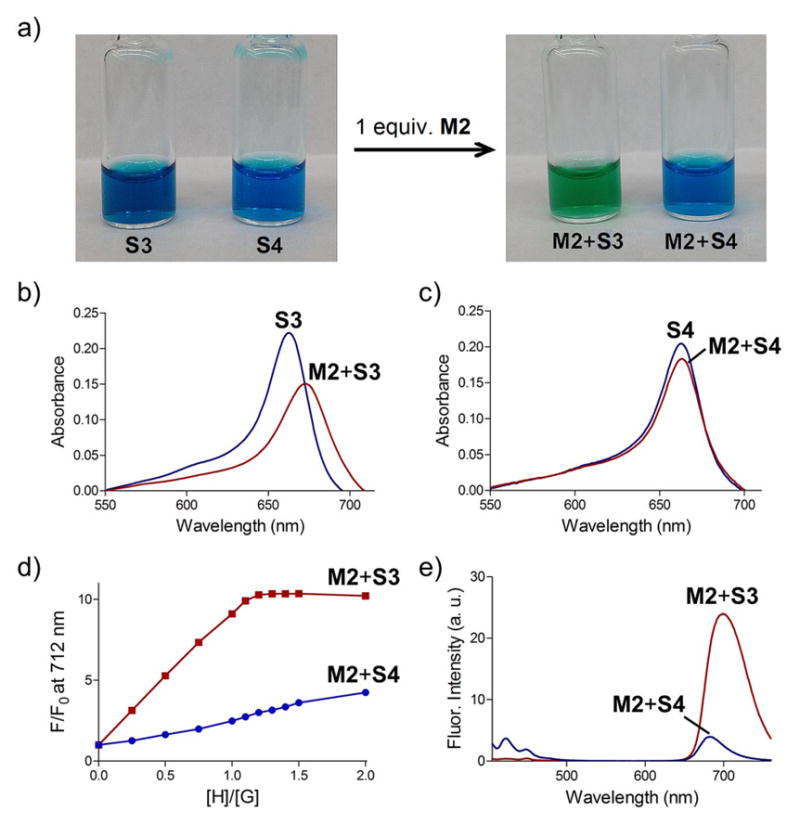
Comparison of the optical changes in H2O. a) color change achieved by adding M2 to separate equimolar solutions of S3 or control dye S4 (200 μM each); b) absorbance spectrum of S3 (3.0 μM) or M2+S3 (3.0 μM each); c) absorbance spectrum of S4 (3.0 μM) or M2+S4 (3.0 μM each); d) fluorescence titration (ex: 690 nm, em: 712) of M2 into separate solutions of S3 or S4 (1.0 μM); e) fluorescence emission upon excitation of M2 anthracene band (ex: 390 nm) in separate samples of M2+S3 or M2+S4 (1.0 μM each).
The complexation-induced changes in optical properties enabled titration experiments that measured kinetic and thermodynamic constants in three solvents, chloroform, methanol, and water. The weaker binding in chloroform and methanol was monitored by absorption, whereas the stronger binding in water was measured at lower concentration using fluorescence methods. As summarized in Table 1, host-guest binding in the organic solvents was moderate (Ka = 0.4–2.0 x 106 M−1) and comparable to previous reports using analogous squaraine dyes.11 However, the association constants in water were ~1000 times higher. For example, the association constant to form M2⊃S3 in water was 1.1 x 109 M−1 at 20 °C. This remarkably strong association was confirmed by independent guest displacement experiments. Guided by literature precedent,16 we prepared the water-soluble bis-fumaride F1 and determined by NMR and fluorescence titration experiments with M2 that a 1:1 complex was formed with Ka = 1.6 ×104 M−1 at 20 °C (Figures S19 and S20). Competitive titration experiments were then conducted that added S3 to a sample of M2⊃F1 and observed unambiguous fluorescence and NMR evidence for displacement of F1 from the macrocycle and confirmation of nanomolar affinity for M2⊃S3 (Figures S21 and S23).
Table 1.
Thermodynamic and kinetic data for host/guest association at 20 °C.
| Guest | Host | Solvent | Ka (M−1) | kon (M−1s−1) |
|---|---|---|---|---|
| S1 | M1 | CHCl3 | (5.9±1.6)×105 | 8.6±0.4 |
| S3 | M1 | CHCl3 | (2.0±0.5)×106 | 11.9±0.9 |
| S1 | M2 | MeOH | (4.0±0.6)×105 | (1.2±0.1)×104 |
| S2 | M2 | MeOH | (4.0±0.6)×105 | (1.8±0.2)×103 |
| S3 | M2 | MeOH | (4.1±1.0)×105 | (5.1±0.6)×103 |
| S2 | M2 | H2O | (6.0±1.2)×108 | (1.2±0.1)×107 |
| S3 | M2 | H2O | (1.1±0.4)×109 | (4.3±0.3)×106 |
| F1 | M2 | H2O | (1.6±0.1)×104 | - |
To gain additional thermodynamic insight, the aqueous titrations were repeated and monitored by Isothermal Titration Calorimetry (ITC) at 27 °C (Figures S24–S27). Association of M2 and F1 was determined to be highly favored enthalpically (ΔH = −11.3 kcal/mol) and moderately disfavored entropically (TΔS = −5.1 kcal/mol). The association constant for M2 and S3 was too high for accurate measurement using our microcalorimeter, but a single injection experiment determined ΔH to be −11.7 kcal/mol. A fluorescence titration experiment at this temperature provided ΔG = −11.3 kcal/mol and thus TΔS = −0.4 kcal/mol. These thermodynamic data support a model where F1 and S3 both form enthalpically favored hydrogen bonds with the four NH residues inside M2, a picture that is supported by computational modeling (Figure S4) and several analogous X-ray crystal structures.11,16–18 The structure of squaraine S3 is more rigid and more hydrophobic than fumaride F1 which is likely a major reason why complexation of S3 does not suffer as large an entropic penalty.
The initial rates of host-guest association, kon, were measured using standard fluorescence or absorption methods (see Supporting Information). Association in chloroform was slow enough for standard mixing experiments in a single cuvette but the much faster formation of M2⊃S3 in methanol and water required stopped-flow instrumentation. Inspection of the rate constants in Table 1 reveals two notable trends. One is that the initial rate constant for association of M2 with squaraine dye S3 in water (kon = (4.3±0.3) x 106 M−1s−1 which corresponds to a half-life of 7 seconds when both components are mixed at 30 nM) is almost 1,000 times faster than the same process in methanol and >300,000-fold faster than the rate constant for analogous association of M1 with S3 in chloroform (Table 1). The lubricating effect of water was further investigated by measuring the rates of association in water/methanol mixtures.19 The rate of association increased dramatically once the mole fraction of water was >0.9 (Figure S34). Another notable trend in Table 1 is that the length of the ethyleneglycol chains at the ends of the squaraine dye does not greatly affect the rate of macrocycle threading in any specific solvent. For example, threading of M2 by S2 in water, which requires macrocycle passage along a relatively short triethyleneglycol chain, is only three times faster than threading by S3, which requires macrocycle passage along a PEG2000 chain that is twenty times longer.
The threading of cyclodextrin and cucurbituril by long PEG chains in water is well known20 and threading of cucurbituril by a dye flanked with PEG chains has also been reported.21 In addition, an organic-soluble threaded macrocycle/polymer system has been studied in detail.22 In comparison, the high affinities and rapid kinetics of the current association system are noteworthy, and so are the large ratiometric changes in near-infrared optical properties, including turn-on fluorescence. The potential utility of these properties was demonstrated by conducting fluorescence imaging studies of Chinese Hamster Ovary (CHO) cells that had been incubated for 8 hours at 37 °C in culture media with S3 or a mixture of M2 + S3.23 The live cell micrographs in Figure 3 show that uptake of S3 into intracellular endosomes is visualized using a Red excitation/emission filter set, whereas endosomal uptake of M2⊃S3 is observed selectively using a Near-Infrared filter set. The M2⊃S3 complex can also be imaged microscopically or on the mesoscale using an alternative filter set that excites the surrounding anthracene units with blue light and detects near-infrared emission from the encapsulated squaraine dye (Figures S36–S37). A time resolved movie of the live cells containing M2⊃S3 clearly shows the expected endosome tracking (see Supporting Information). These imaging results demonstrate that the M2⊃S3 complex is stable in complex biological environments and can be selectively visualized by different types of fluorescence imaging protocols.
Figure 3.
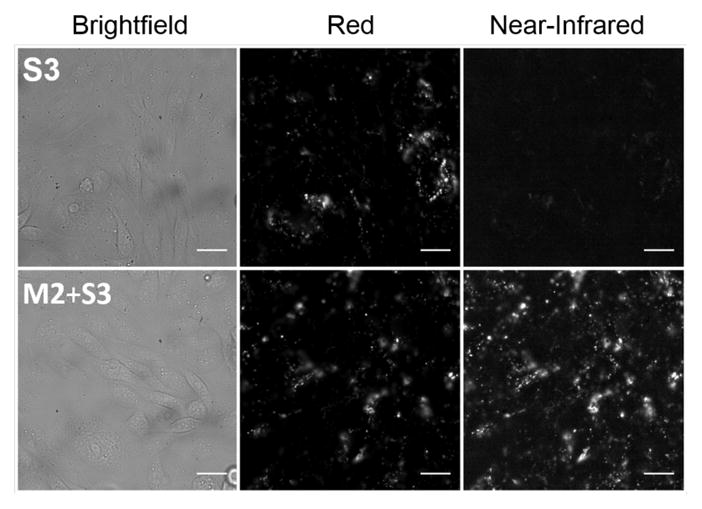
Micrographs of live CHO cells in culture media at 8 hours after treatment with: (top row) S3 (10 μM); (bottom row) mixture of M2+S3 (10 μM each). Epifluorescence images acquired using Red (ex: 620±60 nm, em: 700±75 nm) or Near-Infrared (ex: 710±75 nm, em: 810±90 nm) filter sets. Scale bar = 10 μm.
In conclusion, we report a host-guest pair which employs an amphiphilic cavity to achieve nanomolar binding in water. The structures of both components are readily tuneable, and conjugation to biomolecules or surfaces should also be straightforward, leading to optically active alternatives to the streptavidin-biotin association system. The distinctive recognition properties of the host-guest pair suggests orthogonality to other strong binding pairs (e.g., cucurbituril/alkylammonium),5 and hence the potential for simultaneous use. The ability to engineer multiple strong but controllable noncovalent interactions raises interesting possibilities for applications in biomedical science, materials science, and nanotechnology.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the University of Bristol’s EPSRC Impact Acceleration Account, the NSF (CHE1401783), the NIH (T32GM075762) and a Walther Cancer Foundation Advancing Basic Cancer Research Grant administered by the Harper Cancer Research Institute (USA).
Footnotes
Notes
The authors declare no competing financial interests.
Chemical synthesis and characterization, thermodynamic and kinetics data, cell imaging. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Persch E, Dumele O, Diederich F. Angew Chem, Int Ed. 2015;11:3290. doi: 10.1002/anie.201408487. [DOI] [PubMed] [Google Scholar]; (b) Zhao Y. ChemPhysChem. 2013;14:3878. doi: 10.1002/cphc.201300744. [DOI] [PubMed] [Google Scholar]; (c) Homans SW. Drug Discov Today. 2007;12:534. doi: 10.1016/j.drudis.2007.05.004. [DOI] [PubMed] [Google Scholar]; (d) Lemieux RU. Acc Chem Res. 1996;29:373. [Google Scholar]
- 2.(a) Stayton PS, Freitag S, Klumb LA, Chilkoti A, Chu V, Penzotti JE, To R, Hyre D, Le Trong I, Lybrand TP. Biomol Eng. 1999;16:39. doi: 10.1016/s1050-3862(99)00042-x. [DOI] [PubMed] [Google Scholar]; (b) Klumb LA, Chu V, Stayton PS. Biochemistry. 1998;37:7657. doi: 10.1021/bi9803123. [DOI] [PubMed] [Google Scholar]; (c) Weber PC, Ohlendorf D, Wendoloski J, Salemme F. Science. 1989;243:85. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]
- 3.Houk K, Leach AG, Kim SP, Zhang X. Angew Chem, Int Ed. 2003;42:4872. doi: 10.1002/anie.200200565. [DOI] [PubMed] [Google Scholar]
- 4.(a) Grishina A, Stanchev S, Kumprecht L, Buděšínský M, Pojarová M, Dušek M, Rumlová M, Kříáová I, Rulíšek L, Kraus T. Chem -Eur J. 2012;18:12292. doi: 10.1002/chem.201201239. [DOI] [PubMed] [Google Scholar]; (b) Uhlenheuer DA, Milroy LG, Neirynck P, Brunsveld L. J Mater Chem. 2011;21:18919. [Google Scholar]; (c) Yang Z, Breslow R. Tetrahedron Lett. 1997;38:6171. [Google Scholar]
- 5.(a) Smith LC, Leach DG, Blaylock BE, Ali OA, Urbach AR. J Am Chem Soc. 2015;137:3663. doi: 10.1021/jacs.5b00718. [DOI] [PubMed] [Google Scholar]; (b) Cao L, Šekutor M, Zavalij PY, Mlinarić-Majerski K, Glaser R, Isaacs L. Angew Chem, Int Ed. 2014;53:988. doi: 10.1002/anie.201309635. [DOI] [PubMed] [Google Scholar]; (c) Masson E, Ling X, Joseph R, Kyeremeh-Mensah L, Lu X. RSC Adv. 2012;2:1213. [Google Scholar]; (d) Rekharsky MV, Mori T, Yang C, Ko YH, Selvapalam N, Kim H, Sobransingh D, Kaifer AE, Liu S, Isaacs L. Proc Natl Acad Sci USA. 2007;104:20737. doi: 10.1073/pnas.0706407105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Hagiwara K, Akita M, Yoshizawa M. Chem Sci. 2015;6:259. doi: 10.1039/c4sc02377c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu G, Zhou X, Zhang Z, Han C, Mao Z, Gao C, Huang F. J Am Chem Soc. 2012;134:19489. doi: 10.1021/ja3099905. [DOI] [PubMed] [Google Scholar]; (c) Venturi M, Dumas S, Balzani V, Cao J, Stoddart JF. New J Chem. 2004;28:1032. [Google Scholar]; (d) Gibb CL, Gibb BC. J Am Chem Soc. 2004;126:11408. doi: 10.1021/ja0475611. [DOI] [PubMed] [Google Scholar]
- 7.Lehn JM. Angew Chem, Int Ed. 1988;27:89. [Google Scholar]
- 8.Diederich F. Cyclophanes. Royal Society of Chemistry; Cambridge: 1991. pp. 261–263. [Google Scholar]
- 9.Meyer EA, Castellano RK, Diederich F. Angew Chem, Int Ed. 2003;42:1210. doi: 10.1002/anie.200390319. [DOI] [PubMed] [Google Scholar]
- 10.(a) Verdejo B, Gil-Ramírez G, Ballester P. J Am Chem Soc. 2009;131:3178. doi: 10.1021/ja900151u. [DOI] [PubMed] [Google Scholar]; (b) Ferrand Y, Klein E, Barwell NP, Crump MP, Jiménez-Barbero J, Vicent C, Boons GJ, Ingale S, Davis AP. Angew Chem, Int Ed. 2009;48:1775. doi: 10.1002/anie.200804905. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ferrand Y, Crump MP, Davis AP. Science. 2007;318:619. doi: 10.1126/science.1148735. [DOI] [PubMed] [Google Scholar]; (d) Hunter C, Thomas J, Bernad P., Jr Chem Commun. 1998;22:2449. [Google Scholar]
- 11.Gassensmith JJ, Arunkumar E, Barr L, Baumes JM, DiVittorio KM, Johnson JR, Noll BC, Smith BD. J Am Chem Soc. 2007;129:15054. doi: 10.1021/ja075567v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke C, Destecroix H, Crump MP, Davis AP. Nat Chem. 2012;4:718. doi: 10.1038/nchem.1409. [DOI] [PubMed] [Google Scholar]
- 13.(a) Avirah RR, Jayaram DT, Adarsh N, Ramaiah D. Org Biomol Chem. 2012;10:911. doi: 10.1039/c1ob06588b. [DOI] [PubMed] [Google Scholar]; (b) McEwen JJ, Wallace KJ. Chem Commun. 2009;45:6339. doi: 10.1039/b909572a. [DOI] [PubMed] [Google Scholar]; (c) Sreejith S, Carol P, Chithra P, Ajayaghosh A. J Mater Chem. 2008;18:264. [Google Scholar]
- 14.A simple TLC study showed that the presence of M2 completely prevented elution of S3, indicating formation of a very polar M2⊃S3 complex, but the presence of M2 had no effect on the retention factor of S4, indicating that a threaded complex was not formed (see Figure S12).
- 15.Arunkumar E, Forbes CC, Noll BC, Smith BD. J Amer Chem Soc. 2005;127:3288. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]
- 16.Gatti FG, Leigh DA, Nepogodiev SA, Slawin AMZ, Teat SJ, Wong JKY. J Am Chem Soc. 2001;123:5983. doi: 10.1021/ja001697r. [DOI] [PubMed] [Google Scholar]
- 17.Gassensmith JJ, Baumes JM, Smith BD. Chem Commun. 2009;45:6329. doi: 10.1039/b911064j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.We also characterized the association of control guest N1 with M2 in water. The naphthalene core of N1 has two attached oxygen atoms with similar spacing as the two oxygens in S3 and F1, but with much weaker hydrogen bond accepting ability. 1H NMR chemical shift data indicated threading of N1 by M2 (Figure S8) and ITC titration studies in water at 27 °C determined that a 1:1 complex was formed with Ka = 7.7 ×103 M−1, ΔH = −5.9 kcal/mol, and TΔS = 0.8 kcal/mol (Figure S27). The change in guest from S3 (or F1) to N1 thus lowers the enthalpic driving force by ~6 kcal/mol. It seems likely that part of this is due to the weaker hydrogen bonding of N1 with the four NH residues inside M2, although other factors presumably contribute since the structures of S3, F1, and N1 have different hydrophobic surface areas and hydration shells. For a recent discussion of these factors, see: Biedermann F, Nau WM, Schneider HJ. Angew Chem, Int Ed. 2014;53:11158. doi: 10.1002/anie.201310958.
- 19.Panman MR, Bakker BH, den Uyl D, Kay ER, Leigh DA, Buma WJ, Brouwer AM, Geenevasen JA, Woutersen S. Nat Chem. 2013;5:929. doi: 10.1038/nchem.1744. [DOI] [PubMed] [Google Scholar]
- 20.(a) Harada A, Takashima Y, Yamaguchi H. Chem Soc Rev. 2009;38:875. doi: 10.1039/b705458k. [DOI] [PubMed] [Google Scholar]; (b) Buschmann HJ, Jansen K, Schollmeyer E. J Incl Phenom Macrocycl Chem. 2000;37:231. [Google Scholar]
- 21.Biedermann F, Elmalem E, Ghosh I, Nau WM, Scherman OA. Angew Chem, Int Ed. 2012;51:7739. doi: 10.1002/anie.201202385. [DOI] [PubMed] [Google Scholar]
- 22.(a) Deutman ABC, Cantekin S, Elemans JAAW, Rowan AE, Nolte RJM. J Am Chem Soc. 2014;136:9165. doi: 10.1021/ja5032997. [DOI] [PubMed] [Google Scholar]; (b) Deutman ABC, Monnereau C, Elemans JAAW, Ercolani G, Nolte RJM, Rowan AE. Science. 2008;322:1668. doi: 10.1126/science.1164647. [DOI] [PubMed] [Google Scholar]
- 23.Cell vitality assays showed no evidence of toxicity for concentrations of S1, S3, and M2 up to 50 μM (see Figure S35).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.