Abstract
Purpose of the review
Prescribing the most appropriate dose of motor therapy for individual patients is a challenge because minimal data are available and a large number of factors are unknown. This review explores the concept of dose and reviews the most recent findings in the field of neurorehabilitation, with a focus on relearning motor skills post stroke.
Recent findings
Appropriate dosing involves the prescription of a specific amount of an active ingredient, at a specific frequency and duration. Dosing parameters, particularly amount, are not well-defined or quantified in most studies. Compiling data across studies indicates a positive, moderate dose-response relationship, indicating that more movement practice results in better outcomes. This relationship is confounded by time post stroke however, where longer durations of scheduled therapy may not be beneficial in the first few hours, days, and/or weeks.
Summary
These findings suggest that substantially more movement practice may be necessary to achieve better outcomes for people living with the disabling consequences of stroke. Preclinical investigations are needed to elucidate many of the unknowns and allow for a more biologically-driven rehabilitation prescription process. Likewise, clinical investigations are needed to determine the dose-response relationships and examine the potential dose-timing interaction in humans.
Keywords: Motor, stroke, neurorehabilitation, dose-response relationships
Introduction
There has been a growing consensus, albeit with some contradictions, that increased dose of rehabilitation may lead to better outcomes for individuals experiencing stroke.[1–6] There are minimal data available however, to address the questions of what optimal doses might be and when to deliver these optimal doses post stroke. Progress has been hampered by the fact that the concept of dose in stroke rehabilitation is not well defined and thus dose has often not been quantified or controlled. Many clinical trials have compared higher doses of an experimental intervention to lower doses of a control intervention. Despite well-executed trials showing benefit of more therapy,[7–9] there remains a large disconnect between recommendations from these scientific results and what is actually delivered in routine therapy sessions.[10–14] Answers to the questions of optimal dosing and timing are needed to guide clinical care for the hundreds of thousands of people per year who must live with the disabling consequences of stroke.
This review examines what is currently known about dosing in neurorehabilitation. The review focuses on motor rehabilitation post stroke because that is where the most data exist. There are multiple targets of interventions in neurorehabilitation, such that overall patient management might include interventions to address a variety of impairments and activity limitations. Here, we focus on dosing with respect to interventions selected to re-train or re-learn lost functions. We exclude discussion of rehabilitation focused on improving physical fitness and strengthening muscles, as there is already a great deal of information available to guide dosing for these interventions.[15]
Parameters of dose in stroke rehabilitation
What is meant by dose when it is applied to neurorehabilitation? With pharmaceutical agents, the dose prescribed describes the amount of active ingredient(s) expected to produce the desired effect, and the frequency and duration at which the agent is taken. For approved pharmaceutical agents, the biological mechanism of action, its target, and the desired effect are largely known (e.g. eliminate bacteria in the case of an antibiotic, control blood pressure in the case of an anti-hypertensive). Furthermore, the half-life of agents is known from pharmacokinetic and pharmacodynamics studies, allowing the prescriber to readily determine the appropriate frequency and duration at which the agent is taken (e.g. burst of the agent for a short time for an antibiotic, steady control for an anti-hypertensive). The challenge of dosing for neurorehabilitation is that these essential pieces: the active ingredients, their targets and mechanisms of action, and their half-lives, remain unclear. The Table provides a summary of what is known and unknown related to dosing in neurorehabiliation compared to dosing with pharmaceutical agents.
Table.
Information needed to make an appropriate prescription with a comparison of what is known and unknown for pharmaceutical agents vs. neurorehabilitation.
| Known for approved pharmaceutical agents | Known for neuro-rehabilitation | |
|---|---|---|
| Active ingredient | Yes | Task-specific behavioral training has been identified; others are unknown |
| Mechanism of action, including specific therapeutic target(s) | Yes | No |
| Desired outcome | Yes | Yes |
| Pathway through which active ingredient acts to achieve desired outcome | Yes | No |
| Half-life, derived from pharmacokinetic and pharmacodynamic data | Yes | No |
| Side effects | Mostly | No |
| Toxicity | Yes | No |
| Interactions with other commonly prescribed agents | Mostly | No |
Neuroscience and rehabilitation literature are converging to strongly support the idea that a key active ingredient is task-specific, or task-oriented practice. Repeated practice of a challenging movement can produce lasting physiological changes in motor neural networks, and behavioral changes in motor learning and motor function.[for review see 16] A general mechanism of action is the potentiation of specific neuronal connections that are utilized repeatedly during challenging behavioral practice. The persistence of potentiation due to practice over days and weeks facilitates motor system connectivity via synaptogenesis, axonal sprouting, angiogenesis, and potentially neurogenesis in animal models of stroke.[16,*17] These molecular and cellular changes manifest as enhanced motor representations of the newly acquired movement. Enhanced motor representations due to task-specific training have been demonstrated for several decades in both human and animal studies.[for examples see 18,19–22] And finally, the efficacy of this active ingredient, task-specific practice, is demonstrated across studies, body parts, and time periods post stroke in a recent meta-analysis.[*23]
While task-specific training is known to be a key active ingredient,[*24,25] it is unlikely that it is the only one. Nearly two decades of research have exposed the general mechanisms of action by which task-specific training might result in improved functional outcomes after stroke. A causal pathway across genetic, molecular, cellular, and systems levels of action, however, is not yet understood.[16,*17] Without a precise picture of the mechanisms of action and the timing of those actions, it is nearly impossible to determine the half-life of task-specific training. Thus, one is left to guess what dosing parameter, i.e. amounts, frequencies, and durations, might be most appropriate.
Frequency and duration are readily definable for stroke rehabilitation in terms of number of sessions per day or per week, and the time period, in days or weeks, over which the intervention is delivered. Amount however, is harder to quantify. Studies investigating neuroplastic adaptations post stroke typically require animals to complete hundreds of repetitions of a task daily or twice daily.[16,26,27] The optimal dose of practice needed for animal stroke models is unknown. But even if these data were available, they would not directly translate to humans because: 1) relative contributions of various motor system structures (e.g. rubrospinal tract [28]) are different in humans compared to non-human primates and rodents [29,30], and 2) animal stroke models are not exact replications of the human experience of stroke.
Amount can be quantified as number of repetitions in humans as well.[31,32] This approach takes effort because repetitions of the enormous array of human movements are harder to define and repetitions of task-specific practice need to be counted separately from repetitions of practice of other potential active ingredients (e.g. strengthening exercises).[10,11] An alternative approach is to quantify the number of minutes of active therapy.[33] When the sole intervention applied is task-specific training and the algorithms for determining the challenge point of the training (i.e. difficulty level) are held constant, then minutes of active therapy and number of repetitions are very strongly correlated (unpublished data, Lang et al). If more than one intervention is delivered and/or algorithms vary, then minutes of active therapy and number of repetitions would not be interchangeable approaches for quantifying amount. The simplest and most common approach to quantifying amount has been time scheduled for therapy.[**34] Time scheduled for therapy, however, is not the same as time actually attending therapy, and time attending therapy is not equivalent to minutes of active therapy or number of repetitions. Thus, quantification as time scheduled for therapy is likely an inaccurate and imprecise quantification of the true amount of the active ingredient.
One additional issue complicates the quantification of amount: the challenge point of practice.[35,36] In animal models, the difficulty of the repetitions is carefully titrated, across sessions and days, to produce sufficient motor challenge to continually improve performance on the task. Indeed, it is repetition of continuously challenging tasks that result in changed cortical representations and skill acquisition, not repetition of overlearned movements.[37–39] In human studies of upper limb actions, the term challenge usually reflects task difficulty with respect to skill. In human studies of lower limb actions, i.e. gait, the term challenge can also reflect level of physical intensity.[35,40] For gait in particular, the level of physical intensity may be a key parameter for improving outcomes.[41,42] Human stroke rehabilitation literature does not agree on standard terms to describe challenge or intensity level, despite recent good efforts.[43] Readers are encouraged to look at each study carefully for methodological information to describe how behavioral training is graded and progressed (or shaped, adapted) and the physical intensity (e.g. target cardiovascular parameters) at which it is delivered.
While each parameter of dose can influence outcomes, data are accumulating to suggest that amount may be the primary parameter, with frequency and duration as secondary parameters. These data come largely from studies of constraint-induced movement therapy (CIMT), within which task-specific practice is a critical component.[*24] Looking across 51 randomized controlled trials of CIMT, similar outcomes were obtained from large amounts of task-specific practice (along with other CIMT components) regardless of whether they were provided in the original form, 6 hours daily for 10 days, or the modified form, 1 hour daily, 3 days/week for 10 weeks.[*24] The idea that amount is primary to frequency and duration is already well-established within the general cardiovascular exercise field,[44] where the goal is to achieve the recommended cardiovascular stimulus amount in one or in multiple bouts.
In sum, task-specific training is one known active ingredient for stroke rehabilitation. The exact mechanisms by which task-specific training change the nervous system and improve outcomes is unclear. Quantification of amount of task-specific training is challenging. With these limitations in mind, the next section looks across published studies to determine what is currently known about dose-response relationships, primarily using time scheduled for therapy as a proxy for dose.
Amount of rehabilitation: Is more better?
There has been a general understanding of dose for stroke rehabilitation that more practice is likely better, but “how much more?” and “for whom?” remain unanswered questions. This vague understanding is largely derived from several decades of testing experimental interventions at arbitrarily-set doses and from testing higher-dose experimental interventions against lower-dose control interventions.[1,7,36,45–56] As discussed above, doses examined are quantified as time scheduled for therapy. In several small samples, dose was quantified as repetitions of upper limb tasks or gait steps.[31,32,57]. When carefully quantified, dose had a consistent, moderate relationship (r = 0.5 – 0.6) with outcome, regardless of the target of rehabilitation (upper limb function or mobility) or setting (inpatient or outpatient). These preliminary data suggest that dose of stroke rehabilitation could potentially account for about one third of the variance in outcomes. The idea that more may be better was further supported by the results of the multi-site, Phase III LEAPS trial.[8] In the LEAPS trial, the groups that received more therapy sessions of locomotor training or home physical therapy had substantially better mobility outcomes compared to the group that received fewer therapy sessions (delay group receiving standard care in the first 6 months).
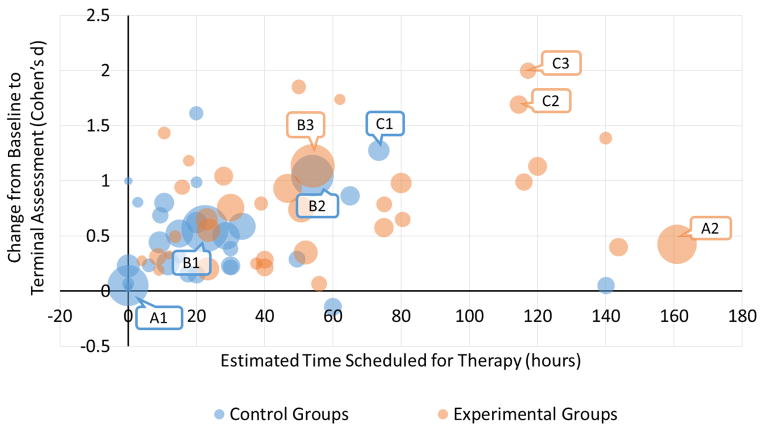
We have recently used a more quantitative, meta-regression approach to examine the effect of dose across stroke rehabilitation studies.[**34] Studies were included if they compared one dose of stroke rehabilitation to another, regardless of the specific interventions delivered. Meta-regression results from 1750 participants (30 studies) indicated a modest benefit of more time scheduled for therapy, with a Hedges’ g effect size of 0.35, which was statistically significant, 95% CI [0.26, 0.45]. (Hedges’ g is a standardized effect-size in which the difference between groups is divided by the pooled standard deviation. At large sample sizes, g is equivalent to Cohen’s d, but g is more conservative in smaller samples.) On average, the higher dose, experimental groups had 57 hours of therapy compared to 24 hours for the lower dose, comparison groups. For every additional 10 hours of therapy, effect sizes increased a small amount (0.034 from model 3). The Figure illustrates the data included in the meta-regression. Control group data (blue circles) are represented separately from experimental group data (orange circles) for each study. The relative size of the circle represents sample size. Collectively, the data points indicate a moderate relationship between time scheduled for therapy and response, as measured by effect size. No interaction was found between time scheduled for therapy and time post stroke, although the majority of studies were conducted months or years post stroke. Overall, meta-data provide solid evidence of a positive dose-response relationship, as the effects were found across studies using different interventions addressing a variety of functional targets and measuring outcomes with different assessments.[*23,**34] The conclusion reached so far is that more is better, and the benefit derived from more (either precisely or grossly quantified) is a moderate improvement in outcomes.
Figure.
Scatterplot of studies included in meta-regression of the dose-response relationship in stroke rehabilitation (Lohse et al. 2014). Control group data (blue circles) are represented separately from experimental group data (orange circles) for each study. The relative size of the circle represents sample size. Collectively, the data points indicate a moderate relationship between time scheduled for therapy and response, as measured by effect size. Three studies are labeled to aid in interpretation:
- A1: Standard care, prior to crossover
- A2: Constraint induced movement therapy
- B1: Standard care, prior to crossover
- B2: Home physical therapy, focused on functional strengthening and balance
- B3: Early locomotor training, body-weight supported treadmill training + over-ground training
- C1: Standard/conventional therapy
- C2: Intensive arm-focused training
- C3: Intensive leg-focused training
[Note to Editors: this figure also exists as an interactive figure, using Tableau visualization software, such that hovering over each data point brings up a pop-up box with study citation and key study parameters. If you are interested, we would be happy to explore with you ways to place this in an online version, or elsewhere on the journal website.]
Timing of rehabilitation: Does it matter?
The conclusion that more is better may be too simple. One Phase II trial[58] and two recent Phase III trials have produced unexpected results suggesting that timing may interact with dose. First, the Phase II VECTORS results[58] indicated that more CIMT, starting an average of 9 days post stroke, led to smaller improvements than less CIMT at the primary endpoint of 90 days post stroke. By 1 year however, the groups were equivalent. Second, despite the Phase II AVERT study[59–61] suggesting benefit of aggressive mobilization within 24 hours of stroke onset, recent Phase III trial data[**62] indicate a higher probability of worse outcomes in the group that was mobilized very early after stroke onset. The third study, ICARES[**63], compared an experimental upper limb retraining program (28 ± 6 hours) to a dose-matched standard care group (27 ± 6 hours) and a non-dose-matched standard care group (11 ± 9 hours), with subjects enrolled 14 – 106 days post stroke. All three groups made large improvements over the course of the study. The three groups were equivalent at the 1 year primary endpoint, despite a 16–17 hour average difference in the amount of therapy time. It is obvious that these three studies differ greatly across their designs, timing of intervention, sample sizes, and types of intervention. Collectively however, they suggest an important interaction between timing and dose that clearly warrants further exploration. More therapy may not be better in the first few hours and days after stroke and could lead to slower recovery. Given that stroke rehabilitation is prescribed to improve the lives of people living with stroke, then at a minimum, what is prescribed must do no harm.
Conclusions
The most appropriate dose at the appropriate time for post stroke rehabilitation remains a mystery. Preclinical investigations are sorely needed to understand the specific mechanisms of action of task-specific training, the time course of those mechanisms, and to identify other critical active ingredients. This knowledge would allow a more biologically-driven rehabilitation prescription process. In the meantime, clinical studies that specifically investigate dose are pending. Our ongoing Phase II parallel, dose-response trial (NCT 01146379) investigates four different doses of task-specific training to address the questions of “how much more is better?” and “better for whom?” in people who are 6 months or more post stroke. Additional studies are clearly needed, as the societal burden of disability post stroke is enormous. Even if optimal timing and dosing produce only a modest benefit at the individual level, at the population the optimal timing and dosing could go a long way in lessening the overall burden of stroke.
Key Points.
Larger amounts of therapy result in better outcomes for people beyond 2–3 months post stroke.
Timing and amount of therapy may interact, such that larger amounts of therapy may not result in better outcomes for people in the first hours and days after stroke.
Optimal dosing will not likely be a single value for everyone, but will vary based on clinical presentation of each individual.
Preclinical and clinical studies are sorely needed to create a biologically-driven and effective prescription process for stroke rehabilitation.
Acknowledgments
Funding acknowledgements: This work is supported by NIH R01HD068290.
Footnotes
Conflicts of interest: none
References
*of special interest
**of outstanding interest
- 1.Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke. A randomised trial. Lancet. 1999;354(9174):191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 2.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor Neurol Neurosci. 2004;22(3–5):281–299. [PubMed] [Google Scholar]
- 3.Galvin R, Murphy B, Cusack T, Stokes E. The impact of increased duration of exercise therapy on functional recovery following stroke--what is the evidence? Top Stroke Rehabil. 2008;15(4):365–377. doi: 10.1310/tsr1504-365. [DOI] [PubMed] [Google Scholar]
- 4.Veerbeek JM, Koolstra M, Ket JC, van Wegen EE, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: A meta-analysis. Stroke. 2011;42(11):3311–3315. doi: 10.1161/STROKEAHA.111.623819. [DOI] [PubMed] [Google Scholar]
- 5.Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise-based therapies to enhance motor recovery after stroke: A systematic review and meta-analysis. BMC Med. 2010;8(60) doi: 10.1186/1741-7015-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris CL, Taylor NF, Shields N. Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: A systematic review. Arch Phys Med Rehabil. 2011;92(9):1490–1500. doi: 10.1016/j.apmr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The excite randomized clinical trial. Jama. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 8.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, Hayden SK. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh YW, Wu CY, Lin KC, Yao G, Wu KY, Chang YJ. Dose-response relationship of robot-assisted stroke motor rehabilitation: The impact of initial motor status. Stroke. 2012;43(10):2729–2734. doi: 10.1161/STROKEAHA.112.658807. [DOI] [PubMed] [Google Scholar]
- 10.Lang CE, MacDonald JR, Gnip C. Counting repetitions: An observational study of outpatient day treatment for people with hemiparesis. Journal of Neurologic Physical Therapy. 2007;31(1):1–8. doi: 10.1097/01.npt.0000260568.31746.34. [DOI] [PubMed] [Google Scholar]
- 11.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, Hornby TG, Ross SA, Scheets PL. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connell LA, McMahon NE, Simpson LA, Watkins CL, Eng JJ. Investigating measures of intensity during a structured upper limb exercise program in stroke rehabilitation: An exploratory study. Arch Phys Med Rehabil. 2014;95(12):2410–2419. doi: 10.1016/j.apmr.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayward KS, Brauer SG. Dose of arm activity training during acute and subacute rehabilitation post stroke: A systematic review of the literature. Clin Rehabil. 2015 doi: 10.1177/0269215514565395. [DOI] [PubMed] [Google Scholar]
- 14.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: Is the intensity adequate to induce a training effect? Arch Phys Med Rehabil. 2002;83(10):1378–1383. doi: 10.1053/apmr.2002.35089. [DOI] [PubMed] [Google Scholar]
- 15.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, Shaughnessy M, et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45(8):2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 16.Nudo RJ. Recovery after brain injury: Mechanisms and principles. Front Hum Neurosci. 2013;7(887) doi: 10.3389/fnhum.2013.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Overman JJ, Carmichael ST. Plasticity in the injured brain: More than molecules matter. Neuroscientist. 2014;20(1):15–28. doi: 10.1177/1073858413491146. This paper reviews the mechanisms for activity-, experience- and injury-dependent neural plasticity as they related to recovery following stroke. [DOI] [PubMed] [Google Scholar]
- 18.Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, Wittenberg GF. Differential patterns of cortical reorganization following constraint-induced movement therapy during early and late period after stroke: A preliminary study. NeuroRehabilitation. 2014;35(3):415–426. doi: 10.3233/NRE-141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawaki L, Butler AJ, Xiaoyan L, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, Wittenberg GF. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22(5):505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional mri evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 21.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263(5151):1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 23*.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987. doi: 10.1371/journal.pone.0087987. This is a large scale meta-analysis exploring the efficacy of many different physical/occupational therapy interventions for adults with stroke. Meta-data suggest a benefit of higher doses of therapy measured in a binary manner (more therapy versus less therapy) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14(2):224–234. doi: 10.1016/S1474-4422(14)70160-7. This is a systematic review and meta-analyses examining a variety of forms of constraint induced movement therapy on outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page SJ, Boe S, Levine P. What are the “ingredients” of modified constraint-induced therapy? An evidence-based review, recipe, and recommendations. Restor Neurol Neurosci. 2013;31(3):299–309. doi: 10.3233/RNN-120264. [DOI] [PubMed] [Google Scholar]
- 26.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 27.Bell JA, Wolke ML, Ortez RC, Jones TA, Kerr AL. Training intensity affects motor rehabilitation efficacy following unilateral ischemic insult of the sensorimotor cortex in c57bl/6 mice. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314553031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105(Pt 2):223–269. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- 29.Lang CE, Reilly KT, Schieber MH. Human voluntary motor control and dysfunction. In: Selzer ME, Clarke S, Cohen LG, Duncan PW, Gage FH, editors. Textbook of neural repair and rehabilitation: Medical rehabiliation. Vol. 2. Cambridge University Press; Cambridge: 2006. pp. 24–36. [Google Scholar]
- 30.Nudo RJ, Masterton RB. Descending pathways to the spinal cord: A comparative study of 22 mammals. J Comp Neurol. 1988;277(1):53–79. doi: 10.1002/cne.902770105. [DOI] [PubMed] [Google Scholar]
- 31.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waddell KJ, Birkenmeier RL, Moore JL, Hornby TG, Lang CE. Feasibility of high-repetition, task-specific training for individuals with upper-extremity paresis. Am J Occup Ther. 2014;68(4):444–453. doi: 10.5014/ajot.2014.011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Host HH, Lang CE, Hildebrand MW, Zou D, Binder EF, Baum CM, Freedland KE, Morrow-Howell N, Lenze EJ. Patient active time during therapy sessions in postacute rehabilitation: Development and validation of a new measure. Phys Occup Ther Geriatr. 2014;32(2):169–178. doi: 10.3109/02703181.2014.915282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053–2058. doi: 10.1161/STROKEAHA.114.004695. This is the first meta-analysis of randomized controlled trials to measure dose in a continuous manner. Meta-data suggest a benefit of increased time in therapy, controlling from the time from the stroke to the beginning of the trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornby TG, Straube DS, Kinnaird CR, Holleran CL, Echauz AJ, Rodriguez KS, Wagner EJ, Narducci EA. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil. 2011;18(4):293–307. doi: 10.1310/tsr1804-293. [DOI] [PubMed] [Google Scholar]
- 36.Wolf SL, Newton H, Maddy D, Blanton S, Zhang Q, Winstein CJ, Morris DM, Light K. The excite trial: Relationship of intensity of constraint induced movement therapy to improvement in the wolf motor function test. Restor Neurol Neurosci. 2007;25(5–6):549–562. [PubMed] [Google Scholar]
- 37.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101(6):1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 38.Peterson GB. A day of great illumination: B. F. Skinner’s discovery of shaping. J Exp Anal Behav. 2004;82(3):317–328. doi: 10.1901/jeab.2004.82-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taub E. The behavior-analytic origins of constraint-induced movement therapy: An example of behavioral neurorehabilitation. Behav Anal. 2012;35(2):155–178. doi: 10.1007/BF03392276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther. 2006;86(11):1466–1478. doi: 10.2522/ptj.20050266. [DOI] [PubMed] [Google Scholar]
- 41.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther. 2015;39(2):95–102. doi: 10.1097/NPT.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 42.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabil Neural Repair. 2014;28(7):643–651. doi: 10.1177/1545968314521001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page SJ, Schmid A, Harris JE. Optimizing terminology for stroke motor rehabilitation: Recommendations from the american congress of rehabilitation medicine stroke movement interventions subcommittee. Arch Phys Med Rehabil. 2012;93(8):1395–1399. doi: 10.1016/j.apmr.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: Recommendation from the american college of sports medicine and the american heart association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 45.Ross LF, Harvey LA, Lannin NA. Do people with acquired brain impairment benefit from additional therapy specifically directed at the hand? A randomized controlled trial. Clin Rehabil. 2009;23(6):492–503. doi: 10.1177/0269215508101733. [DOI] [PubMed] [Google Scholar]
- 46.Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered graded repetitive arm supplementary program (grasp) improves arm function during inpatient stroke rehabilitation: A multi-site randomized controlled trial. Stroke. 2009;40(6):2123–2128. doi: 10.1161/STROKEAHA.108.544585. [DOI] [PubMed] [Google Scholar]
- 47.Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2006;87(1):1–9. doi: 10.1016/j.apmr.2005.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woldag H, Waldmann G, Heuschkel G, Hummelsheim H. Is the repetitive training of complex hand and arm movements beneficial for motor recovery in stroke patients? Clin Rehabil. 2003;17(7):723–730. doi: 10.1191/0269215503cr669oa. [DOI] [PubMed] [Google Scholar]
- 49.Duncan P, Richards L, Wallace D, Stoker-Yates J, Pohl P, Luchies C, Ogle A, Studenski S. A randomized, controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke. 1998;29(10):2055–2060. doi: 10.1161/01.str.29.10.2055. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers H, Mackintosh J, Price C, Wood R, McNamee P, Fearon T, Marritt A, Curless R. Does an early increased-intensity interdisciplinary upper limb therapy programme following acute stroke improve outcome? Clin Rehabil. 2003;17(6):579–589. doi: 10.1191/0269215503cr652oa. [DOI] [PubMed] [Google Scholar]
- 51.Platz T, Winter T, Muller N, Pinkowski C, Eickhof C, Mauritz KH. Arm ability training for stroke and traumatic brain injury patients with mild arm paresis: A single-blind, randomized, controlled trial. Arch Phys Med Rehabil. 2001;82(7):961–968. doi: 10.1053/apmr.2001.23982. [DOI] [PubMed] [Google Scholar]
- 52.Lincoln NB, Parry RH, Vass CD. Randomized, controlled trial to evaluate increased intensity of physiotherapy treatment of arm function after stroke. Stroke. 1999;30(3):573–579. doi: 10.1161/01.str.30.3.573. [DOI] [PubMed] [Google Scholar]
- 53.Sunderland A, Tinson DJ, Bradley EL, Fletcher D, Langton Hewer R, Wade DT. Enhanced physical therapy improves recovery of arm function after stroke. A randomised controlled trial. J Neurol Neurosurg Psychiatry. 1992;55(7):530–535. doi: 10.1136/jnnp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74(4):347–354. [PubMed] [Google Scholar]
- 55.Lo AC, et al. Randomized trial of robot-assisted rehabilitation for chronic stroke. International Stroke Conference; San Antonio, TX. 2010. [Google Scholar]
- 56.Hsu SS, Hu MH, Wang YH, Yip PK, Chiu JW, Hsieh CL. Dose-response relation between neuromuscular electrical stimulation and upper-extremity function in patients with stroke. Stroke. doi: 10.1161/STROKEAHA.109.574160. [DOI] [PubMed] [Google Scholar]
- 57.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41(1):129–135. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 58.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very early constraint-induced movement during stroke rehabilitation (vectors): A single-center rct. Neurology. 2009;73(3):195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (avert). Phase ii safety and feasibility. Stroke. 2008;39(2):390–396. doi: 10.1161/STROKEAHA.107.492363. [DOI] [PubMed] [Google Scholar]
- 60.Bernhardt J, Thuy MN, Collier JM, Legg LA. Very early versus delayed mobilisation after stroke. Cochrane Database Syst Rev. 2009;(1):CD006187. doi: 10.1002/14651858.CD006187.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, Bernhardt J. Very early mobilization after stroke fast-tracks return to walking. Further results from the phase ii avert randomized controlled trial. Stroke. 2011;42(1):153–158. doi: 10.1161/STROKEAHA.110.594598. [DOI] [PubMed] [Google Scholar]
- 62**.Efficacy and safety of very early mobilisation within 24 h of stroke onset (avert): A randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60690-0. The paper provides results from a multisite, international, Phase III trial of early mobilization (< 24 hrs) after stroke. The primary result is that early mobilization reduced the odds of a favorable outcome at 90 days post stroke. [DOI] [PubMed] [Google Scholar]
- 63**.Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, Cen SY, Azen SP, Winstein CJ. Icare primary results: A phase iii stroke rehabilitation trial. International Stroke Conference. 2015:Abs LB15. This abstract (all that is available as of June 2015) provides results for the a multisite, Phase III trial of upper limb rehabiliation, starting an average of 45 days post stroke. The primary result is that all groups improved, regardless of whether or not they receive the innovative upper limb intervention or usual care, and regardless of amount of therapy provided. [Google Scholar]