Abstract
Objectives
To determine the maximum tolerated dose (MTD) of weekly paclitaxel and cisplatin chemotherapy concurrent with extended field irradiation in women with cervical cancer metastatic to the para-aortic nodes.
Methods
Patients with carcinoma of the cervix and histologically documented para-aortic node metastases were eligible for this phase I/II trial. Chemotherapy agents were administered weekly concurrent with extended field radiation with escalating doses of paclitaxel from 30–50 mg/m2 in each of three cohorts of three patients each. A phase II cohort was then evaluated at the selected maximum tolerated dose (MTD).
Results
The MTD was determined to be cisplatin 40 mg/m2 (maximum dose of 70 mg) and paclitaxel 40 mg/m2 administered weekly for six cycles concurrent with extended field radiation therapy. There were 19 evaluable patients for the phase II analysis of toxicity and efficacy. Grade three and four gastrointestinal toxicity was seen in 6 and neutropenia in 7. Radiation therapy was successfully completed in 36.8% of patients at eight weeks and in 68.4% of patients at nine weeks, with a median time to completion was 56 days. A total of 27 evaluable patients were enrolled, twelve are dead (mean survival of those deceased is 25 months), and 15 (56%) are alive, and have been followed for a mean of 48 months (range 25–68; median of 46 months).
Conclusions
Paclitaxel and cisplatin combination chemotherapy concurrent with extended field pelvic para-aortic irradiation can be administered at the described MTD and shows a higher than previously reported disease-free survival in relation to historical data. The 56% survival to date, and 50% estimated 48 month survival, warrants validation in a larger prospective cohort. Central radiation dose reduction is being considered in the next trial to decrease late toxicity of regimen.
INTRODUCTION
Approximately 11,150 cases of invasive cervical cancer were expected to be diagnosed in the United States in 2007, resulting in approximately 3,670 deaths.[1] Historically, treatment of invasive cervical carcinoma has been limited to either surgery or radiotherapy{2}. In early stage disease (FIGO stage IIA or lower) these modalities have demonstrated comparable efficacy, however with differing toxicity profiles. In locally advanced stage disease, the role of surgery is limited and the primary mode of treatment, since the publication of five randomized clinical trials [3–7] and the 1999 National Cancer Institute Clinical Announcement [8], has been concurrent cisplatin-based chemoradiation, including a combination of external beam radiotherapy and intracavitary brachytherapy. [2]
Although the above referenced studies have covered many of the possible clinical stages of cervical cancer, there remains a subset of patients with tumors metastatic to the para-aortic lymph nodes, not traditionally included in the above studies, due to their poor prognosis and requirement for extended field radiotherapy with its associated morbidities. Berman et al, reported the results of the GOG experience involving 621 cervical cancer patients and noted 5% of Stage IB, 17% of Stage IIB patients, and 25% of Stage IIIB patients had biopsy confirmed para-aortic lymph node metastases.[9] Survival rates for this patient population are routinely reported as poor, with the above study reporting a median survival of 15.2 months and a probability of survival of 25% at 3 years. The current understanding that concurrent chemo-radiation can reduce both local and distant failures, makes further study of this population with our best chemotherapy agents worth pursuing.
The efficacy of paclitaxel as a chemotherapeutic agent in the treatment of gynecologic malignancies has been well documented, especially for adenocarcinomas of ovarian origin. Phase II data of paclitaxel’s efficacy in the treatment of recurrent squamous cell carcinomas of the cervix has been encouraging. [11] The combination of paclitaxel and cisplatin has been evaluated in patients with recurrent and advanced cervical cancer. Moore, et al reported on a randomized phase III trial of paclitaxel and cisplatin versus cisplatin alone in which combination chemotherapy yielded an overall response rate of 36.2% and a median progression free survival (PFS) of 4.8 months versus 19.4% and 2.8 months, respectively, for cisplatin alone.[12]
At the time the current study was initiated, paclitaxel had been combined with radiation primarily in the treatment of non-small cell carcinoma of the lung. The paclitaxel dosing regimens ranged from 10 mg/m2 weekly to 90 mg/m2 with minimal toxicity. [13,14] There had been one previous trial looking at the combination of paclitaxel and cisplatin with radiation therapy in cervical cancer. Chen, et al. reported on a phase I study of escalating doses of paclitaxel as a radiation sensitizer in combination with cisplatin.[15] The results demonstrated a well-tolerated dose range starting at 10 mg/m2 weekly and advancing to 50 mg/m2 weekly in combination with cisplatin 50 mg/m2 every three weeks. A 93% response rate was seen in this study of previously untreated patients. The radiation therapy included both primary as well as adjuvant treatment for patients at high risk for recurrence after radical hysterectomy and lymphadenectomy.
Based on the reported activity of paclitaxel and cisplatin in advanced, recurrent cervical cancer and prior data supporting the use of these agents as radiation sensitizers in both cervical and lung cancer, this study (GOG 9804) was initiated to establish a maximally tolerated dose (MTD) for this combination regimen given concurrently with extended field pelvic and para-aortic irradiation and intracavitary brachytherapy to treat cervical cancer metastatic to the para-aortic lymph nodes without evidence of distant metastasis. Based on the dosing of cisplatin by Rose et al. and in the study by Keys et al, a dose of cisplatin of 40 mg/m2 (with a maximum weekly dose of 70 mg) was utilized in this study with a planned dose escalation of weekly paclitaxel [5,7]. The goal was to maintain the current standard dose of cisplatin at 40 mg/m2 weekly and add paclitaxel at the maximum tolerated dose.
MATERIALS AND METHODS
The study was initiated after review and approval by the Cancer Therapy Evaluation Program of the National Cancer Institute. Participating institutions’ Institutional Review Boards approved the study prior to enrolling any patient, and all patients provided written informed consent meeting all federal, state and local requirements before receiving any protocol therapy.
Patients with clinical stages IB, II, IIIB and IVA invasive carcinoma of the cervix with histologically positive para-aortic nodes were eligible. Acceptable modes of evaluation for para-aortic adenopathy included fine needle aspiration and extraperitoneal or laparoscopic lymph node sampling. Lymphadenectomy was not required. Additional eligibility criteria included an absolute neutrophil count (ANC) of ≥1500/μl, platelets ≥ 100,000/μl, creatinine <2 mg%, bilirubin ≤ 1.5 times the upper limit of normal, SGOT (serum glutamic oxaloacetic transaminase)≤ 3 times the upper limit of normal, and a GOG performance status of 0–2. Entry onto the study was required to be within eight weeks of diagnosis. Patients with obstructive uropathy must have been treated with ureteral stenting or percutaneous nephrostomy. CT (computed tomography) scan of the chest, abdomen and pelvis were required to exclude patients with metastatic cancer outside of the radiation treatment field. Patients were excluded who had received previous pelvic or abdominal radiation or cytotoxic chemotherapy, or who had evidence of any cancer within the preceding three years.
The objective of the study’s first phase was to identify the maximum-tolerated dose (MTD) of concurrent weekly paclitaxel and cisplatin chemotherapy administered with radiation. Three patients were to be enrolled at each dose level and observed for 30 days following completion of therapy. If one patient out of three experienced a dose limiting toxicity (DLT), an additional three patients were to be enrolled at that dose level. DLT was defined as the occurrence of a grade 4 toxicity within 30 days of treatment, or the occurrence of a toxicity that caused a dose delay/modification/reduction in any of the six planned cycles. The MTD was to be defined as the dose level at which ≤ 1 patient (among 6) experienced a DLT. Late toxicity is still being collected at the time of this publication.
Paclitaxel was administered as a one hour continuous intravenous (IV) infusion prior to cisplatin administration on a weekly basis for five cycles, with the sixth cycle given concurrent with parametrial boost. The initial paclitaxel dose level was 30 mg/m2 for the first three patients, with a planned 10 mg/m2 incremental increase in subsequent cohorts of three patients, until the appearance of DLT or the dose reached 70 mg/m2. Subsequent dose levels were initiated only if a 30-day post-treatment observation period of all three patients had passed. Cisplatin was initially dosed at 40 mg/m2 and infused at 1 mg/minute, to a maximum dose of 70 mg per week, with appropriate pre- and post-treatment hydration. Infusions were to be completed approximately four hours prior to radiation therapy on Mondays. Pretreatment steroids, histamine blocking agents and antiemetics were recommended as appropriate. The specific dosing levels are found in Table 1. Dose level IV was modified in an attempt to decrease gastrointestinal toxicity by reducing cisplatin to 30 mg/m2 while allowing for the escalation of paclitaxel to 50 mg/m2 (Table 1).
TABLE 1.
DOSE LEVEL SCHEMA
| Dose Level | Cisplatin (mg/m2) | maximum weekly dose (mg) | Paclitaxel (mg/m2) | maximum weekly dose (mg) | Planned Number of Patients |
|---|---|---|---|---|---|
| - I | 30 | 60 | 20 | 40 | 3 |
| I | 40 | 70 | 30 | 60 | 3 |
| II | 40 | 70 | 40 | 80 | 3 |
| III | 40 | 70 | 50 | 100 | 3 |
| IV | 30 | 60 | 50 | 100 | 3 |
| V | 30 | 60 | 60 | 100 | 3 |
| VI | 30 | 60 | 70 | 100 | 3 |
The purpose of the trial’s second phase was to evaluate the feasibility and tolerability of the MTD in a larger cohort of approximately 20 patients prior to moving this regimen forward to a phase III clinical trial.
External beam radiation therapy was administered to the para-aortic nodes and pelvis utilizing 6–20 MV photons. The para-aortic and pelvic regions received 1.5 Gy per fraction using anterior and posterior parallel opposed fields and the pelvic region was supplemented by lateral pelvic ports to bring the tumor dose to 1.8 Gy per fraction. Alternatively, the pelvis and para-aortic regions together could be treated with a 4-field technique. The para-aortic region received 45 Gy in 30 fractions, while the pelvic region received 45 Gy in 25 fractions. A separate para-aortic field was required after the 25 days of pelvic radiation were completed to reach the required total dose. A parametrial boost of 5.4 to 9.0 Gy in 1.8 Gy fractions utilizing AP/PA fields was given based on the extent of parametrial involvement at the discretion of the treating radiation oncologist. The superior border for the para-aortic fields was the L1-L2 interspace, but could be extended superiorly by two vertebral heights if necessary to encompass known disease. The inferior border was at mid-obturator foramen or at least 2 cm. beyond the lower extent of disease. The lateral border for the para-aortic region covered known para-aortic disease with a 2 cm. margin at least to the tips of the transverse processes of the lumbar vertebrae. The pelvis lateral border was one centimeter beyond the widest plane of the bony pelvic wall. The lateral pelvic field borders were the L4-5 interspace superiorly, anterior to the symphysis pubis, and at the posterior border of the sacrum.
Patients were also to undergo intracavitary brachytherapy utilizing either low dose rate (LDR) or high dose rate (HDR) techniques. LDR brachytherapy was prescribed to deliver 40 Gy to point A in one or two implants, resulting in a cumulative dose of 85 Gy to point A. HDR brachytherapy was prescribed to deliver 30 Gy in five weekly fractions beginning in week 4, resulting in a total dose to point A of 75 Gy. The total elapsed treatment time including external beam radiotherapy and brachytherapy was not to exceed eight weeks.
During treatment, patients were evaluated weekly including clinical assessment of toxicity, with complete blood counts and pertinent serum chemistries. Radiation therapy was interrupted for ANC ≤ 500/μl lasting > 7 days, platelets ≤ 20,000/μl or GI toxicity requiring intravenous hydration or hospitalization. Chemotherapy administration required an ANC > 500/μl and platelets > 50,000/μl. Toxicities were graded according to the NCI Common Toxicity Criteria version 2.0. Upon completion of protocol treatment, patients were to be assessed for disease status and treatment-related toxicity every three months for two years, then every six months for a total of five years.
RESULTS
Eligible patients were enrolled onto GOG 9804 from March, 2000 through September, 2005. A total of 29 patients were enrolled, three on dose level I, 20 on dose level II, 3 on dose level III, and 3 on dose level IV. Distribution of patients by stage was: IB (n=6), II (n=7), IIIB (n=12) and IVA (n=4).
Three patients were entered on each of the first 4 dose levels. At dose level I, one patient completed without delays or significant toxicities. Another patient had grade 4 metabolic toxicities at cycles 4 and 5 (hypomagnesemia and hypokalemia). The last patient had a delay at cycle 4 for a grade 3 infection due to a urinary tract infection. All three patients completed the prescribed six cycles of chemotherapy. The median length of radiation therapy in this group was 48 days. Based on this demonstrated tolerance, dose level II was opened for accrual and no dose limiting toxicities encountered. The three patients treated at dose level II in the phase I component are included in the summary of the expanded phase II cohort.
Three patients were enrolled at dose level III. One completed six cycles of chemotherapy without delays or significant toxicities. One experienced grade 3 GI toxicity at cycle 3 but completed six cycles of chemotherapy, and the other experienced grade 4 GI toxicity at cycle 4, and her paclitaxel was stopped for cycles 5 and 6. The median length of radiation therapy for this cohort of patients was 57 days.
Based on the relative intolerability of dose level III, dose level IV was modified to decrease the cisplatin dose to 30 mg/m2 in order to maintain paclitaxel at 50 mg/m2. Three patients were enrolled at dose level IV. One completed without delays or significant toxicities. One experienced a grade 4 cardiovascular toxicity in the form of a pulmonary embolus at cycle 6. The other experienced grade 3 hematologic toxicity, and a seizure with paclitaxel on cycle 1 and was taken off study. The median length of radiation therapy for this cohort of patients was 56.5 days. The decision was to accept level II as the maximum tolerated dose which maintains the cisplatin dose at the current standard of 40 mg/m2, and adds paclitaxel at 40 mg/m2..
Based on the planned dose escalation schema and the toxicities observed at dose levels III and IV, the MTD was identified as dose level II. Twenty patients were enrolled in the phase II component of the study at the selected MTD. Including the patients treated at the MTD in the phase I component, 19 of the 20 patients were evaluable for toxicity, response, recurrence, and survival. One patient was not evaluable secondary to complications of her ureteral stents interfering with study compliance.
Ninety percent of patients on dose level II completed all six cycles of chemotherapy. One patient was not evaluable secondary to being removed from the study after 3 cycles of chemotherapy secondary to complications related to ureteral stents.
The predominant reported acute toxicities were hematologic and gastrointestinal in nature. The frequency and grade of the most common toxicities for the patients treated in the expanded phase II cohort are summarized in Table 2. Grade 3 or 4 GI toxicity occurred in 10.4% of administered cycles in the expanded (dose level II) cohort; these toxicities manifested mainly as dehydration requiring IV fluid secondary to nausea, vomiting, or diarrhea. Among the evaluable patients treated at the MTD, six had grade 3 or 4 gastrointestinal (GI) toxicities (representing 12 of 115 cycles administered to this cohort) and 7 experienced grade 3 or 4 neutropenia (11 out of 112 cycles administered), none occurring prior to the third cycle. Grade 3 or 4 thrombocytopenia was rare (2 of 115 cycles administered)
TABLE 2.
ADVERSE EVENTS - DOSE LEVEL II (DATA AVAILABLE FOR 115/116 CYCLES)
| Grade
|
|||||
|---|---|---|---|---|---|
| Toxicity | 0 | 1 | 2 | 3 | 4 |
| Leukopenia* | 43 | 14 | 25 | 25 | 5 |
| Neutropenia* | 70 | 12 | 19 | 7 | 4 |
| Thrombocytopenia | 93 | 15 | 5 | 2 | 0 |
| Anemia | 33 | 38 | 38 | 2 | 1 |
| Allergy | 113 | 2 | 0 | 0 | 0 |
| Auditory | 112 | 1 | 2 | 0 | 0 |
| Cardiovascular | 108 | 2 | 0 | 1 | 4 |
| Coagulation | 110 | 1 | 0 | 3 | 1 |
| Constitutional Symptoms | 66 | 37 | 9 | 3 | 0 |
| Dermatology | 98 | 14 | 3 | 0 | 0 |
| Endocrine | 108 | 3 | 4 | 0 | 0 |
| GI | 42 | 39 | 22 | 9 | 3 |
| GU | 86 | 26 | 2 | 1 | 0 |
| Hemorrhage | 95 | 13 | 6 | 1 | 0 |
| Hepatic | 106 | 9 | 0 | 0 | 0 |
| Infection | 106 | 1 | 5 | 3 | 0 |
| Lymphatics | 115 | 0 | 0 | 0 | 0 |
| Metabolic | 89 | 20 | 0 | 6 | 0 |
| Musculoskeletal | 110 | 2 | 0 | 3 | 0 |
| Neurology | 96 | 15 | 3 | 0 | 1 |
| Ocular | 112 | 0 | 3 | 0 | 0 |
| Pain | 87 | 18 | 8 | 2 | 0 |
| Pulmonary | 108 | 3 | 4 | 0 | 0 |
| Sexual Function | 113 | 2 | 0 | 0 | 0 |
Data available for 112/115 cycles.
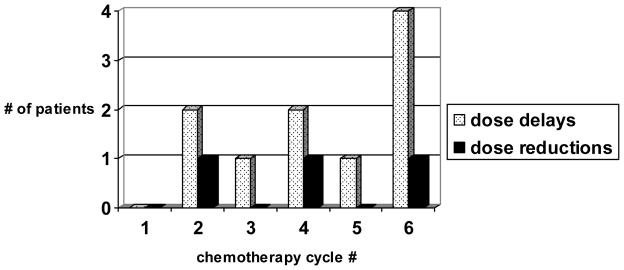
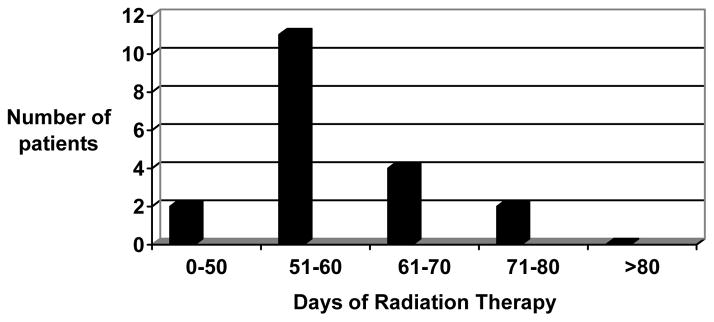
Dose delays and reductions tended to occur in the later chemotherapy cycles. Figure 1 demonstrates this finding for the expanded (dose level II) cohort. Nine patients experienced a total of ten chemotherapy treatment delays ranging from 1 to 21 days. Three patients required dose reductions, two of which were for gastrointestinal toxicity. The median length of radiation therapy was 56 days (range 42 to 76 days) in those patients completing the prescribed therapy. Radiation therapy was successfully completed in 36.8% of patients at eight weeks and in 68.4% of patients at nine weeks, with a median time to completion was 56 days (Figure 2).
Figure 1.
Timing of dose delays and reductions on dose level II
Figure 2.
Length of radiation therapy for dose level II patients
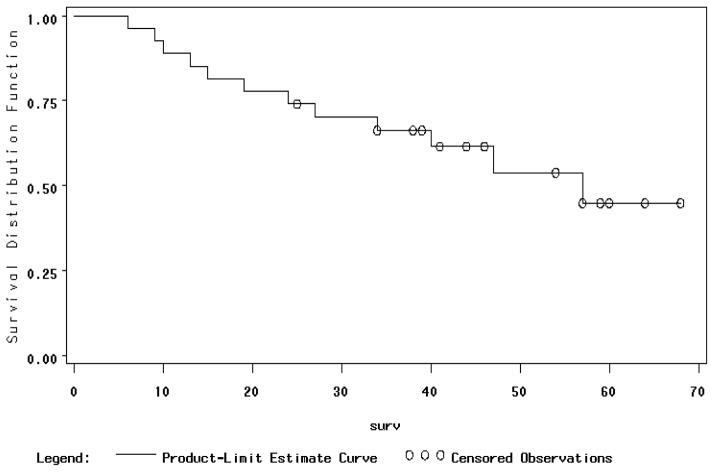
There are a total of twenty-seven patients evaluable for survival, of which fifteen are alive (56%), two of which are alive with disease, and the follow-up ranges from 25 to 68 months (mean of 48, median of 46 months). Those who have died of disease had a survival ranging from 6 to 57 months with a mean of 25 months and median of 24 months. Kaplan-Meier estimates of 36 month survival for all twenty-seven evaluable patients is 66% and 48 month survival is 54%, and estimated 5 year survival is 45% (Figure 3). Sites of recurrent disease include scalene nodes (4), lung (3), peritoneal (2), para-aortic nodes (1), and uncertain (3).
Figure 3.
Overall Survival for Para-Aortic Node Positive Patients
Table 3 outlines the method used for detecting the positive para-aortic lymph nodes and the survival information. Fine needle aspiration only, was used in four cases and 75% died of disease. The remainder had retroperitoneal lymph node dissection with various levels of effort and number of positive nodes detected. The associated survival is reported for number of positive nodes. Table 4 demonstrates the lack of effect of clinical stage for women with positive para-aortic lymph nodes.
TABLE 3.
DIAGNOSTIC MODALITY FOR POSITIVE PARA-AORTIC NODES AND RESULTS
| Positive PA Node Result | N | Alive | Dead | Survival Months |
|---|---|---|---|---|
| FNA only | 4 | 1 | 3 | 29 |
| PA excision = 1 pos | 10 | 5 | 5 | 36 |
| PA excision = 2 pos | 4 | 3 | 1 | 46 |
| PA excision ≥ 3 pos | 9 | 6 | 3 | 37 |
TABLE 4.
| Stage Of Disease | Alive | Months Of Follow-Up | Dead | Average Months Until Death |
|---|---|---|---|---|
| IB | 2 | 44 | 4 | 29 |
| IIB | 4 | 49 | 3 | 14 |
| IIIB | 6 | 54 | 6 | 28 |
| IVA | 3 | 45 | 0 |
Chronic toxicity is common, and the patients with hydronephrosis, ureteral stents or nephrostomy tubes required continued maintenance of those conditions, which pre-dated their treatment. Major surgical interventions included two urinary diversions for vesico-vaginal fistula within three months of completing treatment due to Stage IVA disease with gross tumor in the bladder prior to initiation of therapy. One patient had a colostomy and ileo-conduit performed for a complicated entero-vesical fistula after two years of follow-up. One patient had a colostomy performed for a rectosigmoid stricture in year four of follow-up. The last case, which was on level IV, underwent an ileal resection and anastamosis for stricture after 16 months of follow-up.
DISCUSSION
In an effort to improve upon radiotherapy alone for treatment of advanced stage cervical carcinoma, the concept of radiation with concurrent chemotherapy (chemoradiation) has been evaluated by several cooperative groups, including the Gynecologic Oncology Group (GOG), National Cancer Institute of Canada (NCIC) Southwest Oncology Group (SWOG) and the Radiation Therapy Oncology Group (RTOG). Based on reports of a 30–50% reduction in mortality from the results of five randomized phase III trials [3–7], the National Cancer Institute released a clinical alert in February of 1999 recommending that “…strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiation therapy in women who require radiation therapy for treatment of cervical cancer”.[8] Along with survival benefit, excellent local control rates of approximately 80% in locally advanced disease have been demonstrated with cisplatin and radiation. [4,5] These studies by Morris et al and Rose et al did not include patients with positive para-aortic lymph nodes and did not utilize the current best systemic therapy available. The current study documents efficacy of the combination of weekly cisplatin and paclitaxel, concurrently administered with extended field radiation to women with histologically positive para-aortic metastasis. The dose and schedule is the maximum tolerated dose based on acute toxicity. Radiation dosing has been constant and similar to the dosing prior to the addition of chemotherapy. Radiation dose reductions may prevent chronic toxicity and needs to be considered in the era of chemoradiation for cervical cancer.
Combination chemotherapy with paclitaxel and cisplatin administered concurrently with extended field para-aortic and whole pelvic irradiation can be safely administered at the prescribed MTD of cisplatin 40 mg/m2 (maximum weekly dose of 70 mg) and paclitaxel 40 mg/m2 weekly for six cycles. Response data (Table 3) demonstrate a complete response rate of 76.5%. The overall survival rate among the 27 cases evaluable patients is an encouraging 56% of which two are alive with disease at 25 and 46 months. Assuming the death of these two women, this still leaves a survival of 48% with the mean follow-up of 48 months (range 25 to 68 months; median 46 months). Kaplan-Meier estimate of 5 year survival is 45%, which is superior to the results reported by Varia et al, with concurrent cisplatin and 5-fluorouracil and extended field radiation [10]. The previous GOG positive para-aortic study of 86 evaluable patients had a 3 year progression free survival of 34% and overall 3 year survival of 39%.
There was not a prolongation in the length of the radiation treatment time with the concurrent addition of the weekly paclitaxel and cisplatin regimen administered in this trial relative to the previously reported chemoradiation treatment times. Varia et al[10] in 1998, treating positive para-aortic lymph node patients, had a prescribed treatment time of 10 weeks (77 days), whereas current pelvic only disease required treatment times of less than 8 weeks (56 days). The median length of treatment of 56 days, for those patients treated at the MTD of the current study, is comparable to the median of 63 days in the study by Rose et al[5] and the median of 58 days in the study by Morris et al[4]. The impact of total length of radiation treatment time is critical in respect to ultimate outcome, and the development of chemoradiation regimens should be done through clinical trials which will allow for the close monitoring of the impact of these regimens on total radiation treatment times.
Para-aortic radiation is given at 1.5 Gy over 30 treatment days to a total dose of 45 Gy. This contributes somewhat to the increased the treatment time. The treatment delays during chemotherapy were mainly for hydration secondary to diarrhea, nausea, and vomiting in three participants, which is presumed to be a combination of cisplatin toxicity and secondary to increased small bowel in the para-aortic field. One had delays secondary to ureteral obstruction and stent change, but she successfully completed trial in 67 days. Two patients had compliance or scheduling problems, without documented toxicity, and there were delays over the holidays. The chemotherapy and initial teletherapy was generally completed on time, however, multiple delays were secondary to brachytherapy and parametrial boost scheduling. One patient had phone messages stating she was delaying radiation due to nausea, but refused IV fluids or additional antiemetics. It should be noted that this patient population was usually uninsured and had difficulty filling prescriptions for home antiemetics such as ondansetron. Aprepitant, a new antiemetic recommended with cisplatin, was not yet available during this study period. High dose rate brachytherapy has reduced total treatment times, but may contribute to long term toxicity. Attention to scheduling of brachytherpy and parametrial boost, after completion of the concurrent chemo-radiation could potentially improve treatment times when low dose rate is utilized.
Gastrointestinal toxicity was manageable with close attention to hydration status and the use of prophylactic antiemetic, antidiarrheal therapies, and hydration when necessary. There was one documented case of Clostridia difficile enterocolitis. Hematologic toxicity was expected and did not lead to excessive delays in therapy. The rate of Grade 3 or 4 GI toxicity (10.4% of all cycles on level II) was higher than that seen in the study by DiSilvestro et al (4.4%) which used the same chemotherapy regimen concurrent with a more limited pelvic radiation field [16]. This is not surprising given the known increased bone marrow and small bowel covered by the extended radiation field. Grade 3 or 4 neutropenia (9.8%), however, occurred at a similar rate as DiSilvestro et al (11.5%)[16]. Thrombocytopenia was rare, and only occurred in 2 treatment cycles.
The late toxicity for this regimen is considerable. The two cases where the patients presented with tumor growing into the bladder lumen resulting in vesico-vaginal fistulas as would be expected and should not be attributed to this regimen. Increased toxicity can be expected with extended field radiation. Central radiation dose reduction is being considered, since no patient failed in the pelvis. The HDR brachytherapy may be excessive, and cause harm to the sigmoid colon and terminal ileum directly behind the cervix when combined with cisplatin and paclitaxel chemotherapy. The GOG is planning to study radiation dose adjustments to decrease late toxicity, and patient reported outcomes to enhance collection of late toxicity information. Based on the encouraging outcome data in this study as well as the previously cited GOG study, a patient population once thought to have a very poor prognosis may well be salvaged with this aggressive strategy. Due to its tolerability and efficacy, paclitaxel and cisplatin administered weekly concurrent with extended field radiation therapy for patients with cervical cancer metastatic to the para-aortic nodes warrants further development in an effort to determine if the combination will help improve both local and distant control and ultimately yield a survival benefit. More research is needed on tailoring the radiation dose to the tumor volume and reduction of late toxicity may be feasible with central dose reduction.
Survival of patients with positive para-aortic lymph nodes may change in the new era of PET (positron emission tomography) imaging. PET scanning was not utilized in the current study, and no follow-up scanning was required in this study. A chest radiograph and CT scan of the abdomen and pelvis was only recommended. Patients with recurrent disease presented with symptoms or clinically detected recurrent disease. PET scanning may improve survival in the population of women with positive para-aortic lymph nodes in future studies by excluding those with more sensitive detection of disease outside of the radiation field. PET may also detect smaller para-aortic disease than that seen on CT alone. This will make comparison of survival difficult on future studies difficult. This study enrolled many patients detected to have positive para-aortic nodes during planned extra-peritoneal resection similarly to previous GOG studies.
Conclusion
This chemo-radiation regimen should be considered in future phase III clinical trials of treatment for cervical carcinoma. Chronic toxicity is an unfortunate consequence of advanced local disease and para-aortic radiation. Isolated central failure is now rare, thus allowing consideration toward reduction of the central radiation dose. This may be feasible in this era of chemo-radiation to obtain both excellent distant control, central control, and fewer late toxicities. The acceptable level of major bowel and bladder morbidity in hopes of providing salvage of this advanced stage of disease is unknown, but deserves further patient reported outcomes research.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517).
The following GOG member institutions participated in this study: Case Western Reserve University, Cleveland Clinic Foundation, Indiana University Medical Center, University of Iowa Hospitals and Clinics, University of Oklahoma and Wake Forest University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Monk BJ, Tewari KST. Invasive cervical cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. 7. Philadelphia PA: Elsevier; 2007. pp. 55–124. [Google Scholar]
- 3.Whitney CW, Sause WT, Bonomi P, Liao S. A randomized comparison of hydroxyurea versus 5-FU infusion and bolus cisplatin as an adjunct to radiation therapy in patients with stages II-B, III and IV-A carcinoma of the cervix and negative para-aortic nodes. J Clin Oncol. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 4.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Eng J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Eng J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 7.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Eng J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute Clinical Announcement. Concurrent Chemoradiation for Cervical Cancer. 1999 Feb; http://www.cancer.gov/newscenter/cervicalcancer.
- 9.Berman ML, Keys H, Creasman W, et al. Survival and Patterns of Recurrence in Cervical Carcinoma Metastatic to Periaortic Lymph Nodes (A Gynecologic Oncology Group Study) Gynecol Oncol. 1984;19:8–16. doi: 10.1016/0090-8258(84)90151-3. [DOI] [PubMed] [Google Scholar]
- 10.Varia MA, Bundy BN, Deppe G, Mannel R, Averette HE, Rose PG, Connelley P. Cervical carcinoma metastatic to para-aortic nodes: Extended filed radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: A Gynecologic Oncology Group Study. Int J Rad Oncol Biol Phys. 1998;42(5):1015–1023. doi: 10.1016/s0360-3016(98)00267-3. [DOI] [PubMed] [Google Scholar]
- 11.McGuire WP, Blessing JA, Moore D, Lenta AA, Photopulos G. Paclitaxel has moderate activity in squamous cervix cancer: a Gynecologic Oncology Group study. J Clin Oncol. 1996;14:792–5. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 12.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–9. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 13.Choy H, Browne MJ. Paclitaxel as a radiation sensitizer in non-small cell lung cancer. Sem in Oncol. 1995;S22:70–4. [PubMed] [Google Scholar]
- 14.Havemann K, Wolf M, Goerg C, Faora C, Pfab R, Diergarten K. Paclitaxel and simultaneous radiation in the treatment of stage III A-B non-small cell lung cancer. Sem in Oncol. 1995;S22:19–22. [PubMed] [Google Scholar]
- 15.Chen MD, Paley PJ, Potish RA, et al. Phase I trial of taxol as a radiation sensitizer with cisplatin in advanced cervical cancer. Gynecol Oncol. 1997;67:131–6. doi: 10.1006/gyno.1997.4851. [DOI] [PubMed] [Google Scholar]
- 16.DiSilvestro PA, Walker JL, Morrison A, Rose PG, Homesley H, Warshal D. Radiation Therapy with Concomitant Paclitaxel and Cisplatin Chemotherapy in Cervical Carcinoma Limited to the Pelvis: A Phase I/II Study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;103(3):1038–42. doi: 10.1016/j.ygyno.2006.06.017. [DOI] [PubMed] [Google Scholar]