Abstract
Silicon and boron share many similarities, both chemically and biochemically, including having similar effects on bone, although their mechanisms of action are not known. Here we compared the loading of silicon and boron into bone, their localization and how they are influenced by age (growth & development), to obtain further clues as to the biological effects of these elements and, especially, to see if they behave the same or not. Bone samples were obtained from two different studies where female Sprague Dawley rats had been maintained on a normal maintenance diet for up to 43 weeks. Total bone elemental levels were determined by ICP-OES following microwave assisted acid digestion. Silicon and boron levels in the decalcified bones (i.e. the collagen fraction) were also investigated. Silicon and boron showed marked differences in loading and in their localization in bone. Highest silicon and lowest boron concentrations were found in the under-mineralized bone of younger rats and lowest silicon and highest boron concentrations were found in the fully mineralized bone of the adult rat. Overall, however total bone silicon content increased with age, as did boron content, the latter mirroring the increase in calcium (mineral) content of bone. However, whereas silicon showed equal distribution in the collagen and mineral fractions of bone, boron was exclusively localized in the mineral fraction. These findings confirm the reported association between silicon and collagen, especially at the early stages of bone mineralization, and show that boron is associated with the bone mineral but not connective tissues. These data suggest that silicon and boron have different biological roles and that one is unlikely, therefore, to substitute for the other, or at least boron would not substitute for Si in the connective tissues. Finally, we noted that silicon levels in the mineral fraction varied greatly between the two studies, suggesting that one or more nutritional factor(s) may influence the loading of Si into the mineral fraction of bone. This and the nature of the interaction between Si and collagen deserve further attention.
Abbreviations: B, boron; EDTA, ethylenediaminetetraacetic acid; ICP-OES, inductively coupled plasma-optical emission spectroscopy; Si, silicon; SGIF, simulated gastrointestinal fluid; UHP, ultra-high purity
Keywords: Silicon, Boron, Bone, Collagen, Bone mineralisation, Growth and development
Highlights
-
•
Boron and silicon show marked differences in bone loading.
-
•
Boron is exclusively found in the mineral fraction of bone.
-
•
Silicon is distributed equally in the mineral and collagen fractions.
-
•
Data suggest boron and silicon have different biological effects on bone.
-
•
Silicon loading into bone mineral may be influenced by nutritional factors.
1. Introduction
Silicon (Si) and boron (B) share many similarities, both chemically and biochemically. Both are non-metals and semi-conductors that can exist in amorphous and crystalline states and form organo compounds containing Si—O (Cox, 2009, West and Barton, 1980) or B—O bonds (Hunt, 2012, Devirian and Volpe, 2003). Both also form weak acids upon dissolution in water that are fully pronated (pKa1 9.6 and 9.3, respectively) and, therefore, neutrally charged at physiological pHs. Silicon and B are quasi-essential/essential for some plants and algae (Camacho-Cristobal and Gonzalez-Fontes, 2008, Ju et al., 2011, Epstein, 1999, Brzezinski et al., 1990, Hildebrand et al., 1997). Both elements are deposited in the cell walls of plants where they are reported to have a structural role, affecting cell wall properties such as porosity and preventing pathogen attack (Camacho-Cristobal and Gonzalez-Fontes, 2008, Epstein, 1999, Fauteux et al., 2006). Specifically, B binds and stabilizes cell wall components containing cis-diol groups such as pectins and the apiose residues of rhamnogalacturonan II (Camacho-Cristobal and Gonzalez-Fontes, 2008). Kinrade et al., and Wang et al., have shown similar binding by Si (Jugdaohsingh et al., 2008a, Wang, 2004). The lack of either Si or B causes cell cycle arrest in diatoms, thus affecting their normal growth (Hunt, 2012, Brzezinski et al., 1990). Boron depletion has also been shown to prevent completion of the life cycle (embryo survival) in zebrafish and frogs, as well as preventing limb development in frogs (Hunt, 2012, Nielsen, 2008). Similar studies with Si have not been reported.
In higher animals, dietary Si or B deprivation is reported to impair normal growth and bone development (Hunt, 2012, Nielsen, 2008, Carlisle, 1972, Schwarz and Milne, 1972). The lack of Si or B is also reported to affect growth plate maturation and to impair growth plate closure (Hunt, 2012, Jugdaohsingh et al., 2008b). Higher intakes of Si, or Si supplementation, are associated with higher bone mineral density (BMD (Jugdaohsingh et al., 2004, Macdonald et al., 2012, Eisinger and Clairet, 1993)) and better trabecular micro-architecture (Jugdaohsingh et al., unpublished data & ref. Nielsen and Stoecker, 2004). Boron supplementation similarly increases BMD, trabecular micro-architecture and bone strength (Hunt, 2012, Nielsen, 2008, Price et al., 2013, Nielsen and Stoecker, 2009).
The exact role of Si or B in bone health is however not known. There is a suggestion that B may increase the efficacy or utilization of vitamin D (Hunt, 2012, Devirian and Volpe, 2003, Nielsen, 2008). Indeed, it has been reported that B can alleviate marginal vitamin D deficiency (Hunt, 2012). Boron has also been reported to increase the levels of steroid hormones (e.g. estrogen and testosterone) in serum by influencing their metabolism (Devirian and Volpe, 2003, Nielsen, 2008). Silicon deficiency also affects the normal development of other connective tissues, not just bone (Carlisle, 1972, Schwarz and Milne, 1972), and thus it has been suggested that Si may be involved in the synthesis and/or stabilization of the collagen matrix.
Thus while the above findings suggest that Si and B share similar effects on bone, they may bring about these effects through different pathways/routes. Here we show that dietary Si and B show marked differences in bone loading with age and also differences in bone localization, providing further clues as to the biological effects of these non-metals.
2. Materials and methods
2.1. Bone samples
Bone samples were obtained from two studies utilizing female Sprague Dawley rats. In both studies, rats were maintained under standard conditions; i.e. in plastic cages with stainless steel cover at 22 °C, with 12/12 h light/dark cycle and with ad libitum access to a standard maintenance diet.
2.1.1. Study
Paired adult humerus bones (n = 5 pairs) were obtained from a reference group of adult (19 week old) Sprague Dawley rats from a previous study (Jugdaohsingh et al., 2008b). In brief, female Sprague Dawley rats (B&K Universal Ltd.; Hull, UK) were maintained, from three weeks of age, on B&K Rat and Mouse Standard Diet (B&K Universal Ltd.) and tap water for 26 weeks and thereafter killed (by a Schedule 1 method of euthanasia approved by the Home Office Animals Scientific Procedures Act 1986) following an overnight fast. Bone and other tissues were collected (Jugdaohsingh et al., 2008b). Paired humerus bones were cleaned of excess flesh, tendons and ligaments and stored frozen at − 80 °C until analysis.
Prior analysis had shown that the B&K Rat and Mouse Standard Diet contained, on average, 322 μg Si/g feed and 7.4 μg B/g feed (Table 1 & ref. Jugdaohsingh et al., 2008b). The drinking water contained 5 mg Si/L, while B was undetected.
Table 1.
Silicon, boron and calcium contents of the maintenance diets used in Study 1 and Study 2 analyzed directly by the authors.a
| B&K Rat and Mouse Standard Diet (Study 1) | SDS RM1 Expanded Diet (Study 2) | |
|---|---|---|
| Si (μg/g)b | 322 (47) | 628 (66) |
| B (μg/g) | 7.42 (0.62) | 9.12 (0.50) |
| Ca (mg/g) | 8.85 (1.48) | 11.76 (1.08)c |
Nutrient contents of the diets, supplied by the manufacturers, are listed in Supplementary Table 1.
Bioavailability of Si from the two diets were similar: 37% and 33% for the B&K and SDS RM1 diets, respectively (see Supplementary data).
Note, this is higher than the 7.3 mg/g given by the feed company (Supplementary Table 1).
2.1.2. Study 2
Paired tibia bones were collected from female Sprague Dawley rats of six different ages (3, 5, 8, 12, 26 and 43 weeks), at the National Heart and Lung Institute, Royal Brompton Hospital, London, UK (Jugdaohsingh et al., unpublished data). These rats were ‘surplus to requirement’ and had been maintained on a standard maintenance diet, namely SDS RM1 Expanded Diet (Special Diets Service, UK), and drinking water from three weeks of age. Following overnight fast, rats were sacrificed by Schedule 1 method and bones and tissues were collected. Paired tibia bones were cleaned of excess flesh, tendons and ligaments and stored at − 80 °C until analysis.
Samples of the maintenance diet and drinking water were analyzed for Si, B and Ca (Table 1). The drinking water contained 3.9 mg/L Si and 0.051 mg/L B.
2.2. Materials
Ethylenediaminetetraacetic acid (EDTA, disodium salt dihydrate, 99% purity), high purity nitric acid (69% p.a. plus), 1 M hydrochloric acid, sodium hydrogen carbonate (NaHCO3) and pepsin from porcine gastric mucosa were from Sigma Aldrich Chemical Co. (Gillingham, UK). Sodium chloride was from Fluka Analytical (UK). Dialysis membranes (12.5–14 kDa) were from Spectrum Lab Inc. (UK). ICP standard solutions, 1000 mg analyte/L were from Merck Ltd. (Poole, UK) or Sigma-Aldrich Chemical Co. (Gillingham, UK). Polypropylene sample collection tubes (13 mL & 50 mL) were from Sarstedt Ltd. (Leicester, UK). Ultra high purity (UHP) water was 18 MΩ/cm, from a Branstead Nano-Pure water purifier (Thermo Scientific; Ohio, USA). A 100 mg/L multi-element standard was prepared by pooling 1 mL aliquots of the 1000 mg/L ICP standard solution of the elements of interest.
2.3. Decalcification of bone samples
One of each of the pairs of humerus and tibia bones collected in Studies 1 and 2, respectively, were decalcified to determine what proportions of the total bone Si and B were associated with the collagen component of bone.
Whole right humeri (n = 5, from Study 1) were placed in individual dialysis bags with 5 mL of a saturated EDTA solution (37 g in 500 mL UHP water) inside the bag and 40 mL outside the bag in a 50 mL polypropylene tube. Each bone sample was individually decalcified for 4 weeks, at 4 °C. The EDTA solution, inside and outside, was changed every 2/3 days. The decalcified bones were then incubated in UHP water at 4 °C, for five days and then stored at − 80 °C until analysis.
Whole left tibia (n = 3 per age group, except for the 3-week old rats from Study 2 due to insufficient mass) were similarly decalcified as described above, but over a shorter (2-week) period, as this was found to be more than adequate to remove the mineral phase (Supplementary Fig. 1). The decalcified bones were similarly incubated with UHP water and then stored at − 80 °C until analysis.
2.4. Total elemental analysis
Total elemental analyses were carried out by inductively coupled plasma optical emission spectrometry (ICP-OES: Jobin Yvon 2000-2, Instrument SA, Longjumeau, France), equipped with a concentric nebulizer and cyclonic spray chamber. Sample flow rate was 1 mL/min. Peak profiles were used as previously described (Burden et al., 1995, Sripanyakorn et al., 2009), with a window size of 0.08 nm (0.04 nm either side of the peak) with 21 increments per profile and an integration time of 0.5 s per increment.
Analytical lines were: 251.611 nm (Si), 208.96 nm (B), 213.618 nm (phosphorus, P), 315.887 nm (calcium, Ca), 279.806 nm (magnesium, Mg), 766.490 nm (potassium, K), 257.610 nm (zinc, Zn), 259.940 nm (manganese, Mn) and 324.754 nm (copper, Cu). All samples were analyzed in a blinded fashion.
2.4.1. Bones
Whole left humerus (n = 5) and the paired (right) decalcified humerus (n = 5), from the same rats (Study 1), were thawed at room temperature and then digested with 5 mL 17% nitric acid at 185 °C (12 min ramp to 185 °C and maintained at 185 °C for 20 min) in individual acid-cleaned TFM vessels in a Milestone Ethos Plus microwave digestion system (Milestone Srl, Sorisole, Italy). In this ‘closed’ microwave digestion system, the TFM vessels (containing the digested samples) were vented only when their contents had returned to room temperature. This minimized the loss of boron in the vapor phase. Sample blanks (n = 8), containing just the acid mixture alone (i.e. without any samples), were similarly digested to determine the background contribution of Si, B and other elements analyzed.
Whole right tibia (from n = 8–10 rats per age group) and paired (left) decalcified tibia (n = 3 rats per age group except the 3-week old rats) from Study 2 were similarly digested in 2.5–10 mL 10% nitric acid at 185 °C in the Milestone Ethos Plus microwave digestion system or at 230 °C (5 min ramp to 120 °C, 5 min ramp from 120 °C to 230 °C and 15 min 230 °C) in a Milestone UltraWave microwave digestion system (Milestone Srl). For the 3-week old rats, due to their small size, both the right and left tibias were utilized for the whole bone analysis. Sample blanks were similarly prepared as above.
For both sets of samples, the digested samples and sample blanks were transferred to 13 ml polypropylene tubes for ICP-OES analysis. The whole bone digests were analyzed for Si, B and other bone-related elements using sample-based standards which were prepared by spiking aliquots of a pooled sample of the whole bone digests (i.e. 1 mL was removed from each of the digested whole bone samples and pooled together). The spikes (0–2 mg/L) were prepared from 100 mg/L multi-element standard. The decalcified bone digests were analyzed with similarly prepared sample-based standards. Silicon, B and Ca levels in the sample blanks were determined with multi-element standards (0–10 mg/L) prepared in the 10% nitric acid solution. For each study, all samples, including sample blanks, were analyzed in a single batch.
2.4.2. Feed and drinking water
The Si, B and Ca contents of the SDS RM1 Expanded Diet and, the Si and B contents of the drinking water (Study 2) were also determined. Samples (0.15–0.30 g; n = 6) of the rat feed were digested in 5 mL 69% nitric acid at 180 °C (10 min ramp to 180 °C and then maintained at 180 °C for 20 min) in the Milestone Ethos Plus microwave digestion system. Four sample blanks were similarly prepared. Upon cooling the digested samples and sample blanks were diluted with 10 mL UHP water and transferred into pre-weighed 25 mL polypropylene tubes. The digested samples of feed, sample blanks and drinking water sample were analyzed for Si and other elements with multi-element standards (0–20 mg analyte/L) prepared in 2.5% nitric acid.
2.5. Simulated gastrointestinal dissolution assay
The bioavailability of Si from the feeds, used in Studies 1 and 2, was determined from the dissolution of Si under simulated gastrointestinal conditions as previously described (Sripanyakorn et al., 2009). Briefly, 0.25 g of each feed was weighed in to a 15 mL polypropylene tube and mixed thoroughly with 5 mL of pre-heated simulated gastrointestinal fluid (SGIF) and incubated at 37 °C for 10 min. Simulated gastrointestinal fluid was prepared as previously described (Sripanyakorn et al., 2009) and consisted of: 34.5 mM NaCl, 80 mM HCl and 3.2 g/L pepsin from porcine gastric mucosa (added just prior to use) in ultra-high purity water. The feed solutions were placed in pre-washed dialysis bags (12–14 kDa, Tubing Spectra/Por 4 dialysis membrane 2.0 mL/cm; Fisher Scientific, UK), and placed in a 50 mL tube containing 26.5 mL of pre-warmed SGIF. After 2 h, the SGIF mixture in the surrounding solution was pH adjusted to 7.0 with 1 M NaHCO3. The pH in the dialysis bag was not adjusted. The SGIF surrounding the dialysis bag was sampled (1 mL) into an Eppendorf tube at time intervals (0 min, 2 h, 4 h, 6 h & 24 h) and diluted 1:1 with UHP water before elemental analysis for Si, B and other trace elements by ICP-OES.
2.6. Total hydroxyproline
Total hydroxyproline (HYP) content (Hofman et al., 2011, Marque et al., 2001, Kliment et al., 2011) was used to estimate the collagen content in the decalcified bones from Study 2. Samples (~ 8 mg) of the decalcified bones were freeze dried (LTE Mini Lyotrap, LTE Scientific Ltd., Oldham, UK) for 24 h in 0.3 mL crimp-top borosilicate glass vials (Chromacol, UK) and hydrolyzed by vapor phase HCl in a CEM Discover Protein Hydrolysis system (CEM; Matthews, NC, USA). Samples were hydrolyzed at 150 °C for 45 min under anaerobic conditions (15 psi nitrogen). Vapor phase HCl was generated from 10 mL 6 M HCl added to the reaction vessel. The hydrolyzed samples were reconstituted in 500 μL 50% isopropanol. An aliquot was further diluted (10-fold) in 50% isopropanol and 10 μL aliquots of this diluted sample transferred to a 96-well microwell plate and mixed with buffered chloramine-T reagent for 5 min at room temperature. 0.1 mL of Erlich's Reagent was then added and the chromophore allowed to develop for 30 min at 60 °C before measurement of absorbance at 540 nm with an optical plate reader (Labsystems Multiskan RC). Trans-4-hydroxy-l-proline standards (0 to 0.25 mg/mL) were used to determine hydroxyproline concentration (mg/g sample) in the samples and bovine skin gelatine was used as a quality control. Collagen content was expressed in mg, assuming that collagen contains an average of 13% w/w hydroxyproline (Risteli and Risteli, 2006, Seibel, 2001).
2.7. Statistical analyses
Results are reported as means ± SD unless otherwise stated.
3. Results
3.1. Study 1: silicon and boron in adult humeral bone
Table 2 shows the concentrations (per gram wet weight) and contents of the nine elements analyzed in the humerus of adult (29-week old) female Sprague Dawley rats. Concentrations were in similar ranges to those reported previously for the tibia bone from the same rats, although B was not previously analyzed (ref. Jugdaohsingh et al., 2008b & Supplementary Fig. 2). Of the trace elements, Si was found at the highest concentration, aside from Zn (Table 2). Moreover, the difference in Si and B concentrations, and therefore total contents in bone, was 10-fold higher for Si (Table 2).
Table 2.
Study 1: Bone elemental concentrations and contents in the adult humeral bone of female Sprague Dawley rats.a
| Elements | Concentration |
Contentb |
||
|---|---|---|---|---|
| Mean (SD) | Units | Mean (SD) | Units | |
| Si | 16.39 (3.80) | μg/g | 5.80 (1.68) | μg |
| B | 1.59 (0.13) | μg/g | 0.555 (0.049) | μg |
| Mn | 0.421 (0.020) | μg/g | 0.147 (0.008) | μg |
| Cu | 0.858 (0.176) | μg/g | 0.298 (0.050) | μg |
| Zn | 231.8 (14.6) | μg/g | 80.9 (4.2) | μg |
| Mg | 4.22 (0.25) | mg/g | 1.47 (0.09) | mg |
| K | 1.71 (0.21) | mg/g | 0.594 (0.044) | mg |
| P | 100.8 (4.0) | mg/g | 35.2 (1.6) | mg |
| Ca | 210.9 (3.5) | mg/g | 73.8 (5.0) | mg |
n = 5 rats aged 29 weeks.
Mean wet weight of bones = 0.350 (± 0.023) g.
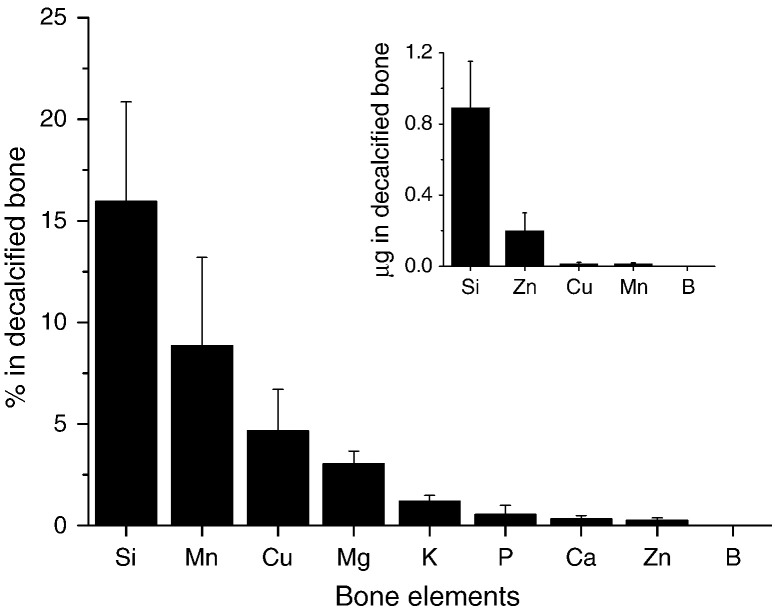
Silicon concentration of the paired decalcified humerus was 3.41 ± 1.01 μg/g wet weight (range: 2.39–4.62 μg/g wet weight) and accounted for 16 ± 5% (range: 11–23%) of the total bone Si content (Fig. 1). Boron was not detected (< 0.12 μg/g) in the decalcified bones. Indeed, of all the bone elements analyzed, the percentage retained in the decalcified bone (i.e. in the collagen fraction) was by far the greatest for Si (Fig. 1). The very low remaining percentage of Ca and P shows that even if the bone could not be totally demineralized, less than 1% mineral remained.
Fig. 1.
Study 1: Distribution of elements in the decalcified adult humerus bone from female Sprague Dawley rats, shown as a percentage of the total (i.e. whole) bone content. A high percentage of silicon was associated with the decalcified bone (i.e. collagen fraction). In contrast, boron was undetected in the decalcified bone. Insert graph shows the content (i.e. amount, in μg) of trace element in the decalcified bone. Data are means ± SD of five bones.
3.2. Study 2: differences in silicon and boron content of tibia with age
We next investigated whether the concentration and distribution of Si and B in bone are influenced by the age of the rat.
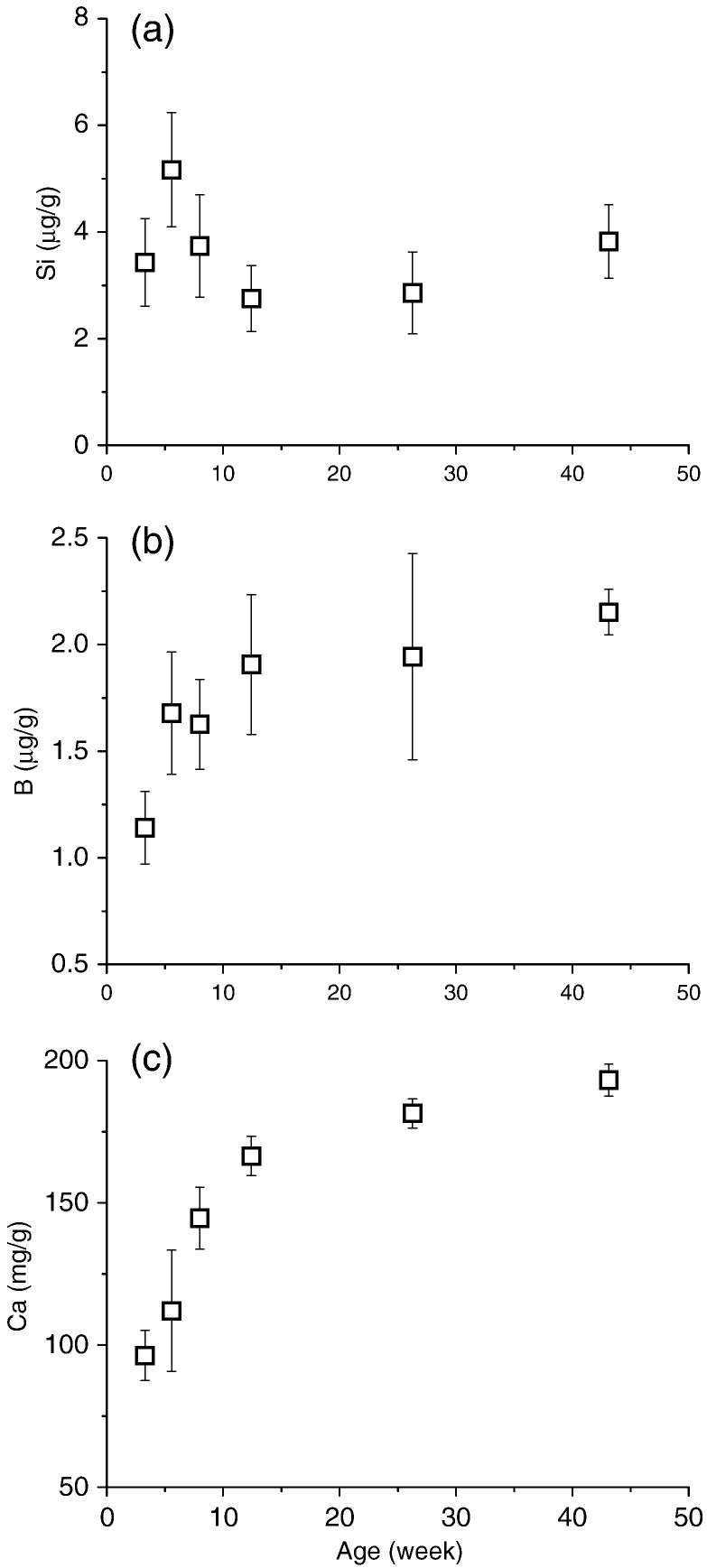
Bone Si concentration was highest in young rats and decreased with age (Fig. 2a). In contrast B concentrations were lowest in the bone of young rats and increased with age (Fig. 2b), mirroring the pattern seen for Ca (Fig. 2c). However, and unexpectedly, in adults, bone Si concentrations were much lower than observed in Study 1 (by 6-fold), while B concentrations were similar.
Fig. 2.
Study 2: Silicon (a), boron (b) and calcium (c) concentrations of the tibia bone from female Sprague Dawley rats of different ages (weanling to adults). Silicon concentrations decreased with age, in contrast to boron and calcium concentrations which increased with age. Data are means ± SD of n = 8–10 bones.
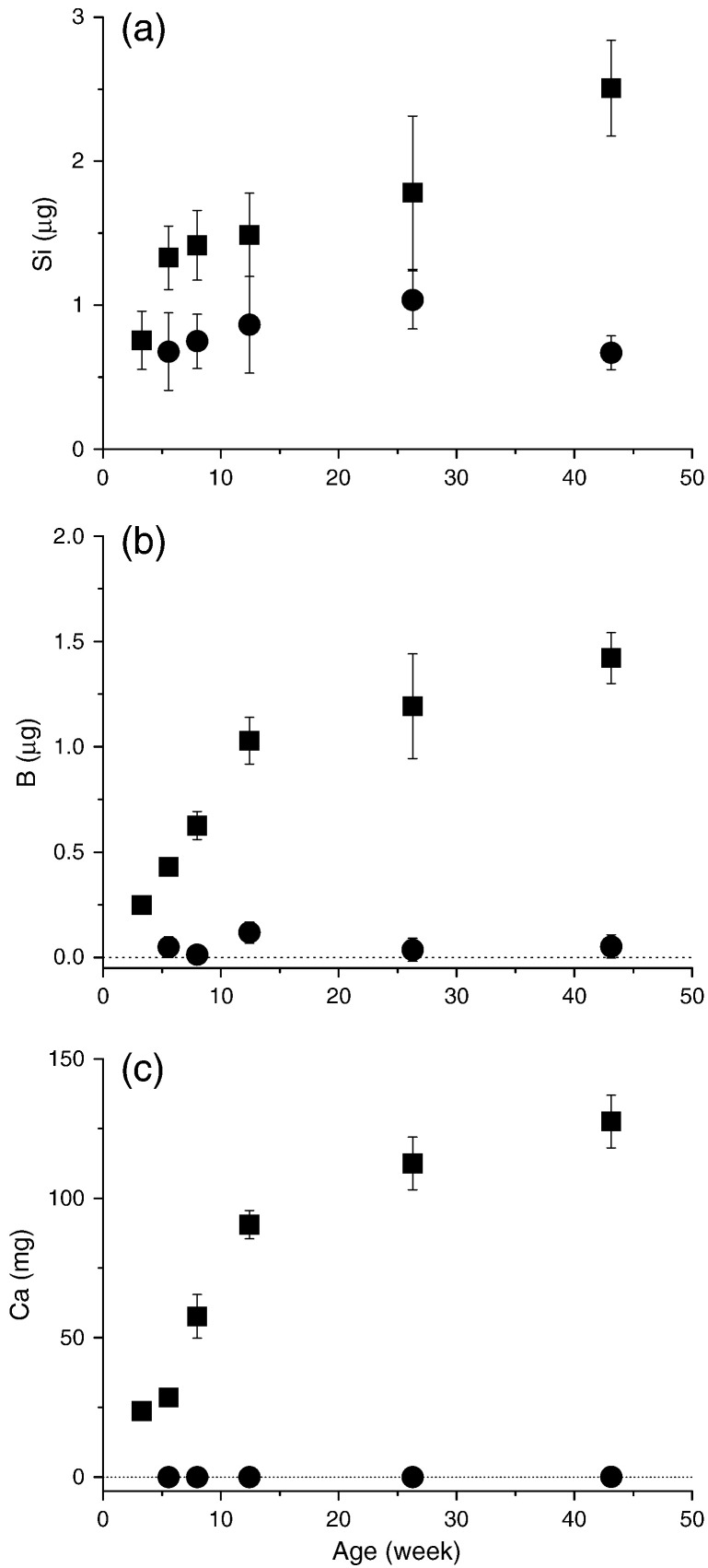
Taking into consideration changes in the size of the bone with age, then an increase in total Si content of the rat bone was observed with age (Fig. 3a). Bone B content showed a more marked increase with age (Fig. 3b) and again, mirrored, the change in bone Ca content (Fig. 3c).
Fig. 3.
Study 2: Total silicon (a), boron (b) and calcium (c) contents of the tibia bone from female Sprague Dawley rats of different ages (weanling to adults); closed squares. Data are means ± SD of n = 8–10 rats. Silicon, boron and calcium contents of the (paired) decalcified tibia bone (closed circles) are also shown. Data are means ± SD of three rats. A high level of Si was associated with the collagen fraction of bone, in contrast boron and calcium were only associated with the inorganic mineral phase.
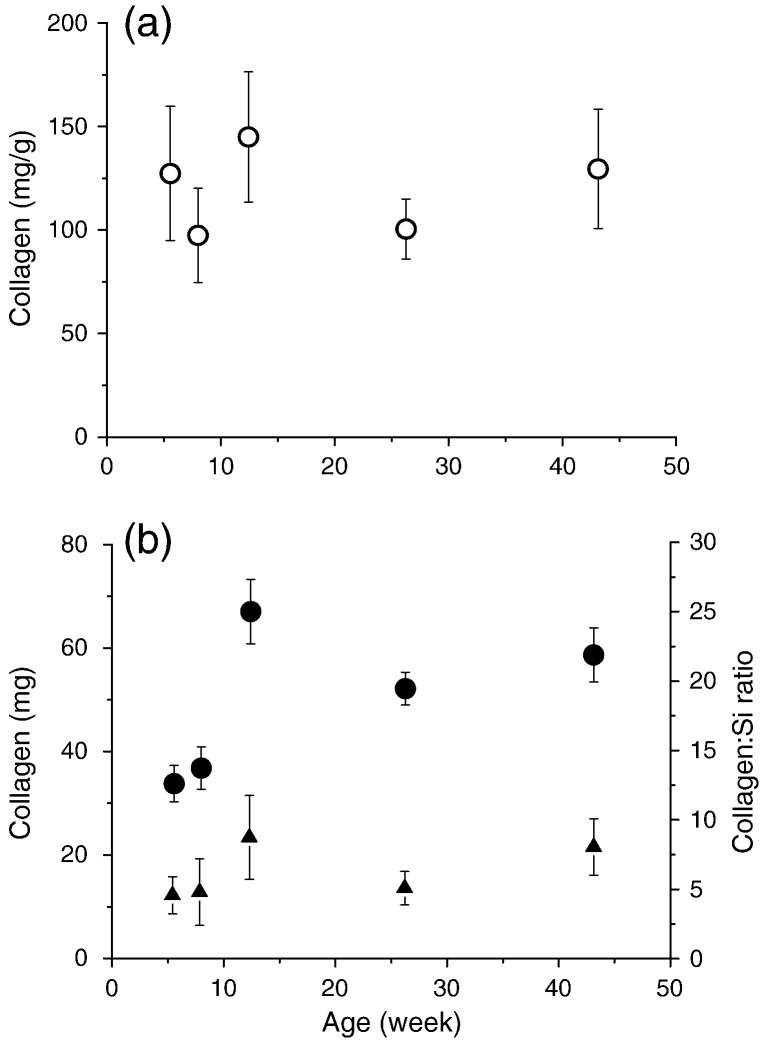
As in Study 1, high concentrations of Si were detected in the (paired) decalcified bone, being 1.89 ± 0.23 μg/g wet weight at 26 weeks (the same age as in Study 1), and there was relatively minor variation with age (range: 1.81–2.64 μg/g wet weight; or 47–71% of total bone Si concentration). Total silicon content of the decalcified bone varied further with age (Fig. 3a), as bone size changed markedly with growth, but as a percentage of total bone silicon it remained reasonably constant (50–58%) except at 43 weeks (27%). At 26 weeks it represented 58 ± 11% of the total bone Si content. Collagen concentration (mg/g wet weight) of the decalcified bone did not change markedly with age (Fig. 4a) but collagen content increased with age due to the increase in bone size (Fig. 4b). An average molar ratio of 1:6 (± 2) Si:collagen was estimated in the decalcified bone, using a molecular weight for type I collagen of 320 kDa (300–340 kDa (Silver and Trelstad, 1980, Zhang et al., 2005)). Boron was virtually undetected in the decalcified bone samples and this was not influenced by age, as per calcium (Fig. 3b & c).
Fig. 4.
Study 2: (a) Collagen concentration (mg/g wet weight; open circles) and (b) collagen content (mg; closed circles) of the decalcified tibia bones from female Sprague Dawley rats of different ages (weanling to adults). (b) The molar ratio of collagen:Si in the decalcified bone is shown (closed triangles). Data are means ± SE of n = 3–5 rat bones per age group.
4. Discussion
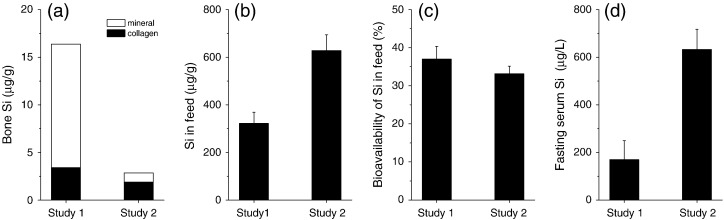
The aim of this study was to investigate and compare the levels of Si and B in bone, their localization between the collagen and mineral phases (fractions), and how these findings are influenced by age. Bones from adult (26 & 29 week old) control (i.e. untreated) female Sprague Dawley rats that were maintained on normal laboratory maintenance feed and drinking water, and collected from two different studies, were employed here and while within (intra-) study variation in bone Si concentration was low, between (inter-) study concentrations of Si was found to be high; namely, 16.4 ± 3.8 μg/g in Study 1 vs. 2.86 ± 0.77 μg/g in Study 2. Interestingly, the concentration of Si associated with the collagen matrix appeared to be relatively consistent between the two studies and it was the Si concentration of the mineral portion of bone that varied so greatly (Fig. 5a). The higher mean Si concentration of the decalcified humeri from Study 1 (3.41 ± 1.01 μg/g) compared to decalcified tibias from Study 2 (1.89 ± 0.23 μg/g) may be due to a higher collagen concentration. Total hydroxyproline concentration of the femurs from Study 1 has previously been reported (Jugdaohsingh et al., 2008b) and at 20.6 ± 0.3 mg/g it is two-fold higher than the total hydroxyproline concentration of the tibia bones from Study 2 (10.3 ± 2 mg/g bone).
Fig. 5.
Comparison of adult (26 and 29 week old) rat bones from Studies 1 & 2: bone Si concentrations in the collagen (black) and mineral (white) phases (a), feed Si concentrations (b), bioavailability of Si in the feed (c) and fasting serum Si concentrations (d). Data are means ± SD of 3–8 samples.
It is not entirely clear why there was this large inter-study variation in bone Si concentration. Certainly it cannot be explained by the difference in Si content of the feeds (Fig. 5b), nor by the bioavailability of Si in the feeds, which were found to be the same (Fig. 5c, see also Supplementary Fig. 3). Indeed, based on the Si content of the feeds and fasting serum Si levels (Fig. 5d) it might have been expected that the bone mineral from rats in Study 2 would have the highest Si concentration of the two studies. Finally, digestion and analysis of the bones from the two studies were carried out in a similar fashion and the levels of the other nine elements analyzed in the bones were found to be similar between the two studies, with the exception of copper and manganese which were more than two-fold higher in Study 2, compared to Study 1 (Supplementary Fig. 2), although their levels in the diets (Supplementary Table 1) and their bioavailability (Supplementary Fig. 4) were similar. Therefore the most plausible explanation is that one or more nutrient/component(s) of the feed may somehow specifically influence the loading of Si into bone mineral. This is not the first occasion we have proposed this. In a previous study we made the conclusion that ‘some co-factor, probably nutritional, is required for maximal Si uptake into bone’ (Jugdaohsingh et al., 2008b) and that this cofactor may be absent or lacking in some diets.
A preliminary examination of the feeds, for their relative levels of differing nutrients, did not find a constituent (factor) that was markedly different between the two although zinc and several vitamins were at levels > 2.5 fold in the feed of Study 1 versus that of Study 2 (Supplementary Table 1). Nonetheless, we believe that this issue deserves more detailed and careful comparison in future studies as identification of such a co-factor may provide important insight into the mechanism of uptake of Si into bone and its homeostasis.
In complete contrast to Si, the concentration of B in bone was found to be consistent between the two studies (1.59 ± 0.13 μg/g in Study 1 vs. 1.94 ± 0.48 μg/g in Study 2) and similar to the levels reported by others (Nielsen and Stoecker, 2009, Chapin et al., 1997). Moreover, unlike Si, B was not found to be associated with the collagen matrix but almost entirely and exclusively located within the mineral portion of bone. Furthermore, B levels in bone mirrored the pattern seen for Ca: i.e. increasing with age and thus the mineral content of bone.
Overall our findings indicate that Si is, in its ‘minimal role’ a collagen seeking element, while B is not. This would imply that their biological roles, with respect to bone, are different and that B cannot substitute for Si in the collagen matrix. Seaborn and Nielsen (Seaborn and Nielsen, 1994), in their investigation of the effect of B on Si deficiency in rats, similarly concluded that B supplementation did not markedly affect the response to Si deprivation, although Si status did influence the response to B supplementation. Furthermore, B is suggested to affect bone/bone mineral by influencing serum steroid hormone levels (estrogen and testosterone) and the metabolism and utilization of Ca (Hunt, 2012, Devirian and Volpe, 2003, EFSA, 2004) and other mineral elements of bone (Nielsen, 2008, EFSA, 2004). Silicon on the other hand, might be involved in the early stages of bone mineralization (Carlisle, 1986, Boonrungsiman et al., 2013, Landis et al., 1986) and most data suggest an involvement with collagen and connective tissue development (Jugdaohsingh, 2007, Reffitt et al., 2003 & Jugdaohsingh et al., unpublished data) as noted above.
The exact nature of the association between Si and collagen is not known. There are suggestions that Si may act as a cofactor for enzymes involved in collagen synthesis, including prolyl hydroxylases (Reffitt et al., 2003, Carlisle et al., 1981). There is also a suggestion that Si may play a structural role in forming cross-links between procollagen chains during collagen synthesis and/or between collagen units (molecules/triple helix) in the extracellular matrix (ECM; (Jugdaohsingh et al., 2008a)). Either of these mechanisms would result in Si being trapped in the ECM and this would explain the high levels of non-dialyzable Si associated with connective tissues and their components (Jugdaohsingh et al., 2008a, Schwarz, 1973). Our finding of a molar ratio of 1:6 Si:collagen in the decalcified bones would support a structural role for Si as a cross-link between collagen units since a higher ratio would have been expected if it was acting as a cross-link between procollagen chains.
Finally, although interactions between Si-containing materials and collagen have been demonstrated (Hench and Greenspan, 2013), a direct interaction between Si(OH)4 and collagen has not. The ex vivo demonstration of complexation between Si(OH)4 and sugars and hydroxyl-amino acids (Kinrade et al., 2003, Kinrade et al., 2001a, Kinrade et al., 2001b, Kinrade et al., 1999), suggests this is a real possibility. Future studies should focus on determining the nature of the interaction between Si(OH)4 and collagen and identify the amino acids or functional groups (most likely hydroxyl groups) responsible for Si binding.
Disclosures
All authors declare no conflict of interest.
Author's contributions
Study design: RJ and JJP. Study conduct: RJ, LDP and AW. Study oversight: RJ. Data analysis: RJ & LDP. Drafting manuscript & revising manuscript content: RJ and JJP. Approving final version of manuscript: RJ, LDP, AW and JJP. RJ takes responsibility for the integrity of the data analysis.
Acknowledgments
We thank the Medical Research Council (grant number MC_US_A090_0008/Unit Programme number U1059) and the charitable foundation of the Institute of Brewing and Distilling and for their support. We also thank the National Heart and Lung Institute (Royal Brompton Hospital, London), Dr. Sarah Ratcliffe, Dr. Katarzyna Kopanska and Ms Katharina Keßler for the help with tissue collection.
The research was designed, executed, analyzed and communicated only by the authors.
Edited by Peter Ebeling
Footnotes
Supported by: The Medical Research Council (grant number MC_US_A090_0008/Unit Programme number U1059) and the charitable foundation of the Institute of Brewing and Distilling (UK).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bonr.2014.10.002.
Appendix A. Supplementary data
Supplementary material.
References
- Boonrungsiman S., Fearn S., Gentleman E., Spillane L., Carzaniga R., McComb D.W., Steven M.M., Porter A.E. Correlative spectroscopy of silicates in mineralised nodules formed from osteoblasts. Nanoscale. 2013;5:7544–7551. doi: 10.1039/c3nr02470a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski M.A., Olson R.J., Chisholm S.W. Silicon availability and cell-cycle progression in marine diatoms. Mar. Ecol. Prog. Ser. 1990;67:83–96. [Google Scholar]
- Burden T.R., Powell J.J., Thompson R.P.H., Taylor P.D. Optimal accuracy, precision and sensitivity of inductive coupled plasma optical emission spectrometry: bioanalysis of aluminium. J. Anal. At. Spectrom. 1995;10:259–266. [Google Scholar]
- Camacho-Cristobal J.J., Gonzalez-Fontes J. Rexach. Boron in plants: deficiency and toxicity. J. Integr. Plant Biol. 2008;50:1247–1255. doi: 10.1111/j.1744-7909.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- Carlisle E.M. Silicon: an essential element for the chick. Science. 1972;178:619–621. doi: 10.1126/science.178.4061.619. [DOI] [PubMed] [Google Scholar]
- Carlisle E.M. Vol. 121. John Wiley and Sons Ltd; Chichester: 1986. Silicon as an essential trace element in animal nutrition; pp. 123–139. (Silicon Biochemistry, Ciba Foundation Symposium). [DOI] [PubMed] [Google Scholar]
- Carlisle E.M., Berger J.W., Alpenfels W.F. A silicon requirement for prolyl hydroxylase activity. Fed. Proc. 1981;40:886. [Google Scholar]
- Chapin R.E., Ku W.W., Kenney M.A., McCoy H., Gladen B., Wine R.N., Wilson R., Elwell M.R. The effects of dietary boron on bone strength in rats. Fundam. Appl. Toxicol. 1997;35:205–215. doi: 10.1006/faat.1996.2275. [DOI] [PubMed] [Google Scholar]
- Cox L. SCI Annual Review Meeting December 2009. University of Birmingham; 2009. Recent advances in organosilicon chemistry. [Google Scholar]
- Devirian T.A., Volpe S.L. The physiological effects of dietary boron. Crit. Rev. Fd. Sci. Nutr. 2003;43:219–231. doi: 10.1080/10408690390826491. [DOI] [PubMed] [Google Scholar]
- EFSA Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the tolerable upper intake level of boron (sodium borate and boric acid). Request # EFSA-Q-2003-018. EFSA J. 2004;80:1–22. [Google Scholar]
- Eisinger J., Clairet D. Effects of silicon, fluoride, etidronate and magnesium on bone mineral density: a retrospective study. Magnes. Res. 1993;6:247–249. [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999;50:641–664. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Fauteux F., Chain F., Belzile F., Menzies J.G., Bélanger R.R. The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17554–17559. doi: 10.1073/pnas.0606330103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hench L.L., Greenspan D. Interactions between bioactive glass and collagen: a review and new perspectives. J. Aust. Ceram. Soc. 2013;49:1–40. [Google Scholar]
- Hildebrand M., Volcani B.E., Gassmann W., Schroeder J.I. A gene family of silicon transporters. Nature. 1997;385:688–689. doi: 10.1038/385688b0. [DOI] [PubMed] [Google Scholar]
- Hofman K., Hall B., Cleaver H., Marshall S. High-throughput quantification of hydroxyproline for determination of collagen. Anal. Biochem. 2011;417:289–291. doi: 10.1016/j.ab.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Hunt C.D. Dietary boron: progress in establishing essential roles in human physiology. J. Trace Elem. Med. Biol. 2012;26:157–160. doi: 10.1016/j.jtemb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Ju Z., Ding L., Zheng F., Zhang Q., Li Y. Effects of silicon, calcium or boron on cell growth and lipid accumulation in Pinnularia gibba var. Linearis. IPCBEE. 2011;22:100–104. [Google Scholar]
- Jugdaohsingh R. Silicon and bone health. J. Nutr. Health Aging. 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- Jugdaohsingh R., Tucker K.L., Qiao N., Cupples L.A., Kiel D.P., Powell J.J. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring Cohort. J. Bone Miner. Res. 2004;19:297–307. doi: 10.1359/JBMR.0301225. [DOI] [PubMed] [Google Scholar]
- Jugdaohsingh R., Kinrade S.D., Powell J.J. vol. 10. 2008. Is there a biological role for silicon? pp. 45–55. (Metal Ions in Biology and Medicine). [Google Scholar]
- Jugdaohsingh R., Calomme M.R., Robinson K. Increased longitudinal growth in rats on a silicon-depleted diet. Bone. 2008;43:596–606. doi: 10.1016/j.bone.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinrade S.D., Del Nin J.W., Schach A.S., Sloan T.A., Wilson K.L., Knight C.T.G. Stable five- and six-coordinated silicate anions in aqueous solution. Science. 1999;285:1542–1545. doi: 10.1126/science.285.5433.1542. [DOI] [PubMed] [Google Scholar]
- Kinrade S.D., Hamilton R.J., Schach A.S., Knight C.T.G. Aqueous hypervalent siliconcomplexes with aliphatic sugar acids. J. Chem. Soc. Dalton Trans. 2001:961–963. (V.) [Google Scholar]
- Kinrade S.D., Schach A.S., Hamilton R.J., Knight C.T.G. NMR evidence of pentaoxo organosilicon complexes in dilute neutral aqueous silicate solutions. J. Chem. Soc. Chem. Commun. 2001:1564–1565. doi: 10.1039/b104713m. [DOI] [PubMed] [Google Scholar]
- Kinrade S.D., Deguns E.W., Gillson A.-M.E., Knight C.T.G. Complexes of pentaoxo and hexaoxo silicon with furanoidic vicinal cis-diols in aqueous solution. Dalton Trans. 2003:3713–3716. [Google Scholar]
- Kliment C., Englert J., Crum L., Oury T.D. A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int. J. Clin. Exp. Pathol. 2011;4:349–355. [PMC free article] [PubMed] [Google Scholar]
- Landis W.J., Lee D.D., Brenna J.T., Chandra S., Morrison G.H. Detection and localization of silicon and associated elements in vertebrate bone tissue by imaging ion microscopy. Calcif. Tissue Int. 1986;38:52–59. doi: 10.1007/BF02556595. [DOI] [PubMed] [Google Scholar]
- Macdonald H.M., Hardcastle A.C., Jugdaohsingh R., Fraser W.D., Reid D.M., Powell J.J. Dietary silicon interacts with oestrogen to influence bone health: evidence from the Aberdeen Prospective Osteoporosis Screening Study. Bone. 2012;50:681–687. doi: 10.1016/j.bone.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Marque V., Kieffer P., Gayraud B., Lartaud-Idjouadiene I., Ramirez F., Atkinson J. Aortic wall mechanics and composition in a transgenic mouse model of Marfan Syndrome. Arterioscler. Thomb. Vasc. Biol. 2001;21:1184–1189. doi: 10.1161/hq0701.092136. [DOI] [PubMed] [Google Scholar]
- Nielsen F.H. Is boron nutritionally relevant? Nutr. Rev. 2008;66:183–191. doi: 10.1111/j.1753-4887.2008.00023.x. [DOI] [PubMed] [Google Scholar]
- Nielsen F., Stoecker B. Nutritional intakes of silicon affect vertebral trabecular microarchitecture and strength, but not femoral or vertebral strength changes induced by ovariectomy, in rats. FASEB J. 2004;18:A919. [Google Scholar]
- Nielsen F.H., Stoecker B.J. Boron and fish oil have different beneficial effects on strength and trabecular microarchitecture of bone. J. Trace Elem. Med. Biol. 2009;23:195–203. doi: 10.1016/j.jtemb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Price C.T., Koval K.J., Langford J.R. Silicon: a review of its potential role in the prevention and treatment of post-menopausal osteoporosis. Int. J. Endocrinol. 2013;2013:316783. doi: 10.1155/2013/316783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reffitt D.M., Ogston N., Jugdaohsingh R. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- Risteli J., Risteli L. Products of bone collagen metabolism. In: Seibel M.J., Sp Robins, Bilezikian J.P., editors. Dynamics of Bone and Cartilage Metabolism: Principles and Clinical Applications. Academic Press; 2006. pp. 391–405. [Google Scholar]
- Schwarz K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc. Natl. Acad. Sci. U. S. A. 1973;70:1608–1612. doi: 10.1073/pnas.70.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K., Milne D.B. Growth-promoting effects of silicon in rats. Nature. 1972;239:333–334. doi: 10.1038/239333a0. [DOI] [PubMed] [Google Scholar]
- Seaborn C.D., Nielsen F.H. Boron and silicon: effects on growth, plasma lipids, urinary cyclic AMP and bone and brain mineral composition of male rats. Environ. Toxicol. Chem. 1994;13:941–947. [Google Scholar]
- Seibel M.J. Molecular markers of bone metabolism in parathyroid disease. In: Bilezikian J.P., Marcus R., Levine M.A., editors. The Parathyroids: Basic and Clinical Concepts. Second Edition. Academic Press; 2001. pp. 399–408. [Google Scholar]
- Silver F.H., Trelstad R.L. Type I collagen in solution. Structure and properties of fibril fragments. J. Biol. Chem. 1980;255:9427–9433. [PubMed] [Google Scholar]
- Sripanyakorn S., Jugdaohsingh R., Dissayabutr W., Anderson S.H.C., Thompson R.P.T., Powell J.J. The comparative absorption of silicon from different foods and food supplements. Br. J. Nutr. 2009;102:825–834. doi: 10.1017/S0007114509311757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Lakehead University; 2004. Molecular Basis of Silicon Bio-Essentiality. MSc Thesis. [Google Scholar]
- West R., Barton T.J. Organosilicon chemistry Part 1. J. Chem. Educ. 1980;57:165–169. [Google Scholar]
- Zhang Z., Li G., Shi B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J. Soc. Leather Tech. Chem. 2005;90:23–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.