The loss of vision during the evolution of cave Mexican tetra considerably reduced the energetic burden of neural tissue.
Keywords: Biology, Brain Evolution, Eye Evolution, Physiological Energetics, Cave, Subterranean
Abstract
One hypothesis for the reduction of vision in cave animals, such as the eyeless Mexican cavefish, is the high energetic cost of neural tissue and low food availability in subterranean habitats. However, data on relative brain and eye mass in this species or on any measure of the energetic cost of neural tissue are not available, making it difficult to evaluate the “expensive tissue hypothesis.” We show that the eyes and optic tectum represent significant metabolic costs in the eyed phenotype. The cost of vision was calculated to be 15% of resting metabolism for a 1-g fish, decreasing to 5% in an 8.5-g fish as relative eye and brain size declined during growth. Our results demonstrate that the loss of the visual system in the cave phenotype substantially lowered the amount of energy expended on expensive neural tissue during diversification into subterranean rivers, in particular for juvenile fish.
INTRODUCTION
The high energetic cost of maintaining neural tissue is proposed to have been a strong selective pressure on the evolution of brain size. When a species increases neural tissue mass during evolution, it will likely require a concomitant increase in nutrient intake, such as in the case of hominin evolution (1). Should food availability decrease, the opposite might also be expected to happen; that is, neural tissue mass decreases over time in an effort to reduce whole-body energy expenditure (2). The effects of nutrient limitation on neural mass evolution appear to be particularly obvious in animals that have evolved on nutrient-poor islands or below ground, and vision is a sensory modality that is commonly reduced under these evolutionary conditions (3). Few studies have convincingly demonstrated an adaptive reduction in neural tissue mass due to an inherent difficulty in studying regressive traits and selective forces. One particular problem is having a measure of the energy budget of the ancestral state to understand how much energy was saved by reducing neural tissue mass.
The Mexican tetra Astyanax mexicanus is an ideal organism for studying the relationships among neural tissue mass, energy demand, and adaptation. Populations of this species diversified from surface rivers into limestone caves on several occasions and evolved into a troglomorphic phenotype with reduced eye size and optic tectum volume (a part of the midbrain that receives visual input from the retina) (4). The eyed surface ecotype of Mexican tetra can be found in rivers that supply cave systems; in addition, intermediate cave-dwelling phenotypes with varying degrees of eye reduction have been found. However, the evolutionary processes that led to the loss of the visual system are debated; one hypothesis states that active selection for regression saved energy in an environment that lacks primary production and is probably food-limited (5). Mexican tetra are particularly amenable to addressing this version of the “expensive tissue hypothesis” because the extant surface ecotype can be used as a proxy for the ancestral surface ecotype that diversified into caves (and continues to do so).
We set out to quantify the energy savings achieved by Mexican tetra with a regressed visual system by comparing organ size and brain and eye metabolic costs in different phenotypes of this species. The phenotypes included the surface ecotype, the Pachón cave ecotype, and two morphs of intermediate eye size (Fig. 1). The Pachón population is the most divergent among the cave ecotypes (6) and is eyeless as a result of the near-complete breakdown and resorption of the embryonic eye. One of the intermediate phenotypes tested were fish from the Micos cave, a phylogenetically young population with a high degree of genetic and phenotypic variability (7). The other intermediate phenotype was a Pachón/surface F2 hybrid (Fig. 1). Besides striking differences in pigmentation and the visual system, the Mexican tetra clade appears to be relatively invariant in traits typically associated with energy-saving adaptations to food-limited environments, such as reduction in body mass or metabolic suppression (8). Surface and cave ecotypes have similar sizes and growth rates (9), limited evidence for specific physiological adaptations to starvation resistance beyond differences in metabolic rate is available (10–12), the cave form is at least as active as the surface form (10, 13), and the surface form is more cannibalistic (14). The mass of the heart, gills, gonads, and digestive system might vary among phenotypes if there has been selective pressure to alter the function or size of these organs, and differences in the relative mass of these organs may play a role in energy saving during diversification underground; however, this has not been investigated. The data on organ size, organ costs, and energy budgets generated in this study are used to infer the likely evolutionary consequences of changes in brain and eye size during Mexican tetra diversification underground.
Fig. 1. A comparison of brain size and body morphology in the four Mexican tetra morphs used in this study.
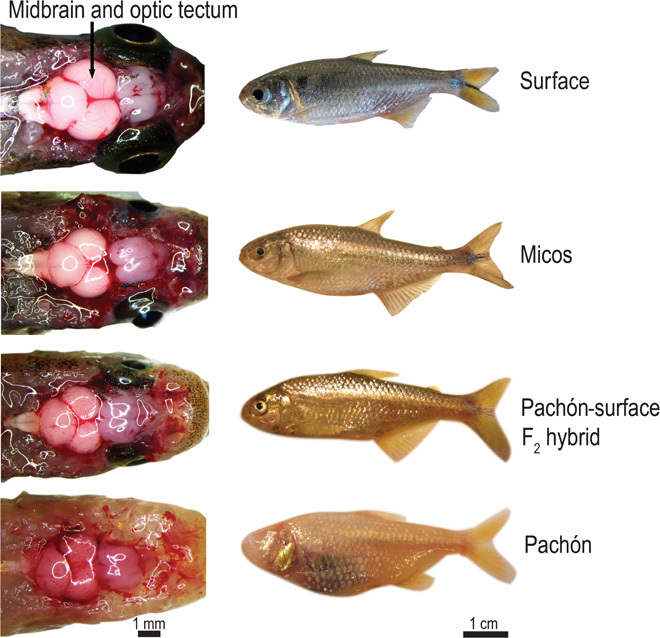
Scale bars are approximate.
RESULTS
Allometric scaling of organ mass
Organ mass measurements were conducted to evaluate whether different morphs of Mexican tetra (Micos cave, Pachón cave, surface, and Pachón/surface F2 full sib) varied in relative organ size. The organs measured included the gills, heart, digestive system, gonads, brain, and eyes; organ–body mass scaling relationships among ecotypes were evaluated using analysis of covariance (ANCOVA). For all organs measured, individual weight had a strong effect on organ mass (ANCOVA, P < 0.001) (table S1). Morph type did not have a significant effect on the heart, digestive system, and gonad mass (P > 0.05) (table S1); however, the gill mass of Pachón fish and Pachón/surface F2 hybrids was significantly different (P < 0.001) (table S1) from that of the other two morphs. The mean relative gill mass of Pachón ecotypes was 3.1% of wet body weight (range, 1.9 to 4.1%) compared to 2.0% (range, 1.1 to 2.7%) for surface ecotypes. Pachón/surface F2 hybrid gill mass was intermediate at 2.6% body weight (range, 1.7 to 3.6%).
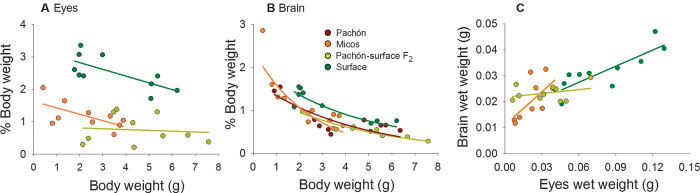
The organs that differed most markedly between ecotypes in terms of mass were the brain and eyes. Relative brain and eye mass decreased with increasing fish size (Fig. 2, A and B), although a notable exception was the largely invariant eye mass of Pachón/surface F2 hybrids (Fig. 2A). As expected, eye mass was considerably higher in surface ecotypes than in Micos and hybrid fish (about 2.5 times larger) (Fig. 2A). Brain mass in surface ecotypes was about 30% larger than in the other ecotypes (Fig. 2B), and the brain–body mass scaling relationship of surface morphs differed significantly from that of the other groups (ANCOVA, P < 0.023) (table S1). Brain and eye mass were positively correlated for surface ecotypes (R2 = 0.69, P = 0.003) and Micos ecotypes (R2 = 0.45, P = 0.033) and followed a similar scaling relationship (Fig. 2C). In contrast to surface and Micos ecotypes, the brain mass of Pachón/surface F2 hybrids was largely invariant despite a 10-fold difference in eye mass (R2 = 0.10, P = 0.37) (Fig. 2C).
Fig. 2. Brain and eye mass allometry in the four Mexican tetra morphs.
(A) Eye mass (percentage of body weight) versus total body weight. (B) Brain mass (percentage of body weight) versus total body weight. (C) Dependence of brain mass on eye mass among eyed morphs.
Eye and brain oxygen consumption rate
We measured the energy demand of the whole brain of Pachón fish and the energy demand of the whole brain and eyes of surface fish using isolated organ oxygen consumption measurements. Whole eyes and brains were dissected out from 10 Pachón and 10 surface ecotypes and placed inside individual respirometry chambers supplied with artificial cerebrospinal fluid. The cerebrospinal fluid was replaced every 10 min, allowing for repeated oxygen consumption measurements to be made over a 24-hour period. In total, we performed 1568 determinations of oxygen consumption from 18 eyes (n = 39 to 97 for a single eye) and 1736 determinations of whole-brain oxygen consumption from 10 surface ecotypes and 10 Pachón ecotypes (n = 66 to 96 for a single brain) (Fig. 3). Mass-specific brain oxygen consumption rates did not differ between ecotypes (F1,18 = 1.43, P = 0.23). The effects of light and dark exposure on eye oxygen consumption were evaluated using nested analysis of variance (light-dark nested in individual organs). The oxygen consumption of eyes was significantly higher (F1,18 = 4.64, P = 0.000) in the dark (mean ± SD, 0.542 ± 0.405 mg O2 hour−1 g wet mass−1) than in the light (mean ± SD, 0.507 ± 0.260 mg O2 hour−1 g wet mass−1); however, the effect size of this variable was small (∂η2 = 0.052) and considerably less than the effect size of the variation between individual eyes (∂η2 = 0.304). Therefore, we decided to ignore light as a variable for modeling purposes, and a single mean oxygen consumption rate was calculated for all eye data (mean ± SD, 0.525 ± 0.342 mg O2 hour−1 g wet mass−1). The mean whole-eye (sclera plus retina minus lens and vitreous) respiration rate (0.525 mg O2 hour−1 g wet tissue−1) was broadly similar to the only other metabolic rate measurement made of a fish retina [retinal tissue of the rainbow trout Oncorhynchus mykiss, 1.498 mg O2 hour−1 g wet tissue−1 (15)], taking into account that an isolated retina contains considerably more metabolically active tissue compared to a retina mounted on a sclera [the latter is largely composed of connective tissue (16) with minimal metabolic activity]. Mass-specific brain oxygen consumption rates did not differ between surface ecotypes and Pachón ecotypes (F1,18 = 1.43, P = 0.232), and the mean brain respiration rate for all experiments (1.603 mg O2 hour−1 g wet tissue−1) fell in the range reported for other fish [0.72 to 2.02 mg O2 hour−1 g wet tissue−1 (17, 18)].
Fig. 3. Summary graphs of respirometry data showing the oxygen consumption rates of eyes and brains in surface and Pachón ecotypes at different times of day.
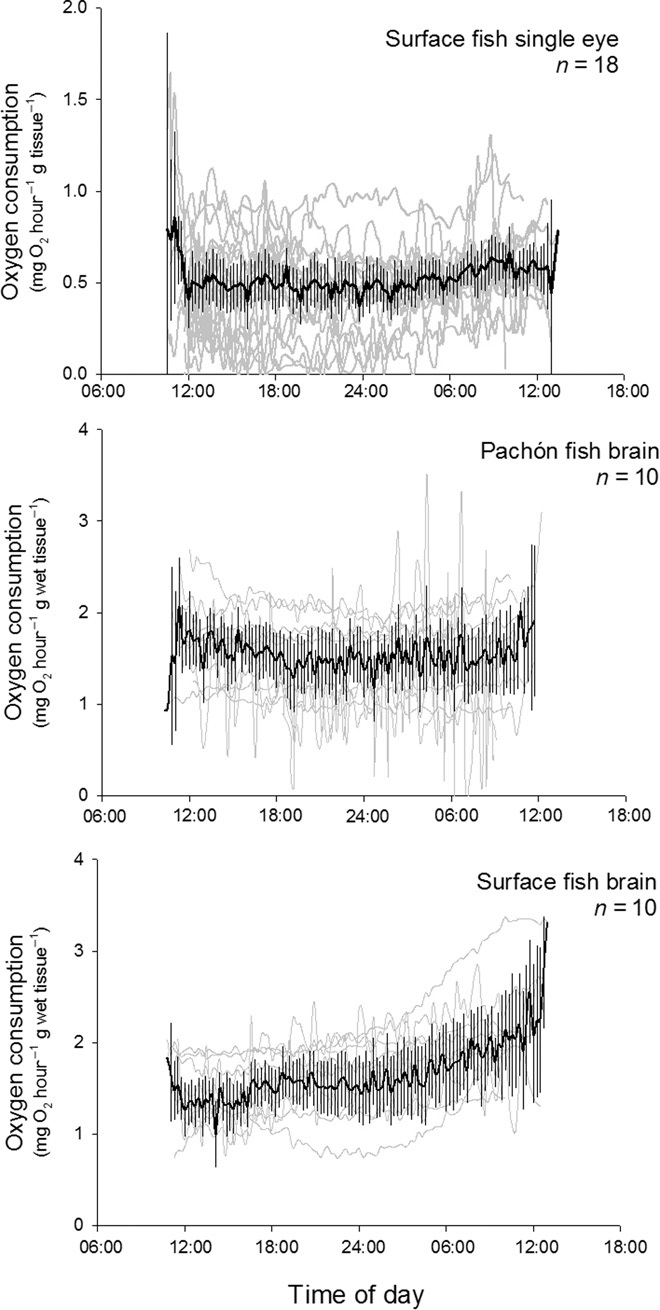
Each gray line represents a single organ measurement. A running mean ± 95% confidence interval is overlaid.
Modeling of neural tissue energetic cost
The oxygen consumption of neural tissue was compared to whole-body oxygen consumption to derive a model of relative neural tissue costs for surface, Micos, and Pachón ecotypes. These costs were modeled for fish body weight ranging from 1 to 8.5 g, the mass range for which relative organ weights were measured and whole-body oxygen consumption data exist (11, 19). Previous research has reported that surface ecotypes have a 19% higher whole-body minimal metabolic rate than Pachón ecotypes (19), whereas Micos ecotypes have an intermediate rate (11) (Fig. 4A). The model predicted that the energetic cost of the whole brain for a 1-g surface fish was 15% of resting metabolism, declining to 5% as relative brain size decreased with body mass (Fig. 4, B and C). Whole-brain energetic costs for Micos and Pachón ecotypes were lower than that for surface ecotypes (reflecting the smaller relative brain size) and decreased from 10 to 6% over the modeled body weight range (Fig. 4C). The energetic cost of eyes in a 1-g surface fish was 8% of resting metabolism, decreasing to 5% in an 8.5-g surface fish (Fig. 4E). The energetic cost of eyes in Micos fish was about 3% over the same mass range (Fig. 4E). The predicted whole-brain energetic costs for larger fish (that is, higher than about 3 g) of the Mexican tetra ecotypes fall in the general range for vertebrates [2 to 8% of resting metabolism (20)]. The cost of neural tissue (that is, eyes plus brain) for a 1-g surface ecotype represented 23% of resting metabolism, whereas for Micos and Pachón ecotypes, the cost was 13 and 10%, respectively (Fig. 4F).
Fig. 4. Output from a model used to calculate the relative energetic costs of eyes, brain, and vision for three Mexican tetra ecotypes.
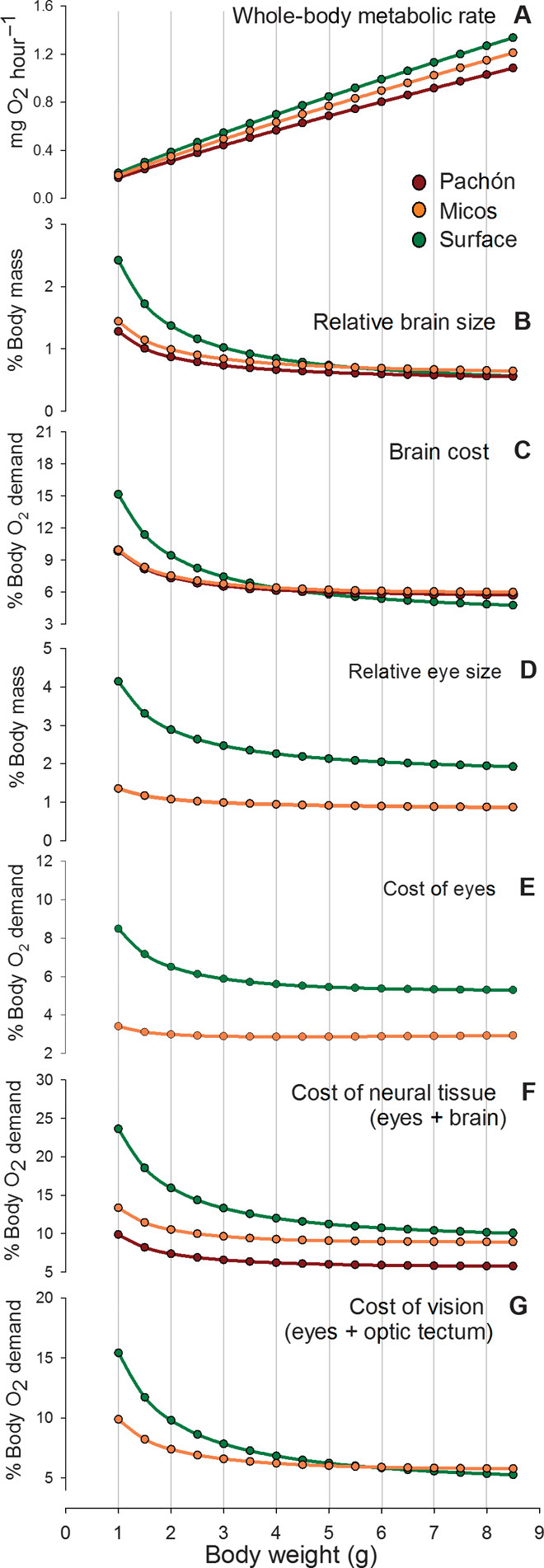
(A) Whole body oxygen consumption (B) relative brain size (C) brain oxygen consumption (D) relative eye size (E) oxygen consumption of eyes (F) oxygen consumption of eyes plus brain (G) oxygen consumption of eyes plus optic tectum. Refer to the Supplementary Materials for the calculative approach.
The whole-body metabolic rates for surface, Micos, and Pachón fish (ecotypes for which these data are available) correlated with neural tissue costs for any given body size (R2 >0.940), suggesting that the reduction and loss of the visual system were major factors contributing to the lowering of maintenance energetic costs in Mexican tetra. By subtracting the total neural tissue cost of Pachón ecotypes from that of surface and Micos ecotypes, it was possible to derive an estimate of the energetic cost of vision for the eyed ecotypes [given that the main neural mass differences between the ecotypes are eye size and optic tectum volume (4, 21)]. The cost of vision for a 1-g surface fish was calculated to be equivalent to 15% of resting metabolism, decreasing to about 5% for surface fish heavier than 6 g (Fig. 4G). For Micos ecotypes weighing 1 to 8.5 g, the cost of vision varied from 4 to 3% of resting metabolism (Fig. 4G).
DISCUSSION
The lack of differentiation among ecotypes in the mass scaling of the heart, digestive system, and gonads suggests that these organs were not under strong selective pressure to change during Mexican tetra diversification underground. The gills were significantly larger in Pachón and Pachón/surface F2 hybrid fish compared to surface and Micos fish, suggesting that the Pachón ecotype may have been under selective pressure to evolve larger respiratory exchange surfaces to cope with hypoxic periods that can occur in caves (22). The observed difference in relative eye and whole-brain size among ecotypes was to be expected, and the brain mass differences given in the present study are in reasonable agreement with a separate study that used high-resolution micro–computed tomography scanning to measure volumetric brain differences in surface and Pachón ecotypes (21). Adult surface ecotypes were reported to have 22% larger brains by volume compared to Pachón ecotypes when the fish were 1 year old (21), and the present study observed a 30% difference in the relative brain mass of adult fish of varying sizes. Rodrigues (21) also reported that the brain region accounting for much of the difference between the ecotypes was the optic tectum, in agreement with an earlier comparative study of brain cross-sectional areas (4).
The strong correlation between eye and brain mass observed for surface and Micos ecotypes reflects the close coupling of retinal size and visual processing requirements. This notion is supported by an earlier study comparing brain region volumes in the two phenotypes, as the midbrain (which receives input from the optic nerve) in Micos fish is 50% smaller than that in surface fish, whereas the forebrain and hindbrain are virtually identical in volume (4). In contrast to surface and Micos ecotypes, the whole-brain mass of Pachón/surface F2 hybrids was largely invariant despite a 10-fold difference in eye mass. The same trend was also reported in a previous study of the brain dimensions and eye diameter of this hybrid (23), indicating a significantly altered eye and brain developmental pathway compared to other reduced-eye phenotypes that developed regressed vision by means of an incremental evolutionary process. This observation has implications for studies that use hybrids to investigate questions about the evolutionary value of varying eye size (24). A weak coupling between the retina and optic tectum volume indicates that the visual capability of hybrids cannot be assumed to be the same as that of nonhybrid conspecifics with similar eye sizes, and that varying eye size may not be an honest signal of the energetic burden of vision (which incorporates both eye size and optic tectum volume).
The substantial energetic cost of the visual system in eyed Mexican tetra reported in this study illustrates the value and importance of this sense in a lit environment. The cost of the visual system as a fraction of minimal metabolism in juvenile surface fish (15%) approaches the cost of the human brain (about 20 to 25%) (20). Vision is therefore likely to be under strong selective pressure to regress in caves, where light is absent and food is limited. The pressure for Mexican tetra to save energy and to eliminate the visual system during diversification into caves is predicted to have been strongest during the early life stages when eyes and brains are proportionately very large, energy reserves are limited, and nutriment is required to sustain the high growth rates typical of larval and juvenile fish (25). The energy-saving advantages of a regressed visual system for cave ecotypes are established before first feeding. The degeneration of the visual system in Pachón ecotypes occurs early in development, with the lens undergoing apoptosis during embryogenesis and the growth of the neural retina arrested soon thereafter (26). Rodrigues (21) reported that, at the onset of feeding, Pachón ecotype larvae have a 50% reduced optic tectum volume compared to surface conspecifics, a difference that is maintained throughout development. The results from the present study suggest that the decreased optic tectum volume lowers the brain energy demand of Pachón ecotypes by about 30% compared to surface ecotypes.
The finding that the visual system of surface Mexican tetra is fairly expensive does not, in itself, help resolve the debate over the proximate or ultimate mechanisms by which eye regression evolved in cave ecotypes (27–29), as the energetic benefits of reducing neural mass occur irrespective of the regressive mechanism. It is also important to emphasize that the energetic burden of the visual system needs to be measured against the degree of food limitation experienced by fish in the wild to establish whether energy intake is a selective pressure driving visual regression in this species. Although cave ecosystems tend to be nutrient-limited because of a lack of primary production (30), some of the caves inhabited by Mexican tetra have a food source in the form of bat guano (31); thus, the idea that energy intake acts as a selective pressure on this species and is a driver of visual regression remains a hypothesis. In addition, the results of the current study should be considered alongside results that have demonstrated that the programmed degradation of the visual system in larval cave ecotypes is intimately coupled with the development of constructive traits for subterranean life, such as jaw shape changes and increases in taste buds and mechanosensory cells (32, 33). A recent study has also reported that the abiotic conditions in caves can induce a heat shock protein–related stress response that unmasks standing genetic variation in eye size among surface ecotypes (34), demonstrating that many factors are involved in the evolution of eyelessness in Mexican tetra.
Eye size in surface Mexican tetra is similar to that in other species with equivalent body weight (35), and it is likely that all eyed fish with small body mass expend a considerable portion of maintenance energetic costs on neural tissue and vision. The strong coupling between the energetic burden of the visual system and the dependence of juvenile fish survival on the visual system for prey capture and predator avoidance is likely to have resulted in significant selective pressure to energetically optimize vision during basal fish (and more generally vertebrate) evolution. Conversely, in habitats where food is limited and vision is unnecessary for feeding or evasion, our study suggests that selection will strongly favor individuals with a reduced visual system to reduce overall energy expenditure, especially for animals with relatively large eye and brain size.
MATERIALS AND METHODS
Experimental design
The objective of this study was to derive the energetic cost of vision of eyed and eyeless Mexican cavefish (A. mexicanus). Whole-body metabolic rate data from previous studies were available. The strategy for measuring the energy expenditure of eyes was to use isolated organ oxygen consumption measurement, as initial attempts using implanted blood flow and oxygen probes in the vasculature around the eye and brain failed because of the small size of blood vessels. Mass-specific brain and eye oxygen consumption measurements were coupled with brain and eye mass allometry data to model the expected energetic costs of these organs compared to whole-body energy expenditure.
Fish stock and husbandry
All experiments were performed at Lund University (Lund, Sweden). Before experimentation, animal ethics approval was obtained from the relevant local Swedish authorities. Pachón, pure surface (Rió Tampaón stock), and Pachón/surface F2 full-sib fish were obtained from the Borowsky Laboratory at New York University. Additional Pachón stock was obtained from A. Ipsen and F. Gloza-Rausch at Noctalis Museum (Bad Segeberg, Germany). Micos fish were obtained from H. Wilkens at the Zoologisches Institut und Zoologisches Museum, University of Hamburg. The fish were between 1 and 3 years old at testing, a mixture of the sexes, and maintained in 100-liter tanks at 20 ± 0.5°C. Fluorescent room lighting provided a 12-hour light/12-hour dark cycle with 30-min artificial sunrise and sunset. Fish were fed a combination of commercially available dry flake diet, frozen mosquito larvae, and liver paste daily.
Organ mass
Organ mass measurements were conducted to evaluate whether different morphs of Mexican tetra varied in relative organ size. Fish were not fed for 3 days before organ mass measurements to ensure that all digesta had been evacuated. Ten fish of each type (Pachón cave, Micos cave, surface, and Pachón-surface hybrid) were euthanized using MS-222 (5 min at 100 mg liter−1 and 5 min at 200 mg liter−1). Body length and blotted wet mass were recorded, and the eyes removed from the orbit using curved scissors. The eyes (and other organs) were blotted dry using low-lint tissue paper and weighed to the nearest 0.1 mg. The fish was placed on its side in a dissecting tray and immersed in saline. Next, the brain was exposed through removal of the dorsal skull area, the cranial nerves were severed, and the brain was carefully excised. The brain was irrigated with saline to remove as much clotted blood as possible and transferred to a scale using a plastic Pasteur pipette. The gills were obtained by removing the opercula and cutting individual gill bars at the dorsal and ventral attachment points. The viscera were exposed through a ventral incision from the anus to the pericardium and through another incision from the anus to the dorsal corner of the operculum. After a cut at the esophagus and urogenital pore, the digestive system and gonads were removed, separated, and weighed. The pericardium was carefully exposed, and the heart was removed through distal cuts to the bulbus arteriosus and sinus venosus. All organs were dried in a dessicator for dry weight measurement. Linear regression was used to describe the relationship between body mass and organ mass for each ecotype (results are presented in table S2). Differences in organ mass among ecotypes were investigated with ANCOVA in Statistica (version 8.0, StatSoft Inc.) using whole-body weight as a covariate. Tukey’s least significant difference test was used to evaluate significant differences between ecotypes.
Respirometry apparatus
The metabolic rates for the isolated eyes and brains of Pachón and surface Mexican tetra were quantified using in vitro whole-organ oxygen consumption measurement. This approach, rather than in situ measurement of organ vasculature blood flow and blood oxygen content, was taken because pilot testing indicated that blood flow probes were too large for a 5-g fish. We used whole-organ measurements rather than tissue slices because the small sizes of Mexican tetra brain and eyes meant that diffusion of oxygen and nutrients to the tissues was unlikely to be a significant limiting factor in a respirometry setup. Pilot testing indicated that proper mixing of artificial cerebrospinal fluid (ACSF) around the organs was very important in obtaining linear oxygen decreases and in maintaining tissue respiratory capacity over 24 to 72 hours. High rates of ACSF mixing in respirometry vials in turn required the tissue to be well attached and supported, hence the tissue support methodology described later. The ACSF used in the present study was based on the composition used by Zhang et al. (36) for studying brain slices in freshwater fish. The composition was as follows: 128.1 mM NaCl2, 2.5 mM KCl, 1.8 mM CaCl2, 22.2 mM NaHCO3, 1.0 mM MgCl2, 10.0 mM Hepes, and 10.0 mM glucose, and pH was adjusted to 7.4 using HCl.
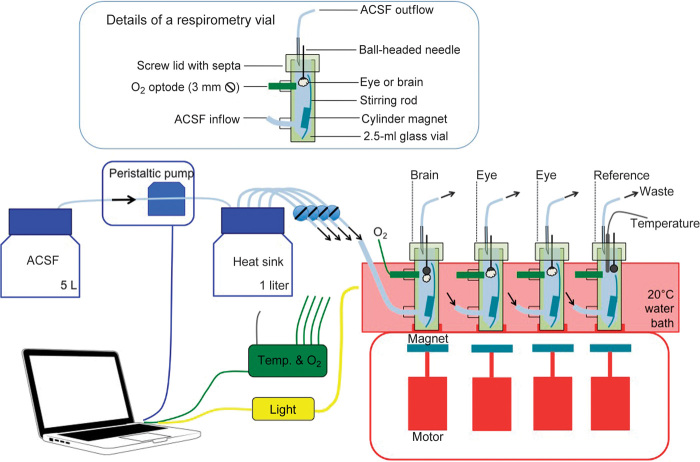
The brain and eye metabolic measurement system was an automated intermittent-flow respirometer (Fig. 5) located in a temperature-controlled (20°C) dark room. Individual whole organs were placed inside respirometry vials, which were modified 3-ml clear-glass microreaction vessels with polytetrafluoroethylene-faced rubber septa and cap. Four respirometry vials were used in the system: one for the brain, two for the eyes, and one as a reference vial. The glass vials had two holes drilled into them to allow for ACSF inflow and an oxygen optode. The brain or eye was attached to a spherical pinhead using cyanoacrylate glue, and the organ was suspended at the center of respirometry vials by puncturing the pin through the septa. An effluent line was inserted into the septa to drain ACSF away during the flushing phase. A peristaltic pump pressurized the ACSF delivery system and replaced the volume of the respirometer in about 1 min. The peristaltic pump rollers noticeably increased the temperature (1° to 2°C) of the ACSF as it passed through the pump; therefore, it was necessary to have a 1-liter bottle between the peristaltic pump and the respirometry vials to act as a heat sink. The respirometry vials were semi-immersed in a water bath, which smoothed out the heat fluctuations associated with the temperature control of the room. The water bath was fixed atop a custom-built magnetic stirring unit in which the motors had a rotational velocity of about 60 rpm. The magnetic stirring bars inside the respirometers were custom-designed to give a high degree of mixing throughout the vial volume at low rotation. The triangular magnetic stirring bars sold as standard accessory for conical base microreaction vessels did not provide sufficient mixing at low revolution. At high revolution, the shear forces were too great for the organs that they rapidly disintegrated.
Fig. 5. A schematic drawing of the respirometry apparatus used to measure the oxygen consumption rates of eyes and brains.
The oxygen saturation in each respirometry vial was measured and recorded with a temperature-compensated oxygen optode system (optically isolated OXROB3 optodes coupled to a FireStingO2 meter, Pyro Science GmbH). The optodes were programmed to make a measurement every 10 s. The peristaltic pump was programmed to a repeat a cycle of 5 min on/10 min off, and the oxygen consumption rate was quantified during the 10-min no-pumping period. A wide-spectrum 35-W metal halide lamp (Olympus ILH-1, Olympus Optical Co.) shone light onto respirometry vials to test the effects of light on oxygen consumption. Care was taken not to direct the light beam straight to the oxygen optodes because although the optodes were optically isolated, a line-of-sight light path was observed to affect the fluorescence signal. The lamp was programmed to repeat a cycle of 1 hour on/1 hour off (1 hour was equivalent to four cycles of oxygen consumption measurement).
Measurements of brain and eye oxygen consumption rates
Fish were not fed 2 days before experimentation to ensure gut clearance and representative body mass measurements. The respirometry equipment was disinfected by flushing through sodium hypochlorite (3% in water) followed by distilled water and ACSF. The respirometry vials were also washed with detergent before disinfection. Before every experiment, the peristaltic pump tubing was replaced to eliminate problems with tube fatigue, and fresh ACSF was bubbled with 1 μm-filtered air for 2 hours to ensure full oxygen saturation. A fish to be used for experimentation was collected from the tank at around 0900 and anesthesized in an aerated MS-222 bath (100 mg liter−1) for 5 min before being euthanized in an ice-cold bath containing MS-222 (200 mg liter−1). After measurement of standard length and total blotted body weight, the fish was momentarily submersed in an ethanol (70%) bath for disinfection, washed in distilled water, and transferred to a dissection tray containing chilled ACSF. The eyes of surface fish were enucleated using 10-cm curved microdissecting scissors, weighed to the nearest 0.1 mg, and attached to the ball head of a pin on the medial surface close to the optic nerve using cyanoacrylate glue (Fig. 6). The needle end of the pin was held in one hand while the other hand made a single puncture into the eye around where the cornea and sclera join. One blade of the microdissecting scissors was inserted into the puncture and made a cut into the center of the cornea. A further three cuts divided the eye into three attached sections, which were held at the cornea and glued onto the ball head of the pin so that the eye was everted (Fig. 6). The lens was carefully removed with 80-mm microscissors, and the everted eye was irrigated with cold ACSF. The pin was inserted into a wax-bottomed container, and the eye was immersed in ice-cold ACSF. This process was repeated for the other eye.
Fig. 6. Details of the method used to attach an enucleated eye to a pinhead.
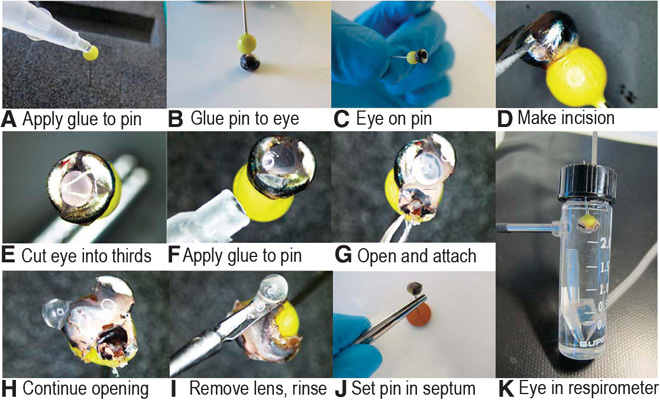
(A) Applying glue to pin (B and C) attaching pin to eye (D) using microscissors to puncture and cut through sclera and retina (E) making two more cuts to have eye in three segments (F) applying glue to pin (G and H) attaching sclera to pin (I) removing lens and irrigating (J) setting pin in septum (K) setting septum in respirometry vial.
The brain was exposed through a series of scissor cuts to the cranial case (Fig. 7). After the cuts, the fish was submersed in ice-cold ACSF, and the cranial case was prized open. The cranial nerves were cut using 80-mm microscissors, and the spinal cord was severed from the medulla. The brain was carefully excised from the cranium through irrigation and fine probing and was drawn into a plastic Pasteur pipette with the tip cut to accommodate the brain dimensions. Next, the brain was gently expelled onto a weighing dish, excess ACSF was drawn away using low-lint tissue paper, and the brain was weighed to the nearest 0.1 mg. The dorsal side of the brain was glued to the ball head of a pin using cyanoacrylate glue (Fig. 7) and immersed in ACSF. The dissection process took 20 to 30 min. The pins holding the brain and eyes were transferred to respirometry vials, and oxygen consumption measurements were taken for about 24 hours (equivalent to 96 metabolic rate determinations per piece of tissue). A pin without any tissue was also inserted into the reference vial. This process was performed on 10 Pachón fish and 10 surface Mexican tetra. The retina detached from the sclera during oxygen consumption measurement for the two eyes; thus, data from these experiments were discarded (total n = 18 eyes). The oxygen consumption rate was determined by calculating the rate of oxygen decrease during the 10-min closed respirometry cycle (an example of a respirometry trace is given in fig. S1). Oxygen mass in respirometry vials was calculated using the ACSF volume (2.94 ml) and solubility constants given by Graham (37). The linearity (coefficient of determination R2) of oxygen decrease in tissue respirometry vials was >0.95, and oxygen saturation remained higher than 85%. Changes in oxygen saturation in the reference vial during the closed respirometry phase were low and variable (R2 < 0.3), indicating that microbial respiration was negligible. Tissue oxygen consumption rates (mg O2 hour−1) were standardized to mass (mg O2 hour−1 g wet mass−1) to account for organ size.
Fig. 7. Details of the method used to dissect and attach the brain to a pinhead.
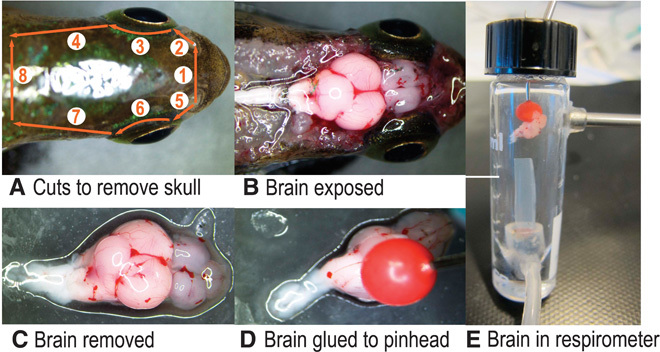
(A) Series of cuts to remove dorsal skull (B) exposed brain (C) excised brain (D) attaching brain to pre-glued pinhead (E) brain set in respirometer.
Modeling the energetic cost of neural tissue
To derive estimated costs of the brain and eyes in three Mexican tetra ecotypes (Pachón cave, surface, and Micos cave), we created a model that incorporated data on relative brain and eye mass, brain and eye oxygen consumption rates, and whole-body oxygen consumption. The model was bound in the body mass range of 1 to 8.5 g, the mass range for which relative organ weights were measured and whole-body oxygen consumption data exist (11, 19). The general approach was to (i) quantify eye and brain size in 0.5-g increments of body weight using the organ–body mass scaling relationships described in this study (table S2); (ii) calculate the expected oxygen consumption rate of those organs using data from isolated organ respirometry experiments; and (iii) compare the predicted oxygen consumption rate of those organs against the whole-body oxygen consumption rate to derive the relative energetic costs of eyes, brains, neural tissue, and vision. The calculations used for each part of the model are given in Table 1.
Table 1. Sample calculations used for modeling eye and brain costs for a 4-g fish.
| Pachón | Micos | Surface | Data origin | ||
| a | Whole-body minimal metabolic rate (mg O2 hour−1 per 5-g fish) |
0.8328* | 0.929† | 1.026* | Published data |
| b | Whole-body oxygen demand (mg O2 hour−1) | 0.686‡ | 0.766‡ | 0.846‡ | a(4 g ÷ 5 g)0.862 |
| c | Brain mass (g) | 0.027 | 0.031 | 0.034 | 4 g × organ mass scaling relationships in table S2 |
| d | Relative brain mass (% body weight) | 0.68 | 0.78 | 0.85 | c ÷ 4 g |
| e | Brain metabolic rate (mg O2 hour−1 g wet mass−1) | 1.603 | 1.603 | 1.603 | Data from the current study |
| f | Brain oxygen demand (mg O2 hour−1) | 0.043 | 0.050 | 0.055 | e × c |
| g | Relative brain cost (% body O2 demand) | 6.27 | 6.52 | 6.50 | f ÷ b |
| h | Eye mass (g) | 0 | 0.038 | 0.090 | 4 g × organ mass scaling relationships in table S2 |
| i | Relative eye mass (% body weight) | 0 | 0.95 | 2.25 | h ÷ 4 g |
| j | Eye metabolic rate (mg O2 hour−1 g wet mass−1) | 0 | 0.525 | 0.525 | Data from the current study |
| k | Eye oxygen demand (mg O2 hour−1) | 0 | 0.020 | 0.047 | j × h |
| l | Relative eye cost (% body O2 demand) | 0 | 2.61 | 5.56 | k ÷ b |
| m | Neural tissue (eyes plus brain) oxygen demand (mg O2 hour−1) |
0.043 | 0.070 | 0.102 | f + k |
| n | Neural tissue cost (% body O2 demand) | 6.27 | 9.14 | 12.06 | m ÷ b |
| o | Cost of vision (% body O2 demand) | 0 | 3.52 | 6.97 | (mMicos − mPachón) ÷ bMicos |
| (msurface − mPachón) ÷ bsurface |
The whole-body oxygen consumption data for Pachón and surface fish were taken from a recent study of ours that used intermittent-flow respirometry to measure the oxygen consumption rate of individual fish over a week under controlled exercise conditions (19). Data on Micos fish were derived from an earlier study that compared the three ecotypes (11) and reported that the routine oxygen consumption rate of Micos fish was equidistant between that of Pachón fish and that of surface fish. The data on minimal oxygen consumption rates from our study were more accurate than those from an earlier study, as our results were based on a larger data set and we used a more robust statistical method to calculate the minimal metabolic rate (lowest decile method instead of lowest observed value) (38). To ensure that the whole-body metabolic rate data were comparable among ecotypes, we modeled the Micos fish minimal oxygen consumption as equidistant between that of Pachón fish and that of surface fish.
The mass-specific brain oxygen consumption rate used in the model was the grand mean value determined from the isolated organ respirometry (1.603 mg O2 hour−1 g wet tissue−1). As the mass-specific brain oxygen consumption rate was not found to vary between Pachón and surface ecotypes, we assumed that Micos fish would have a comparable rate. The mass-specific oxygen consumption of the eyes of surface fish (0.525 mg O2 hour−1 g wet tissue−1) was used to estimate the energetic cost of eyes in Micos fish, again assuming that the per-gram cost of retinal neural tissue is equivalent and that the main difference between ecotypes is relative organ size. The cost of neural tissue was modeled by summing the oxygen consumption rate of eyes and brains (while acknowledging that this figure is an underestimate because it ignores neural tissue distributed in tissues outside of the brain and eyes). The cost of vision for surface and Micos ecotypes was calculated as the oxygen consumption rate of neural tissue minus the brain oxygen consumption rate of an equivalently sized Pachón fish. The rationale for doing this was that a number of studies have demonstrated that the main difference in brain regions among Mexican tetra ecotypes is the size of the optic tectum (4, 21, 23, 39).
Acknowledgments
We thank R. Borowsky, H. Wilkens, F. Gloza-Rausch, and A. Ipsen for supplying the fish used in the study. Funding: D.M. was supported by a Marie Curie Fellowship from the European Research Council (PIEF-GA-2009-251874). R.S. was supported by an Erasmus Work Placement grant. E.J.W. acknowledges the ongoing support of the Swedish Research Council, the Royal Physiographic Society of Lund, and the Knut and Alice Wallenberg Foundation. Author contributions: D.M. and E.J.W. conceptualized the research question and obtained project funding. D.M. and R.S. developed the experimental techniques and collected the data. All authors analyzed the data and wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The oxygen consumption and organ mass data presented in this study are included as Supplementary Materials.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/8/e1500363/DC1
Fig. S1. Sample respirometry data for a surface fish.
Table S1. ANCOVA statistics for organ weight versus body weight regression for four morphs of A. mexicanus.
Table S2. Linear regression statistics for organ weight versus body weight for four morphs of A. mexicanus.
Additional data (separate file)
Data file S1. Organ_weight_data.csv
Data file S2. Eye_respiration_data.csv
Data file S3. Brain_respiration_data.csv
REFERENCES AND NOTES
- 1.Leonard W. R., Snodgrass J. J., Robertson M. L., Effects of brain evolution on human nutrition and metabolism. Annu. Rev. Nutr. 27, 311–327 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Köhler M., Moyà-Solà S., Reduction of brain and sense organs in the fossil insular bovid Myotragus. Brain Behav. Evol. 63, 125–140 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Niven J. E., Laughlin S. B., Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Peters N., Schacht V., Schmidt W., Wilkens H., Gehirnproportionen und Ausprägungsgrad der Sinnesorgane von Astyanax mexicanus (Pisces, Characinidae): Ein Vergleich zwischen dem Flussfisch und seinen Höhlenderivaten «Anoptichthys». Z. Zool. Syst. Evol. 31, 144–159 (1993). [Google Scholar]
- 5.Jeffery W. R., Adaptive evolution of eye degeneration in the Mexican blind cavefish. J. Hered. 96, 185–196 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Bradic M., Teotónio H., Borowsky R. L., The population genomics of repeated evolution in the blind cavefish Astyanax mexicanus. Mol. Biol. Evol. 30, 2383–2400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkens H., Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces). Evol. Biol. 23, 271–367 (1988). [Google Scholar]
- 8.Passow C. N., Greenway R., Arias-Rodriguez L., Jeyasingh P. D., Tobler M., Reduction of energetic demands through modification of body size and routine metabolic rates in extremophile fish. Physiol. Biochem. Zool. 88, 371–383 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Gallo N. D., Jeffery W. R., Evolution of space dependent growth in the teleost Astyanax mexicanus. PLOS One 7, e41443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salin K., Voituron Y., Mourin J., Hervant F., Cave colonization without fasting capacities: An example with the fish Astyanax fasciatus mexicanus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 156, 451–457 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Hüppop K., Oxygen consumption of Astyanax fasciatus (Characidae, Pisces): A comparison of epigean and hypogean populations. Environ. Biol. Fishes 17, 299–308 (1986). [Google Scholar]
- 12.Protas M., Tabansky I., Conrad M., Gross J. B., Vidal O., Tabin C. J., Borowsky R., Multi-trait evolution in a cave fish, Astyanax mexicanus. Evol. Dev. 10, 196–209 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Duboué E. R., Keene A. C., Borowsky R. L., Evolutionary convergence on sleep loss in cavefish populations. Curr. Biol. 21, 671–676 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Borowsky R., Breeding Astyanax mexicanus through natural spawning. Cold Spring Harb. Protoc. 2008, pdb.prot5091 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Waser W. P., Heisler N., Oxygen delivery to the fish eye: Blood flow in the pseudobrachial artery of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 30, 77–85 (2004). [Google Scholar]
- 16.Y. W. Kunz, Developmental biology of teleost fishes, in Fish and Fisheries Series (Springer, Dordrecht, the Netherlands, 2004). [Google Scholar]
- 17.Johansson D., Nilsson G., Törnblom E., Effects of anoxia on energy metabolism in crucian carp brain slices studied with microcalorimetry. J. Exp. Biol. 198, 853–859 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Nilsson G., Brain and body oxygen requirements of Gnathonemus petersii, a fish with an exceptionally large brain. J. Exp. Biol. 199, 603–607 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Moran D., Softley R., Warrant E. J., Eyeless Mexican cavefish save energy by eliminating circadian rhythm in metabolism. PLOS One 9, e107877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mink J. W., Blumenschine R. J., Adams D. B., Ratio of central nervous system to body metabolism in vertebrates: Its constancy and functional basis. Am. J. Physiol. 241, R203–R212 (1981). [DOI] [PubMed] [Google Scholar]
- 21.F. R. Rodrigues, Master’s thesis, Universidade de Lisboa, Spain (2013). [Google Scholar]
- 22.F. Hervant, F. Malard, in Encyclopedia of Caves, D. C. Culver, W. B. White, Eds. (Academic Press, Oxford, UK, 2012), pp. 651–658. [Google Scholar]
- 23.Pfeiffer W., Die korrelation von Augengröße und Mittelhirngröße bei Hybriden aus Astyanax × Anoptichthys (Characidae, Pisces). Wilhelm Roux’ Arch. Entwicklungsmech. Organ. 159, 365–378 (1967). [DOI] [PubMed] [Google Scholar]
- 24.Espinasa L., Bibliowicz J., Jeffery W., Rétaux S., Enhanced prey capture skills in Astyanax cavefish larvae are independent from eye loss. EvoDevo 5, 35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houde E. D., Fish early life dynamics and recruitment variability. Am. Fish. Soc. Symp. 2, 17–29 (1987). [Google Scholar]
- 26.Jeffery W. R., Martasian D. P., Evolution of eye regression in the cavefish Astyanax: Apoptosis and the Pax-6 gene. Amer. Zool. 38, 685–696 (1998). [Google Scholar]
- 27.Jeffery W. R., Pleiotropy and eye degeneration in cavefish. Heredity 105, 495–496 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Wilkens H., Variability and loss of functionless traits in cave animals. Reply to Jeffery (2010). Heredity 106, 707–708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkens H., Genes, modules and the evolution of cave fish. Heredity 105, 413–422 (2010). [DOI] [PubMed] [Google Scholar]
- 30.K. Hüppop, in Encyclopedia of Caves, D. C. Culver, W. B. White, Eds. (Academic Press, Oxford, UK, 2012). [Google Scholar]
- 31.R. W. Mitchell, W. H. Russell, W. R. Elliott, Mexican eyeless Characin fishes, genus Astyanax: Environment, distribution, and evolution (Special Publications The Museum, Texas Tech University, Texas, 1977), pp. 1–89. [Google Scholar]
- 32.Yamamoto Y., Byerly M. S., Jackman W. R., Jeffery W. R., Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol. 330, 200–211 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshizawa M., Yamamoto Y., O’Quin K. E., Jeffery W. R., Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC Biol. 10, 108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohner N., Jarosz D. F., Kowalko J. E., Yoshizawa M., Jeffery W. R., Borowsky R. L., Lindquist S., Tabin C. J., Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342, 1372–1375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howland H. C., Merola S., Basarab J. R., The allometry and scaling of the size of vertebrate eyes. Vision Res. 44, 2043–2065 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Shi Z., Magnus G., Meek J., Han V. Z., Qiao J. T., Functional circuitry of a unique cerebellar specialization: The valvula cerebelli of a mormyrid fish. Neuroscience 182, 11–31 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Graham M., The solubility of oxygen in physiological salines. Fish Physiol. Biochem. 4, 1–4 (1987). [DOI] [PubMed] [Google Scholar]
- 38.Herrmann J. P., Enders E. C., Effect of body size on the standard metabolism of horse mackerel. J. Fish Biol. 57, 746–760 (2000). [Google Scholar]
- 39.Strickler A. G., Soares D., Comparative genetics of the central nervous system in epigean and hypogean Astyanax mexicanus. Genetica 139, 383–391 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/8/e1500363/DC1
Fig. S1. Sample respirometry data for a surface fish.
Table S1. ANCOVA statistics for organ weight versus body weight regression for four morphs of A. mexicanus.
Table S2. Linear regression statistics for organ weight versus body weight for four morphs of A. mexicanus.
Additional data (separate file)
Data file S1. Organ_weight_data.csv
Data file S2. Eye_respiration_data.csv
Data file S3. Brain_respiration_data.csv