The unusually heavy Cl of the Moon is related not to degassing of dry magmas but rather to the loss of Cl from the lunar magma ocean.
Keywords: Moon, Lunar, volatiles, apatite, basalt, chlorine, water, magma ocean
Abstract
The Moon contains chlorine that is isotopically unlike that of any other body yet studied in the Solar System, an observation that has been interpreted to support traditional models of the formation of a nominally hydrogen-free (“dry”) Moon. We have analyzed abundances and isotopic compositions of Cl and H in lunar mare basalts, and find little evidence that anhydrous lava outgassing was important in generating chlorine isotope anomalies, because 37Cl/35Cl ratios are not related to Cl abundance, H abundance, or D/H ratios in a manner consistent with the lava-outgassing hypothesis. Instead, 37Cl/35Cl correlates positively with Cl abundance in apatite, as well as with whole-rock Th abundances and La/Lu ratios, suggesting that the high 37Cl/35Cl in lunar basalts is inherited from urKREEP, the last dregs of the lunar magma ocean. These new data suggest that the high chlorine isotope ratios of lunar basalts result not from the degassing of their lavas but from degassing of the lunar magma ocean early in the Moon’s history. Chlorine isotope variability is therefore an indicator of planetary magma ocean degassing, an important stage in the formation of terrestrial planets.
INTRODUCTION
The Earth and Moon are nonidentical twins, born in the same giant impact (1). Chemically dissimilar from the volatile-rich rocks of Earth, rocks from the Moon are depleted in the volatile elements. This difference expresses across the entire periodic table (2), but more noticeably in the absence in lunar rocks of common terrestrial hydrous minerals such as biotite and amphibole. In contrast to those differences in elemental abundances, rocks from the Moon and Earth have very similar isotopic ratios for many of the most abundant elements (3–10), which strongly ties the two bodies together with respect to their genesis. One of the most significant exceptions to this is lunar chlorine. It recently has been shown that lunar basalts, glasses, and apatite (a common accessory mineral, Ca5[PO4]3[F,Cl,OH], which can contain abundant Cl) have δ37Cl values [reported as δ37Cl relative to standard mean ocean chlorine (SMOC)] that range from typical terrestrial values of 0 ± 1‰ to +81‰ (11, 12), far greater than observed in any other solid materials in our Solar System (11). These elevated δ37Cl values were interpreted as having been produced by isotopic fractionation during extensive outgassing of Cl, probably from eruptions of basalt that were sufficiently poor in H such that HCl was not an important gas species (11). This “lava-outgassing” scenario is consistent with observations that bulk lunar rocks are generally poor in H relative to their terrestrial counterparts (13–15), but is at odds with data suggesting that many lunar magmas contained significant H before eruption (16–19), and still other data suggesting that some lunar magmas apparently had H abundances and D/H ratios similar to their terrestrial counterparts (20, 21).
It has been difficult to reconcile interpretations based on H abundances and isotopic ratios with those based on Cl abundances and isotopic ratios, in part because there have been limited data on H and Cl abundances and isotopic compositions for the same materials. Here, we present measurements of H and Cl abundances as well as 37Cl/35Cl and D/H ratios in apatite crystals from a range of lunar basalts, where measurements are from the same thin sections and, when possible, in the same apatite crystals (for example, Fig. 1). We use these data to place constraints on the origin of the unusual chlorine isotopic composition of the Moon and to place that chlorine within the larger framework of lunar volatiles.
Fig. 1. Photomicrographs of two basalts.
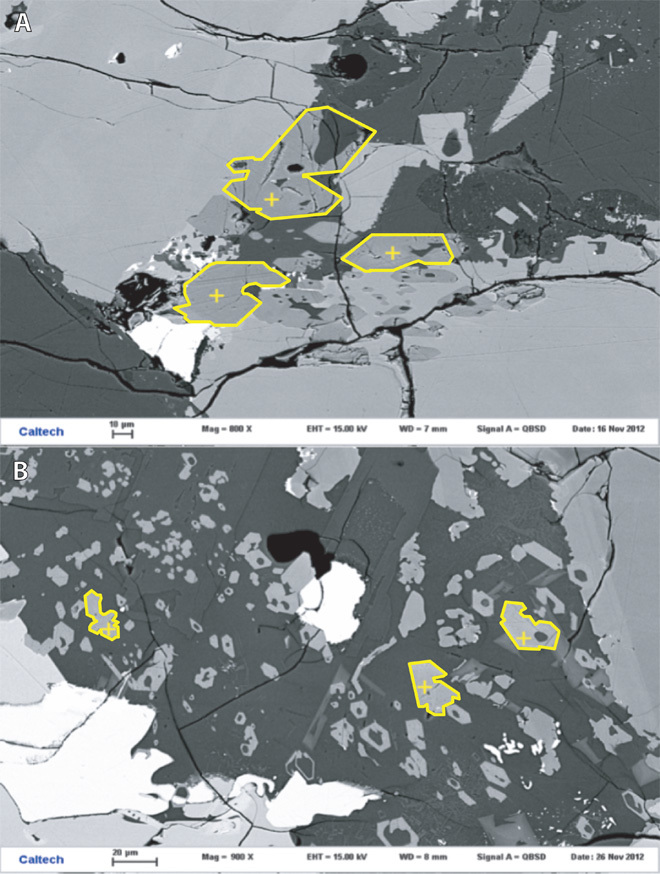
Photomicrographs of two basalts analyzed in this study, with yellow crosses marking the locations of analyses. (A) Subhedral apatite grains (outlined in yellow for clarity) forming a cluster in Apollo 12039,42. (B) Euhedral to subhedral apatite grains in Apollo 12040,211 that show skeletal growth with hollow centers.
RESULTS
Chlorine isotope ratios in the samples analyzed here range from δ37Cl = −4‰ in Miller Range (MIL) 05035 to +18‰ in 12039 (Fig. 2 and Table 1) and are consistent with the few previous analyses of δ37Cl in apatite from mare basalts, with the exception of MIL 05035: The δ37Cl for this sample (−4 ± 2‰) is among the lowest measured in any natural material from anywhere in the Solar System. Lunar apatites also display a wide range of D/H (Fig. 3), with δD values [reported as δD relative to Vienna standard mean ocean water (VSMOW)] of multispot grain averages ranging from −150 to +970‰. Of the basalts, 10044 has the greatest range in δ37Cl (+2 to +15‰), as well as in δD, from +540 to +950‰ (n = 6), where the latter are consistent with other analyses of 10044 (Fig. 3).
Fig. 2. Plots of Cl and H2O versus δ37Cl.
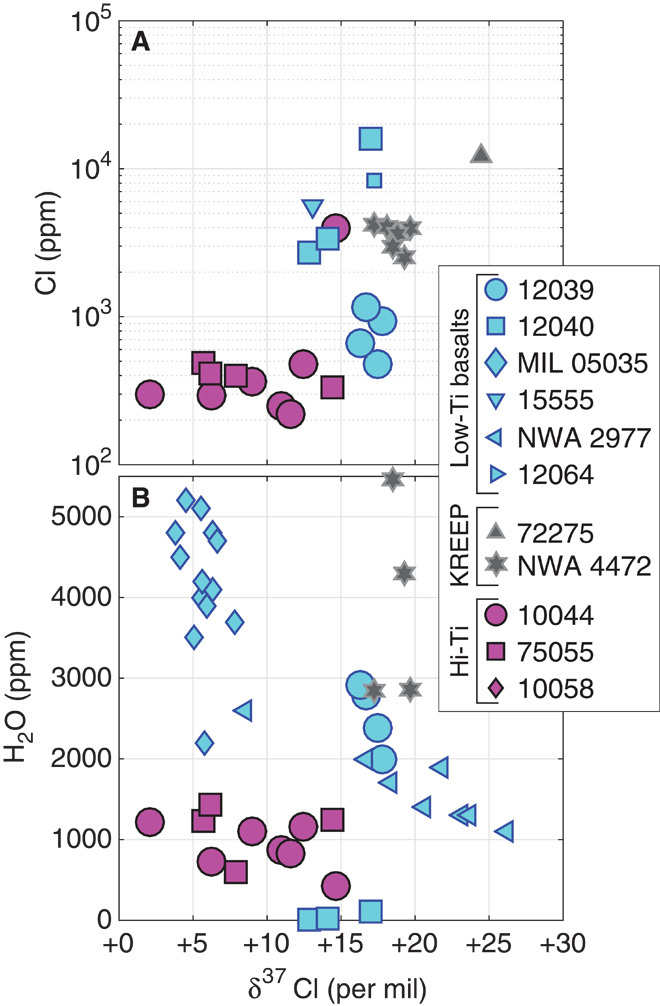
(A and B) Plots of Cl (A) and H2O (B) concentrations versus δ37Cl values in apatite from the Moon. Each data point represents a single apatite grain in a rock, including data from the literature plotted as smaller symbols (11, 12, 33); magenta symbols are high-Ti basalts; blue symbols are low-Ti basalts; gray symbols are KREEP basalts. There are no statistically significant correlations observed for any of the sample types studied here. Elevated δ37Cl values are associated with both high H2O and high Cl concentrations: The former is inconsistent with the idea that elevated δ37Cl values are only associated with anhydrous conditions, and the latter is inconsistent with a simple, single-stage fractionation model.
Table 1. New data generated for this study.
| Sample | Section | Grain | H2O (ppm) | H2O 2σ | Cl (ppm) | Cl 2σ | δ37Cl | δ37Cl 2σ | δD | δD 2σ | Category |
| 10044 | 12 | 10 | 1105 | 29 | 365 | 104 | +9 | 3 | +933 | 31 | High Ti basalt |
| 10044 | 12 | 1a | 728 | 27 | 292 | 11 | +6 | 3 | +954 | 27 | High Ti basalt |
| 10044 | 12 | 1b | — | — | — | — | +6 | 4 | — | — | High Ti basalt |
| 10044 | 12 | 1c | 1220 | 30 | 300 | 11 | +2 | 4 | +781 | 23 | High Ti basalt |
| 10044 | 644 | 2 | 877 | 26 | 250 | 10 | +11 | 3 | +536 | 57 | High Ti basalt |
| 10044 | 644 | 8 | 421 | 22 | 3976 | 122 | +15 | 3 | — | — | High Ti basalt |
| 10044 | 644 | 4a | 826 | 25 | 219 | 10 | +12 | 3 | +606 | 33 | High Ti basalt |
| 10044 | 644 | 4b | 1155 | 29 | 479 | 16 | +12 | 3 | +702 | 28 | High Ti basalt |
| 75055 | 55 | 1 | 604 | 23 | 398 | 14 | +8 | 3 | +621 | 35 | High Ti basalt |
| 75055 | 55 | 2 | 1225 | 35 | 485 | 16 | +6 | 3 | +794 | 26 | High Ti basalt |
| 75055 | 55 | 3 | 1241 | 30 | 334 | 12 | +14 | 3 | +968 | 27 | High Ti basalt |
| 75055 | 55 | 4 | 1430 | 34 | 410 | 14 | +6 | 3 | +794 | 26 | High Ti basalt |
| 75055 | 55 | 101 | — | — | — | — | +5 | 3 | — | — | High Ti basalt |
| 12039 | 42 | 4 | 1996 | 43 | 928 | 29 | +18 | 3 | +720 | 30 | Low Ti basalt |
| 12039 | 42 | 6 | 2379 | 47 | 477 | 16 | +17 | 3 | +830 | 31 | Low Ti basalt |
| 12039 | 42 | 10 | — | — | — | — | +16 | 3 | — | — | Low Ti basalt |
| 12039 | 42 | 11 | — | — | — | — | +17 | 3 | — | — | Low Ti basalt |
| 12039 | 42 | 17a | 2784 | 95 | 1157 | 36 | +17 | 3 | +729 | 28 | Low Ti basalt |
| 12039 | 42 | 17b | 2916 | 57 | 665 | 21 | +16 | 3 | +698 | 28 | Low Ti basalt |
| 12040 | 211 | 1 | 105 | 21 | 15884 | 489 | +17 | 3 | +9 | 163 | Low Ti basalt |
| 12040 | 211 | 4 | 3 | 20 | 2689 | 83 | +13 | 3 | +14 | 84 | Low Ti basalt |
| 12040 | 211 | 5 | 16 | 20 | 3388 | 104 | +14 | 4 | −150 | 26 | Low Ti basalt |
| MIL 05035 | — | 1 | — | — | — | — | −4 | 2 | — | — | Low Ti basalt |
Fig. 3. δD versus H2O.
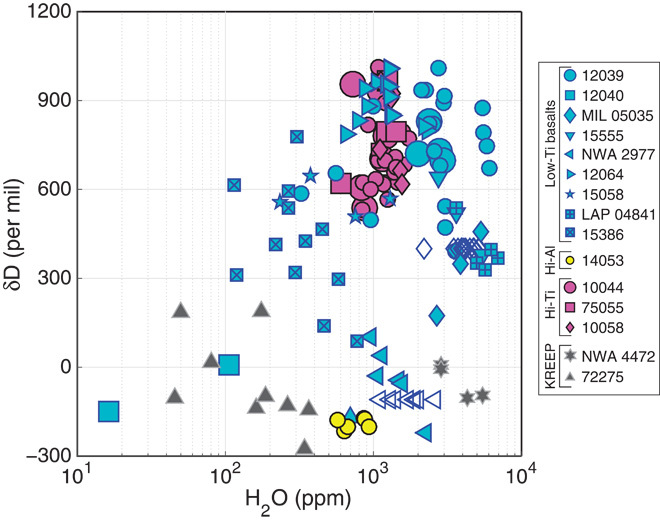
δD versus H2O for new data (large symbols) and data from the literature (small symbols). Hollow symbols are multiple hydrogen abundance measurements plotted with bulk δD values. Data have been previously interpreted as representing mixing between a high-δD, high-H2O end member (possibly comets), and a low-δD, low-H2O end member (possibly solar wind), or evolution from or contamination with a material of chondritic composition (16, 24, 25, 33), though neither explanation explains all the data. Note the large number of analyses with δD values in the range for bulk chondrites (+750 to −200‰) (58).
Chlorine abundances in apatites from low-Ti basalts (Fig. 2A) range from 480 to 16,000 ppm. Chlorine abundances in seven of eight apatites in high-Ti basalt 10044 are between 220 and 480 ppm, with one outlier at 4000 ppm Cl. Apatite grains in low-Ti mare basalts have hydrogen concentrations (always reported here as the oxide H2O equivalent) from below 100 ppm H2O in sample 12040 to 2920 ppm H2O in 12039, with data from the literature extending this to more than 5000 ppm (Fig. 2B). Apatites from high-Ti mare basalts (10044 and 75055) vary from 420 to 1430 ppm H2O.
Lunar rocks are remarkable for their high 37Cl/35Cl values, which imply a distinctive fractionation event in lunar history that is not preserved in rocks of other planetary bodies. The lava-outgassing hypothesis of Sharp et al. (11) requires that the basalt magmas contain minimal hydrogen (otherwise, degassing would be predicted to be of HCl gas, which would cause little fractionation of 37Cl from 35Cl). Independent of the mechanism of fractionating chlorine, the timing of the chlorine loss resulting in the fractionation must also be constrained: The lava-outgassing model suggests that this loss and fraction occur circa eruption.
DISCUSSION
The data reported here allow three tests of the lava-outgassing hypothesis, two based on abundances and one on isotope ratios:
(i) Elevated 37Cl/35Cl ratios should be inversely proportional to Cl abundance.
(ii) Elevated 37Cl/35Cl ratios should not be observed in samples with abundant H.
(iii) Elevated 37Cl/35Cl ratios should be observed only in samples that have enrichments in D/H.
Although the quantitative significance of measured abundances of H and Cl in apatite has been called into question (22), H and Cl concentrations—or even simply their presence or absence—are still valuable constraints on models used to explain observed H and Cl isotopes in lunar basalts. This is especially true of Cl, which does not experience the same order of magnitude of increase in apatite due to fractionation of fluorine.
On the basis of the lava-outgassing hypothesis, one would predict that high δ37Cl values should be found preferentially in samples with low abundances of Cl, because elevated δ37Cl is thought to develop only through extensive Cl loss (11). We find, to the contrary, that apatites with high δ37Cl are among the most Cl-rich, with Cl abundances positively correlated with δ37Cl values for the same samples (Fig. 2A). If the mechanism for increasing δ37Cl involves preferential loss of 35Cl (which seems likely, relative to 37Cl) and is occurring syn- and post-eruption, there must have been a subsequent process that concentrated Cl (with elevated δ37Cl) relative to F and OH. This implies that the fractionation of Cl did not occur in the degassing accompanying eruption and cooling at the surface, because there is no subsequent event after eruption that can preferentially enrich high-δ37Cl samples in their Cl abundance more than that same process enriches low-δ37Cl samples. This is especially problematic given that the samples that are more fractionated (more elevated δ37Cl) likely have experienced greater degrees of fractional loss to achieve higher 37Cl/35Cl values—but they must finish with higher Cl abundances.
A second prediction of the lava-outgassing model is that magmas with high δ37Cl should be nearly anhydrous (11), and thus, the apatite crystals would also be anhydrous. The negative relationship between H abundance and δ37Cl in apatites from some lunar basalts (for example, NWA 2977) could be interpreted as support for this prediction (Fig. 2B). However, nearly all lunar apatites have high δ37Cl relative to the range of terrestrial samples (0 ± 1‰), and many of them are rich in H, some exceeding 5000 ppm H2O. Thus, although it is possible that loss of transition metal chlorides (11) contributes to elevated δ37Cl, it does not seem possible that this signature is entirely generated by degassing from H-free melts. Whether or not this scenario is possible, there is experimental evidence suggesting that it is not necessary: evaporation of HCl at standard temperature and pressure yields an increase in +9‰ in δ37Cl in the residue after 90% evaporation (23). Therefore, it is not impossible to achieve fractionations of the same order of magnitude as those observed in mare basalts from phase transformations of HCl.
Third, we note that if the H-poor magmas required for generation of δ37Cl anomalies were themselves generated by extensive degassing of H2 (favoring the lighter isotope), one should expect that mare basalts with elevated δ37Cl would also have relatively elevated δD. This is not only because—in the context of the lava-outgassing hypothesis—extensive loss of hydrogen is required to generate conditions under which metal chloride degassing dominates over HCl degassing, but also (more generally) because magmas that have outgassed Cl should also have had the opportunity to outgas H. If the building blocks of Earth and its Moon were H-bearing, and the lava-outgassing hypothesis requires the magma to be H-free when Cl loss is initiated syn-eruption, then hydrogen must have been lost before Cl, resulting in an elevated D/H ratio in the residue. This expectation is not met: Instead of the positive correlation one might expect, lunar basalts actually define two separate populations with no apparent relationship between δD and δ37Cl: Some basalts with very high δ37Cl (for example, samples 12040 and NWA 2977, which range up to +20‰) have low δD (≤0‰) (Fig. 4).
Fig. 4. Plot of δD versus δ37Cl.
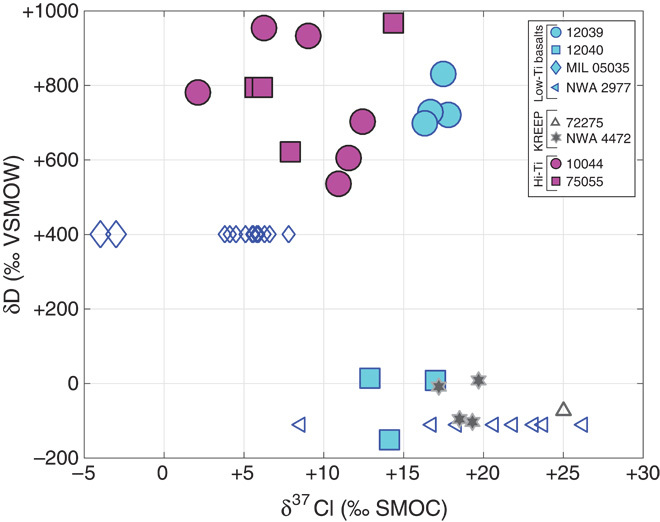
Plot of δD versus δ37Cl for data generated in this study and those from previous studies (plotted as smaller symbols). Hollow symbols indicate in situ δ37Cl measurements plotted versus bulk or mean apatite δD from the literature. The presence of high δ37Cl apatite with low δD values is inconsistent with the prediction that elevated δ37Cl would accompany elevated δD, because both isotopes should shift toward higher δ values during degassing. The lack of correlation between δD and δ37Cl suggests that the two isotope systems are at least partially decoupled and that multiple processes may be at work.
It should also be noted that neither the relationship between δD and H abundance (Fig. 3) nor the presence of apatite with strongly negative δD can be explained by extensive losses of H2 from a chondritic reservoir (24, 25). Given the presence of implanted solar wind, spallogenic D, and H-rich chondritic materials gardened into the upper lunar regolith through repeated impacts, it is plausible that the regolith serves as an exchangeable reservoir of H for adjacent magmatic dikes, sills, and lava flows that can modify the D/H ratio of magmas (26, 27). This form of “crustal contamination” [as previously suggested by Hui et al. (28) to explain major and trace element variability in Apollo 14 basalts] may exert significant leverage on D/H ratios, especially in magmas with low intrinsic H abundances. Apatite grains in individual basalt samples show wide ranges of δD values, with individual samples having ranges as large as 800‰ (10044), which is also consistent with partial late-stage reequilibration with extrinsic hydrogen.
We conclude that none of the three tests of the lava-outgassing hypothesis are consistent with this new data set, and therefore, we develop other explanations, focusing on processes that are implied by correlations between d37Cl and other geochemical properties: The δ37Cl values of apatite from lunar basalts are correlated with Cl content, which is known to be enriched in KREEP. KREEP is the name given to a chemical component—possibly derived from the urKREEP, the last vestige of the lunar magma ocean—rich in potassium (K), the rare earths (REE), and phosphorus (P) and other elements that are incompatible during the crystallization of magmas (29–31). Incompatible elements are those that are preferentially excluded from crystals growing from a melt and therefore concentrated in that residual melt—in this case, the lunar magma ocean. The lunar magma ocean first crystallizes from below, then from both above and below, resulting in a “sandwich horizon” of melt with decreasing volume and increasing abundances of incompatible elements (31). KREEP (and the proposed urKREEP source) have long been implicated in the trace element variations of lunar basalts [for example, (32)], with different units having different contributions of KREEP (sometimes referred to as “KREEPiness”). More KREEP-rich (or “KREEPy”) samples are observed to have higher abundances of trace elements such as Th, Cl, as well as the major and trace elements that make up the acronym KREEP. Model compositions of the urKREEP (29) also indicate that the urKREEP may have elevated La/Lu ratios, relative to the mare basalt source. Thus, trace element abundances and ratios can be used to determine the magnitude of KREEP contamination/addition. The δ37Cl values of mare basalt apatites are also positively correlated with abundances and ratios in their host rocks of the elements that are enriched in the lunar KREEP component, notably bulk Th abundance and La/Lu abundance ratios derived from the literature (Fig. 5) (29, 31).
Fig. 5. Bulk Th and La/Lu versus δ37Cl.
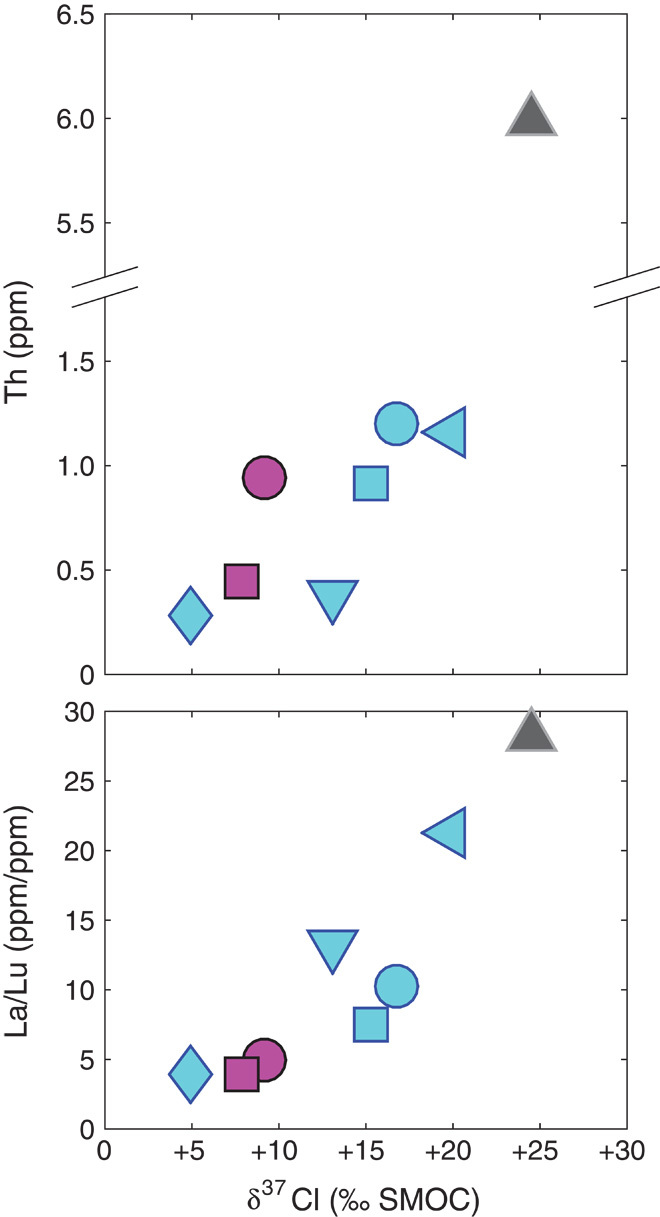
(A and B) Measures of KREEP contribution, bulk rock Th abundance (A), and bulk rock La/Lu (B), as a function of δ37Cl, with trace element data from (43, 54, 59). Symbols are the same as those in previous figures. For all basalts, δ37Cl is strongly correlated with Th and La/Lu, suggesting that pure urKREEP would have δ37Cl ≥+30‰.
The correlations between Cl, Th, La/Lu, and δ37Cl suggest that Cl abundances and δ37Cl of mare basalts are controlled—at least in part—by the abundance of the KREEP component. The highest values of δ37Cl found in previous studies for apatite from lunar basalt were from a KREEPy clast in the highlands breccia 72275 (11), whereas the highest values observed by (33) were also in a KREEPy clast. The trends in Fig. 5 are consistent with the existence of a background lunar mantle reservoir containing Cl with a δ37Cl value similar to the bulk Earth (~0‰) and a KREEP reservoir such as urKREEP containing more abundant Cl with a δ37Cl of ≥+30‰ (34). The lunar mantle end member might be recording an undegassed lunar source, a partially degassed source that did not have the special conditions required for fractionation of Cl isotopes, or meteoritic material that could have a δ37Cl as low as −4‰ (35). Chlorine degassed from a mare basalt at any stage of lunar evolution might be expected to have low δ37Cl relative to its source and could return to the Moon as adsorbates on regolith grains (11), providing another possible explanation for the negative δ37Cl values observed for lunar apatite in MIL 05035.
We interpret the high δ37Cl of urKREEP to have formed during vapor phase loss of Cl from the magma ocean, perhaps by a mechanism akin to that of Sharp et al. (11): loss of transition metal chlorides from a hydrogen-poor melt. However, it is also possible that the mechanism is that of HCl loss, which was also put forth by Sharp et al. (23). Either mechanism of Cl loss is consistent with recent interpretations of Cl, Zn, and K isotopes from lunar materials, subsets of which have similar patterns of fractionations (36). Chlorine that survives the degassing of the lunar magma ocean is enriched in 37Cl relative to 35Cl, and is then concentrated into the urKREEP, because Cl is incompatible in all of the major lunar mantle phases and will choose to stay in the ever-shrinking remnants of the lunar magma ocean. This results in a reservoir that is enriched in Cl and Th, has elevated La/Lu, and still has the fingerprint of the magma ocean degassing preserved in the elevated isotopic ratio of Cl. Basalts may acquire this isotopic signature via incorporation of KREEP-rich materials in the lunar mantle or as the magmas are traversing—and assimilating—crust or regolith materials that have a KREEP signature.
Other planets are inferred to have had magma oceans early in their histories—including Earth—which shows no evidence of elevated δ37Cl (37). It is of course possible that the budget of Cl on Earth is dominated by late delivery of volatile-rich materials (38), but this is inconsistent with the argument that the terrestrial planets received their hydrogen early (39). Even if Earth’s chlorine is primordial, the pressure, temperature, and chemistry of the atmosphere above a magma ocean most likely control—at least in part—the extent to which loss can change the isotope ratio of the residuum (40, 41). To the extent that fractionation due to Cl loss is governed by parent body size—with smaller bodies predicted to have less atmosphere and therefore larger potential fractionations—the magnitudes (if not the directions) of the δ37Cl anomalies for rocks from Earth, Mars, and the Moon are consistent with the relative size and expected atmospheric pressures of those bodies during their magma ocean phases. This model predicts elevated δ37Cl for other small bodies that had magma oceans, as is inferred for 4Vesta (42). Thus, chlorine isotopes may provide a unique tool for exploring the existence, extent, and conditions during the magma ocean phase that is thought to be a significant stage in the formation of terrestrial planetary bodies.
MATERIALS AND METHODS
Experimental design
The purpose of this study was to investigate the relationship between chlorine and other elements (as well as their isotope ratios) in lunar basalts. We set out to test the hypothesis that δ37Cl variations in lunar basalts are due to anhydrous degassing of basalts during eruption (11). Three predictions following from that hypothesis were tested:
(i) Elevated 37Cl/35Cl ratios should be inversely proportional to Cl abundance.
(ii) Elevated 37Cl/35Cl ratios should not be observed in samples with abundant H.
(iii) Elevated 37Cl/35Cl ratios should be reserved for samples that have enrichments in D/H.
These hypotheses were tested by making the H, Cl, D/H, and 37Cl/35Cl measurements in apatite crystals from the same samples and, when possible, in the same crystals. After these predictions were tested, additional hypotheses were formulated, including the hypothesis that the degassing of the lunar magma ocean had generated the high 37Cl/35Cl ratios observed in the samples. This hypothesis makes three additional predictions:
(i) Elevated 37Cl/35Cl ratios should be positively correlated to Cl abundance.
(ii) Elevated 37Cl/35Cl ratios should be positively correlated to Th abundance, which is elevated in urKREEP.
(iii) Elevated 37Cl/35Cl ratios should be positively correlated to La/Lu ratio, which is elevated in urKREEP.
These predictions were tested by direct measurement of δ37Cl and Cl abundance (above), and by comparison with literature values for bulk Th content and La/Lu ratio.
Materials
Six thin sections of five samples were analyzed by ion microprobe for this study: two high-Ti basalts (10044 and 75055) and three low-Ti basalts (12039, 12040, and MIL 05035).
Apollo sample 10044
This is a low-K ilmenite basalt that has a Ti content lower than typical ilmenite basalts from Apollo 11 (43). On the basis of its texture, it was classified as coarse-grained porphyritic basalt (44) and microgabbro [for example, (45)], which consists of subhedral to anhedral pyroxene, set in a matrix of plagioclase, anhedral pyroxene, and ilmenite, with minor apatite, spinel, silica, and symplectitic intergrowth. For a more detailed description of the petrography of 10044, see the Lunar Sample Compendium (43). Here, apatites from two thin sections were examined, 10044,12 and 10044,644, which appear mostly as inclusions in pyroxene and oxides. The grain sizes range from <5 to 200 μm. Most grains are euhedral to subhedral in shape, with some grains appearing as irregular-shaped aggregates. For this study, five apatite grains were analyzed (31 analyses total) with ranges in grain size from 20 to 200 μm.
Apollo sample 75055
This is a medium-grained ilmenite basalt that is more aluminous and less Ti-rich than other Apollo 17 basalts (46). On the basis of its texture, the sample has been described as subophitic (44, 47) with tabular plagioclase intergrown with subhedral to anhedral pyroxene and ilmenite laths. For further petrographic information, see the Lunar Sample Compendium (43). Here, Apollo sample 75055,55 was analyzed. Apatite in this sample appears as inclusions mainly in pyroxene and oxides, but some can be found in plagioclase. Apatite ranges in grain size from <5 to 110 μm and are mostly subhedral in shape. Some grains show skeletal growth with hollow centers similar to apatite from Apollo 12040 (Fig. 1). Five apatite grains have been analyzed for this study (13 analyses total), none smaller than 10 μm in size.
Apollo sample 12039
This is a medium-grained pigeonite basalt/microgabbro (48, 49) and one of the most Fe-rich and Mg-poor Apollo 12 igneous rocks (50). Texturally, it ranges from porphyritic (44), to subophitic, to granular (50). Petrographically, it is mainly composed of plagioclase and pyroxene with long needles of ilmenite and tridymite cutting across the plagioclase and pyroxene. Minor troilite, tranquillityite, chromite, metal, apatite, and symplectitic intergrowths are present (43). Here, apatite in thin section 12039,42 was analyzed. Apatite is subhedral to anhedral, sometimes present as long needles, with grain sizes ranging from <10 to 400 μm long. Some grains occur as clusters, and some grains show skeletal growth (Fig. 1). Seventeen points in five different grains were analyzed.
Apollo sample 12040
This is a coarse-grained olivine basalt with a high proportion of mafic minerals. Texturally, it is equigranular with an average grain size of 1 mm (51). It is mainly composed of olivine and pyroxene with minor plagioclase, ilmenite, chromite, troilite, metal, phosphates, and alkali feldspar (52, 53). Here, three apatite grains were analyzed (14 spots total) in thin section 12040,211. Apatite is mostly subhedral to anhedral, and grain sizes range from <5 to 30 μm and occurs in pyroxene and plagioclase. Small, subhedral to euhedral apatite grains show skeletal growth with hollow centers (Fig. 1).
Lunar meteorite MIL 05035
MIL 05035 is a coarse-grained lunar gabbroic meteorite (54). It mainly consists of pyroxene (54 to 69 volume %) with grain sizes up to 6 mm and plagioclase (17 to 36 volume %) with grain sizes up to 4 mm. It contains minor fayalitic olivine, ilmenite, spinel, FeS, apatite, and silica, which represent crystallized products of its residual melt (54, 55). It also contains symplectic intergrowth that is composed of silica, fayalitic olivine, and hedenbergitic pyroxene (54). Here, a single apatite grain in the thin section MIL 05035,6 was analyzed (two points total).
Methods
Measurements of H, Cl, F, as well as D/H and 37Cl/35Cl were all made in the Caltech Center for Microanalysis using the Cameca 7f-GEO secondary ion mass spectrometer. Standards used for abundance measurements are those described in (56) with the slightly revised values of (57). Isotopic measurements are reported relative to Durango apatite at δ37Cl = +0.40‰ SMOC and δD = −120 ± 5‰ VSMOW (2σ) (16).
Abundances of OH, F, and Cl
Measurements of volatile abundances in lunar apatite are made by measuring 16O1H, 18O (reference element), 19F, 31P (secondary reference element), 32S, and 35Cl using a −0.5-nA, 10-keV Cs+ beam at a mass resolving power of ~5500, sufficient to separate peaks of interest from all known interferences (18). Preanalytical sputtering was performed with a 2-nA beam and 25-μm × 25-μm raster for 300 s, except in cases where abundance measurements followed isotopic measurements, in which case preanalytical sputtering was reduced to 20 s. This was followed by 10 to 30 cycles of measurement with a 2-μm × 2-μm raster. Secondary ions were accelerated to −9 keV, and those passing through a ~100-μm field aperture were measured with dynamic transfer via electron multiplier, except for F and Cl, that in some cases were measured with a Faraday cup. An 80% electronic gating was applied to further reduce contamination. Measurements of less than 100 ppm H2O are very conservatively assumed to be within error of the blank for epoxy-bearing thin sections such as those studied here.
Cl isotopes
Measurements of Cl isotopes were made in two sessions, both normalized to Durango apatite at +0.4‰ SMOC. In the first session, samples were presputtered for 300 s at ~2 nA with a 25-μm × 25-μm raster. Data were collected for 35Cl (1 s) and 37Cl (1 s) using a lower primary beam current (~0.5 nA), rastered over a smaller area (2 μm × 2 μm) for 300 cycles. Data were collected with an electron multiplier (dead time = 44 ns) at low mass resolving power (~2000). Additional settings were as follows: field aperture (400 μm), contrast aperture (150 μm), no dynamic transfer, no E-gating, 45-eV energy window. The second session used similar settings, except that a Faraday cup detector was used instead of an electron multiplier, with a higher primary current (~3 to 3.5 nA for the presputter, ~1.5 to 2 nA), and shorter analysis times (120-s presputter, 30 cycles of measurement). No difference was observed in the reproducibility of the reference materials between the two sessions.
H isotopes
Measurements of H isotopes were made at low mass resolution [mass resolution power (MRP) ~800] with the electron multiplier, with 50 to 100 cycles of ~3-nA, 2-μm × 2-μm rastered beam sputtering, preceded by 180 s of ~3-nA, 25-μm × 25-μm raster presputtering. Durango apatite was used as a δD standard, with a value of −120‰ VSMOW (16). Additional settings include the following: field aperture of 100 μm, no dynamic transfer, electronic gating of 80% (64% by area).
Statistical analysis
All uncertainties are reported as 2 SEMs. For single analyses, uncertainties are reported as 2 SEMs, which consists of the analytical uncertainty. For multiple analyses of the same sample, it consists of either the analytical uncertainty (2 SEMs) or two standard deviations of the mean of the measured values for that sample, whichever is larger. For measurements of hydrogen abundance (always reported as H2O, regardless of speciation), all values below 100 ppm H2O are assumed to be within error of zero, because all of our samples are epoxy-bearing thin sections, which are not ideal for low-blank H2O measurements by ion microprobe. No outliers were discarded: The only data not reported here are due to operator error on the instrument and are noted before data reduction.
Acknowledgments
We are grateful to the institutions and people who made samples available for this research, including the Lunar Sample and Meteorite curators at the Johnson Space Center, and T. Bunch of Northern Arizona University. We are also grateful to F. McCubbin and J. Mosenfelder, who graciously provided apatite and other mineral standard materials, and M. Le Voyer, who was instrumental in developing Cl isotope capabilities at Caltech. The first author would like to acknowledge the support that he received from his coauthors and colleagues during the duration of this project, which was considerably lengthened by a near-fatal illness. The manuscript benefitted from detailed, thoughtful, and constructive criticism from the editor, two anonymous reviewers, and C. Neal - who is perhaps thanked twice in this sentence. Funding: This research was supported by NASA Early Career Fellowships to J.W.B. (NNX13AG40G) and J.G. (NNX13AF54G), as well as NASA grants to A.H.T. (NNX12AH64G) and J.P.G. (NNX11AB29G). Author contributions: J.W.B., A.H.T., J.M.E., and E.M.S. conceived the project. C.M., A.H.T., and J.G. performed the petrographic and petrologic descriptions. Y.G., C.M., A.H.T., and J.W.B. performed the analyses. All authors participated in the data reduction and writing of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All new data generated for this paper are found in Table 1. Samples analyzed were borrowed from several sources (see above) and must be requested directly to those sources.
REFERENCES AND NOTES
- 1.Hartmann W. K., Davis D. R., Satellite-sized planetesimals and lunar origin. Icarus 24, 504–515 (1975). [Google Scholar]
- 2.Albarede F., Albalat E., Lee C.-T. A., An intrinsic volatility scale relevant to the Earth and Moon and the status of water in the Moon. Meteorit. Planet. Sci. 50, 568–577 (2015). [Google Scholar]
- 3.Wiechert U., Halliday A. N., Lee D. C., Snyder G. A., Taylor L. A., Rumble D., Oxygen isotopes and the Moon-forming giant impact. Science 294, 345–348 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Norman M. D., Yaxley G. M., Bennett V. C., Brandon A. D., Magnesium isotopic composition of olivine from the Earth, Mars, Moon, and pallasite parent body. Geophys. Res. Lett. 33, L15202 (2006). [Google Scholar]
- 5.Touboul M., Kleine T., Bourdon B., Palme H., Wieler R., Late formation and prolonged differentiation of the Moon inferred from W isotopes in lunar metals. Nature 450, 1206–1209 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Spicuzza M. J., Day J. M. D., Taylor L. A., Valley J. W., Oxygen isotope constraints on the origin and differentiation of the Moon. Earth Planet. Sci. Lett. 253, 254–265 (2007). [Google Scholar]
- 7.Simon J., DePaolo D. J., Stable calcium isotopic composition of meteorites and rocky planets. Earth Planet. Sci. Lett. 289, 457–466 (2010). [Google Scholar]
- 8.Fitoussi C., Bourdon B., Silicon isotope evidence against an enstatite chondrite Earth. Science 335, 1477–1480 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., Dauphas N., Davis A. M., Leya I., Fedkin A., The proto-Earth as a significant source of lunar material. Nat. Geosci. 5, 251–255 (2012). [Google Scholar]
- 10.Herwartz D., Pack A., Friedrichs B., Bischofff A., Identification of the giant impactor Theia in lunar rocks. Science 344, 1146–1150 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Sharp Z. D., Shearer C. K., McKeegan K. D., Barnes J. D., Wang Y. Q., The chlorine isotope composition of the Moon and implications for an anhydrous mantle. Science 329, 1050–1053 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Y. Wang, Y. Guan, W. Hsu, J. M. Eiler, paper presented at the 75th Annual Meteoritical Society Meeting, Cairns, Queensland, Australia, 2012. [Google Scholar]
- 13.S. Epstein, H. P. Taylor, The isotopic composition and concentration of water, hydrogen, and carbon in some Apollo 15 and 16 soils and in the Apollo 17 orange soil, Proceedings of the 4th Lunar Science Conference, Houston, TX, 5 to 8 March 1973 (Pergamon Press Inc., New York, 1973). [Google Scholar]
- 14.L. Haskin, P. H. Warren, in Lunar Sourcebook (Cambridge Univ. Press, Cambridge, 1991), pp. 357–474. [Google Scholar]
- 15.J. Papike, L. Taylor, S. Simon, Lunar minerals, Lunar Sourcebook (Cambridge Univ. Press, Cambridge, 1991), pp. 121–181. [Google Scholar]
- 16.Greenwood J. P., Itoh S., Sakamoto N., Warren P., Taylor L., Yurimoto H., Hydrogen isotope ratios in lunar rocks indicate delivery of cometary water to the Moon. Nat. Geosci. 4, 79–82 (2011). [Google Scholar]
- 17.McCubbin F. M., Steele A., Hauri E. H., Nekvasilc H., Yamashita S., Hemley R. J., Nominally hydrous magmatism on the Moon. Proc. Natl. Acad. Sci. U.S.A. 107, 11223–11228 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce J. W., Liu Y., Rossman G. R., Guan Y., Eiler J. M., Stolper E. M., Taylor L. A., Lunar apatite with terrestrial volatile abundances. Nature 466, 466–469 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Hui H., Pelsier A. H., Zhang H., Neal C. R., Water in lunar anorthosites and evidence for a wet early Moon. Nat. Geosci. 6, 177–180 (2013). [Google Scholar]
- 20.Saal A. E., Hauri E. H., Cascio M. L., Van Orman J. A., Rutherford M. C., Cooper R. F., Volatile content of lunar volcanic glasses and the presence of water in the Moon’s interior. Nature 454, 192–195 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Saal A. E., Hauri E. H., Van Orman J. A., Rutherford M. C., Hydrogen isotopes in lunar volcanic glasses and melt inclusions reveal a carbonaceous chondrite heritage. Science 340, 1317–1320 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Boyce J. W., Tomlinson S. M., McCubbin F. M., Greenwood J. P., Treiman A. H., The lunar apatite paradox. Science 344, 400–402 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Sharp Z. D., Barnes J. D., Fischer T. P., Halick M., An experimental determination of chlorine isotope fractionation in acid systems and applications to volcanic fumaroles. Geochim. Cosmochim. Acta 74, 264–273 (2010). [Google Scholar]
- 24.Barnes J. J., Franchi I. A., Anand M., Tartèse R., Starkey N. A., Koike M., Sano Y., Russell S. S., Accurate and precise measurements of the D/H ratio and hydroxyl content in lunar apatites using NanoSIMS. Chem. Geol. 337–338, 48–55 (2013). [Google Scholar]
- 25.Tartese R., Anand M., Late delivery of chondritic hydrogen into the lunar mantle: Insights from mare basalts. Earth Planet. Sci. Lett. 361, 480–486 (2013). [Google Scholar]
- 26.Rumpf M., Fagents S. A., Crawford I. A., Joy K. A., Numerical modeling of lava-regolith heat transfer on the Moon and implications for the preservation of implanted volatiles. J. Geophys. Res. Planets 118, 382–397 (2013). [Google Scholar]
- 27.Stephant A., Robert F., The negligible chondritic contribution in the lunar soils water. Proc. Natl. Acad. Sci. U.S.A. 111, 15007–15012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui H., Oshrin J. G., Neal C. R., Investigation into the petrogenesis of Apollo 14 high-Al basaltic melts through crystal stratigraphy of plagioclase. Geochim. Cosmochim. Acta 75, 6439–6460 (2011). [Google Scholar]
- 29.Neal C. R., Taylor L. A., Metasomatic products of the lunar magma ocean: The role of KREEP dissemination. Geochim. Cosmochim. Acta 53, 529–541 (1989). [Google Scholar]
- 30.B. Jolliff, paper presented at the 20th Lunar and Planetary Science Conference, Houston, TX, 1989. [Google Scholar]
- 31.Warren P. H., Wasson J. T., The origin of KREEP. Rev. Geophys. 17, 73–88 (1979). [Google Scholar]
- 32.Reed G. W. J., Jovanovic S., Fuchs L., Trace element relations between Apollo 14 and 15 and other lunar samples, and the implications of a moon-wide Cl-KREEP coherence and Pt-metal noncoherence. Proc. Third Lunar Sci. Conf. 2, 1989–2001 (1972). [Google Scholar]
- 33.Tartèse R., Anand M., Joy K. H., Franchi I. A., H and Cl isotope systematics of apatite in brecciated lunar meteorites Northwest Africa 4472, Northwest Africa 773, Sayh al Uhaymir 169, and Kalahari 009. Meteorit. Planet. Sci. 49, 2266–2289 (2014). [Google Scholar]
- 34.Treiman A. H., Boyce J. W., Gross J., Guan Y., Eiler J. M., Stolper E. M., Phosphate-halogen metasomatism of lunar granulite 79215: Impact-induced fractionation of volatiles and incompatible elements. Am. Mineral. 99, 1860–1870 (2014). [Google Scholar]
- 35.J. T. Williams, Z. D. Sharp, C. K. Shearer, C. B. Agee, paper presented at the 46th Lunar and Planetary Science Conference, Houston, TX, 2015. [Google Scholar]
- 36.Day J. M. D., Moynier F., Evaporative fractionation of volatile stable isotopes and their bearing on the origin of the Moon. Philos. Trans. R. Soc. A 372, 20130259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Z. D. Sharp, C. K. Shearer, F. M. McCubbin, C. Agee, K. D. McKeegan, paper presented at the 44th Lunar and Planetary Science Conference, Houston, TX, 2013. [Google Scholar]
- 38.Wang Z., Becker H., Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 499, 328–331 (2013). [DOI] [PubMed] [Google Scholar]
- 39.A. Sarafian, H. Marschall, S. Nielsen, F. McCubbin, B. Monteleone, 45th Lunar and Planetary Institute Science Conference, Houston, TX, 2014. [Google Scholar]
- 40.Richter F. M., Davis A. M., Ebel D. S., Hashimoto A., Elemental and isotopic fractionation of type B calcium-, aluminum-rich inclusions: Experiments, theoretical considerations, and constraints on their thermal evolution. Geochim. Cosmochim. Acta 66, 521–540 (2002). [Google Scholar]
- 41.Young E. D., Galy A., The isotope geochemistry and cosmochemistry of magnesium. Rev. Mineral. Geochem. 55, 197–230 (2004). [Google Scholar]
- 42.Righter K., Drake M. J., A magma ocean on Vesta: Core formation and petrogenesis of eucrites and diogenites. Meteorit. Planet. Sci. 32, 929–944 (1997). [Google Scholar]
- 43.C. Meyer, Lunar Sample Compendium (NASA, Houston, TX, 2009). [Google Scholar]
- 44.P. E. McGee, J. L. Warner, C. H. Simonds, Introduction to the Apollo Collections. Part 1: Lunar Igneous Rocks (NASA, Washington, DC, 1977). [Google Scholar]
- 45.J. V. Smith et al., paper presented at the Apollo 11 Lunar Science Conference, Houston, TX, 1970. [Google Scholar]
- 46.J. M. Rhodes et al., paper presented at the 7th Lunar Science Conference, Houston, TX, 1976. [Google Scholar]
- 47.R. F. Dymek, A. L. Albee, A. A. Chodos, paper presented at the 6th Lunar and Planetary Science Conference, Houston, TX, 1975. [Google Scholar]
- 48.J. M. Rhodes, D. P. Blanchard, M. A. Dungan, J. C. Brannon, K. V. Rodgers, paper presented at the 8th Lunar and Planetary Science Conference, Houston, TX, 1977. [Google Scholar]
- 49.Neal C. R., Hacker M. D., Snyder G. A., Taylor L. A., Liu Y.-G., Schmitt R. A., Basait generation at the Apollo 12 site, Part 2: Source heterogeneity, multiple melts, and crustal contamination. Meteoritics 29, 349–361 (1994). [Google Scholar]
- 50.Bunch T. E., Keil K., Prinz M., Mineralogy, petrology and chemistry of lunar rock 12039. Meteoritics 7, 245–255 (1972). [Google Scholar]
- 51.P. E. Champness, A. C. Dunham, F. G. F. Gibb, H. N. Giles, W. S. MacKenzie, E. F. Stumpel, J. Zussman, paper presented at the 2nd Lunar and Planetary Science Conference, Houston, TX, 1971. [Google Scholar]
- 52.B. M. French, L. S. Walter, K. F. J. Heinrich, P. D. Lowman Jr., A. S. Doan Jr., I. Adler, Compositions of Major and Minor Minerals in Five Apollo 12 Crystalline Rocks (NASA, Greenbelt, MD, 1972). [Google Scholar]
- 53.G. M. Brown, C. H. Emeleus, J. G. Holland, A. Peckett, R. Phillips, paper presented at the 2nd Lunar Science Conference, Houston, TX, 1971. [Google Scholar]
- 54.Joy K. H., I. A. Crawford, M. Anand, R. C. Greenwood, I. A. Franchi, S. S. Russell, The petrology and geochemistry of Miller Range 05035: A new lunar gabbroic meteorite. Geochim. Cosmochim. Acta 72, 3822–3844 (2008). [Google Scholar]
- 55.Arai T., Hawke B. R., Giguere T. A., Misawa K., Miyamoto M., Kojima H., Antarctic lunar meteorites Yamato-793169, Asuka-881757, MIL 05035, and MET 01210 (YAMM): Launch pairing and possible cryptomare origin. Geochim. Cosmochim. Acta 74, 2231–2248 (2010). [Google Scholar]
- 56.McCubbin F. M., Hauri E. H., Elardo S. M., Vander Kaaden K. E., Wang J., Shearer C. K. Jr, Hydrous melting of the martian mantle produced both depleted and enriched shergottites. Geology 40, 683–686 (2012). [Google Scholar]
- 57.Boyce J. W., Eiler J. M., Channon M. C., An inversion-based self-calibration for SIMS measurements: Application to H, F, and Cl in apatite. Am. Mineral. 97, 1116–1128 (2012). [Google Scholar]
- 58.Alexander C. M. O. D., Bowden R., Fogel M. L., Howard K. T., Herd C. D. K., Nittler L. R., The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723 (2012). [DOI] [PubMed] [Google Scholar]
- 59.K. Righter, J. Gruener, Northwest Africa 773, 2700, 2727, 2977, 3160, The Lunar Meteorite Compendium; http://curator.jsc.nasa.gov/antmet/lmc/index.cfm.


