X-ray crystallography of the first and the largest AgAu nanoparticles with a doping shell and its structure-related property.
Keywords: Crystallographic structure, Alloy nanoparticle, Surface Doping, Atomically precise, Thiol, Bi-metallic, Stucture-related, Catalysis
Abstract
The structure effect is widely present in the catalysis of alloy systems. However, the surface structure of this system is still ambiguous because of the limitations of the current surface characterization tools. We reported the x-ray crystallographic structure of the first and the largest AgAu alloy nanocluster with a doping shell formulated as [Ag46Au24(SR)32](BPh4)2. This nanocluster consists of an achiral bimetallic Ag2@Au18@Ag20 core protected by a chiral Ag24Au6(SR)32 shell. The catalysis experiments further revealed that the surface structure affects the selectivity of products significantly. This is the first case to find the structure effect in atomically precise alloy nanoclusters. Our work will benefit the basic understanding of bimetal distribution, as well as the structure-related catalytic property of alloy nanoclusters at the atomic level.
INTRODUCTION
Alloy nanoparticles have emerged as a new category of nanomaterials in recent years (1). These nanoparticles were widely applied across a diverse range of fields from catalysis to sensing and biolabeling (2–12). Recent research revealed that physical and chemical properties such as catalytic activity and selectivity (13–17) as well as electrical (18) and optical properties (19) are highly structure-dependent. Thus, uncovering the structures of nanoparticles is of extreme significance and has attracted intense research efforts. Thiolate-protected atomically precise Au or Ag nanoparticles (also called nanoclusters) represent an important class of noble metal nanoparticles due to precise determination of their structures. In the past few years, great improvement has been made in understanding the surface structures and metal packing modes of these nanoclusters by using x-ray crystallography (20). On the basis of these efforts, the structural construction and the related catalytic, magnetism, luminescence, and other properties of homogold or homosilver nanoclusters have been well studied (20–33). Compared to the homometal nanoclusters, the structures of bimetallic nanoclusters have been rarely investigated. Recently, three thiolate-protected Au-Ag nanoclusters have been successfully determined by x-ray crystallography (34–36). It is interesting to find that these alloy nanoclusters retain the same frameworks as their homometal counterparts with homometallic shells (37–40). This phenomenon raises questions such as whether alloy nanoclusters with doped surfaces exist or not and how the atoms are packing within these alloy nanoparticles. The unique surface structure may provide opportunities for a better understanding of packing modes of alloy nanoparticles and structure-related properties.
Herein, we accomplished surface doping in Ag-Au nanoclusters and obtained a chiral [Ag46Au24(SR)32](BPh4)2 (R=tBu) nanocluster, which constitutes the largest bimetallic nanocluster structure thus far. The atomic structure of [Ag46Au24(SR)32](BPh4)2 nanocluster can be described as a three-shelled achiral Ag2@Au18@Ag20 core surrounded by a chiral bimetallic shell comprising of six heart-like units. The free valence electrons were calculated to 36 (70-32-2). The total structure of this thiolate-protected bimetallic nanocluster uncovers the unprecedented bimetallic Ag2Au1SR unit as well as the Ag4SR unit as the unique surface-protecting motifs. This newly found surface doping [Ag46Au24(SR)32](BPh4)2 bimetallic nanocluster provides an opportunity to study the packing mode and structure-related catalytic property in a bimetallic system at the atomic level.
RESULTS AND DISCUSSION
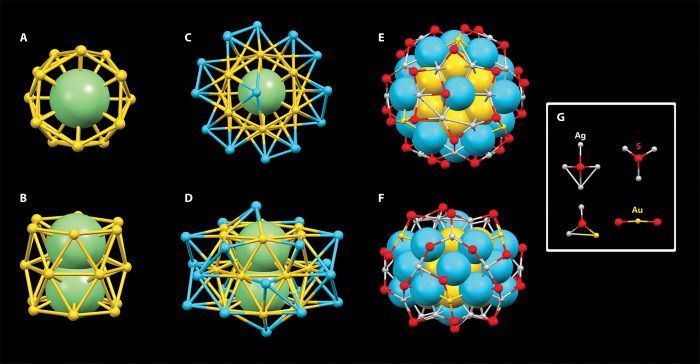
The structure of [Ag46Au24(SR)32]2+ nanocluster (counterion: two BPh4− ions) was solved by single-crystal x-ray crystallography (Fig. 1). The 70 metal atoms in this nanocluster are distributed in three shells. The central two silver atoms are surrounded by a tubbiness structure composed of 18 gold atoms (Fig. 2, A and B), which are capped with 20 silver atoms (Fig. 2, C and D). The 18 core gold atoms are distributed into three hexagons, and the overall shape resembles a barrel. Within this 18–gold atom shell, the average Au-Au distance is 2.7764 Å. The second shell consists of 20 silver atoms, with the top and bottom two silver atoms as covers of 18 core gold atoms. Among the remaining 18 silver atoms in the second shell, every six silver atoms form concentric hexagons with six gold atoms in the first shell. The average M-M distance in the Ag20Au18 core is 2.8821 Å. The Ag20Au18 core can also be considered as two types (types A and B) of layers (Fig. 2D): type A is one silver atom; type B is a hexagonal Au6 with another six vertex caps (Ag6). These two types of layers give rise to the 1:12:1:12:1:12:1 layers and can be described as nearly hexagonal close-packed A:B:A layering. Note that the similar 9:1:9 layer structure was also reported in [Au39(PPh3)14Cl6]Cl2 by Teo et al. (41).
Fig. 1. Total structure of bimetallic chiral [Ag46Au24(SR)32](BPh4)2 nanocluster (one of enantiomer) by x-ray crystallography.
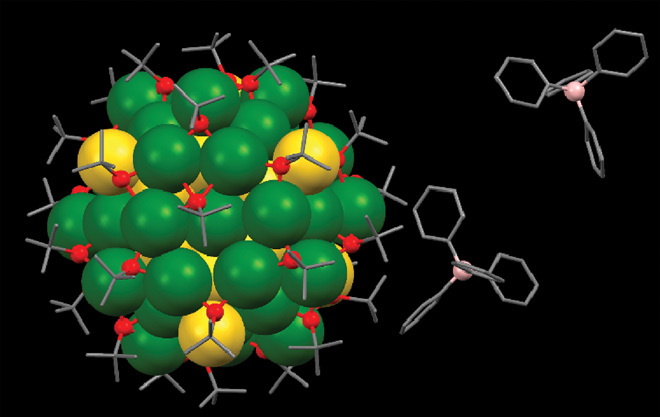
Gray, carbon; red, sulfur; green, silver; yellow, gold; pink, boron. The hydrogen atoms were omitted for clarity reasons.
Fig. 2. The three-shell structure of [Ag46Au24(SR)32]2+.
(A and B) Top and side [Ag46Au24(SR)32](BPh4)2 views of the Ag2Au18 core (which is not connected with any thiolate ligands). (C and D) Top and side views of the Ag2@Au18@Ag20 core. (E and F) Top and side views of the Ag2@Au18@Ag20 core protected by Ag24Au6(SR)32 bimetallic shell. (G) Four bonding modes in the motif structure. Light green/blue/gray, silver; yellow, gold; red, sulfur.
The Ag22Au18 core is protected by an Au-Ag bimetallic shell. The doped shell composition in our case can be seen as six eight–metal atom motifs Ag7Au1(SR)8 resembling a “heart” shape, and each motif shares an Ag3(SR)3 unit with the neighboring two hearts (Fig. 2, E and F). Four types of bonding modes are found in this nanocluster (Fig. 2G): (i) One RS connects with three Ag atoms to form Ag3SR, and this bonding mode is common in other thiolate-protected silver nanoclusters, such as Ag44 and Ag62 (40, 42, 43). (ii) One RS connects with four Ag atoms to form a Ag4SR unit. The Ag-S distance in this unit is very unusual. For example, the distance of S-Agcore bond was much shorter (2.330 Å) than that of in Ag44 nanoclusters (~2.6 Å), indicating the strong interaction between Ag and RS groups. The two longer S-Agshell (2.911 and 2.950 Å) indicates the fairly weak bonding therein, which has never been previously reported in homosilver and bimetallic nanoclusters. (iii) One RS is bonded with two Ag atoms and one Au atom to form an Ag2Au1SR, and this mode is unique in the alloy nanocluster. In this context, this mode represents the first example to identify the capability of the thiolate group in forming the bimetallic surface. (iv) Staple-like Au1(SR)2, one Au atom, and two S atoms are almost on a line, similar to the bonding mode in Au18(SR)14, Au23(SR)16, Au30(SR)18, Au102(SR)44, and Au133(SR)52 nanoclusters (44–50).
The previously reported structures of Ag-Au alloy nanoclusters are based on the same framework of the same-sized homometal nanoclusters [for example, Ag32Au12(SR)32 versus Ag44(SR)30 (34, 40), AgxAu38–x(SR)24 versus Au38(SR)24 (36, 39), and AgxAu25–x(SR)18 versus Au25(SR)18) (35, 37, 38)]. The same situation was also predicted in the case of AgxAu144–x(SR)60 nanoclusters (51). In these nanoclusters, the second metal could only be doped in the core, whereas the surface motifs are homometallic. By contrast, the [Ag46Au24(SR)32]2+ nanocluster represents the first example, which shows that silver and gold coexist in the surface motifs.
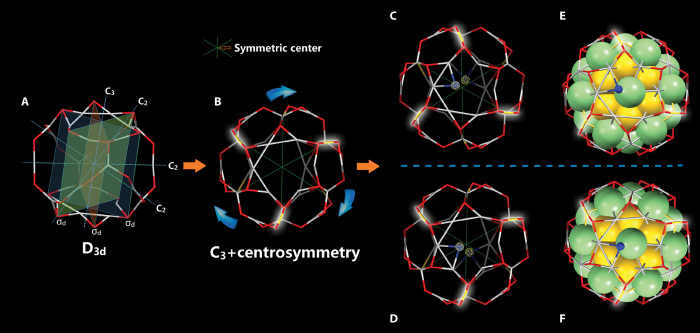
As shown in Fig. 2 (C and D), the metal core of this nanocluster is achiral. To further identify the origin of chirality in this nanocluster, we remove the metal core, the AuRS unit in the shell, as well as the top and bottom RS groups. As shown in Fig. 3A, the point group of a Ag-RS shell is D3d after removing these groups, suggesting that this shell is achiral. The tilted AuSR units reduce the symmetry by eliminating three σd, with a symmetric center remaining (Fig. 3B). The top and bottom RS groups give rise to chirality (Fig. 3, C and D) in the [Ag46Au24(SR)32](BPh4)2 nanocluster (Fig. 3, E and F). The findings on chirality in this nanocluster are remarkable. In general, two major effects might be responsible for the chiral of homometal nanoclusters: (i) the chiral ligands can induce the chiral of achiral nanoclusters (52) and (ii) asymmetric arrangement of an RS-Au-RS group on the achiral gold nanoclusters (39, 48, 49, 50, 53). In our case, asymmetric arrangement of the two RS groups changed the chirality of the nanoclusters.
Fig. 3. Chiral structure of [Ag46Au24(SR)32](BPh4)2 nanocluster.
(A and B) Top view of the shell structure without the AuSR unit and the top/bottom RS group (A) and with the addition of three AuSR groups (B). (C and D) Top views of two-enantiomer shell. (E and F) Top views of two-enantiomer nanoclusters without H and C atoms. Gray/green, silver; yellow, gold; red/blue, sulfur.
The nanocluster formula and charge state were further confirmed by nuclear magnetic resonance (NMR) analysis (fig. S1). In the 1H NMR spectrum of [Ag46Au24(SR)32](BPh4)2, the peaks at 6.5 to 7.5 ppm (40 H) are assigned to –C6H5 of Ph4B−, and the peaks at 0.5 to 4.5 ppm (288.7 H) are assigned to –CH3 in the –SBut ligands. This result is consistent with the proton ratio (C6H5:CH3=40:288) and thus indicates the 1:2 ratio of Ag46Au24(SR)32 to Ph4B−, in agreement with the x-ray crystallographic result.
With the successfully determined Ag46Au24(SR)32 nanocluster structure, a comparison with the previously reported core-shell structured Ag-Au bimetallic nanoclusters is now available. This could provide a simple model for understanding the structural effect in styrene oxidation catalyzed by the bimetallic silver-gold nanocluster.
We synthesized a series of structure-determined homometal and alloy nanoclusters, such as Au25(SR)18, Ag44(SR)30, Ag32Au12(SR)30, and Ag46Au24(SR)32 nanoclusters (see the Supplementary Materials for details). Multiwalled carbon nanotubes (CNTs) were used as the common carrier because of the fact that all of these nanoclusters can be efficiently adsorbed on the CNT surface. In addition, CNTs have an advantage to prevent diffusion of naked nanoclusters (54). Transmission electron microscopy (TEM) (figs. S2 and S3) showed that the clusters were evenly spread on CNTs and that all nanoclusters have average sizes of about 2 nm before and after reaction. This indicates negligible aggregation of clusters owing to sufficient interaction between the nanocluster and CNT (9).
Table 1 summarizes the results. Epoxide and benzaldehyde were the major products in the styrene oxidation. All catalysts showed high activity compared to plain CNT. Homogold Au25/CNT showed the highest conversion of styrene, that is, 72.8%, whereas the selectivity for benzaldehyde is 66.4%. The homosilver Ag44/CNT showed a much lower conversion (that is, 43.6%) than the homogold nanocluster did but it exhibited a better selectivity for benzaldehyde (that is, 92.6%). Compared with the homometal nanoclusters, surface doping bimetallic Au24Ag46/CNT (Table 1, entry 3) catalysts could increase the selectivity for epoxide (that is, >95%) and give much better conversion (that is, ~70%) than the homosilver nanocluster does. Therefore, the advantages of both the silver (high selectivity for benzaldehyde) and the gold (high conversion) have been well reflected on the surface doping Ag46Au24/CNT catalyst. In contrast, the core-shell structured bimetallic Ag32Au12 nanocluster shows a much lower benzaldehyde selectivity (that is, 37.6%) than does the surface doping catalyst. This finding is remarkable in pioneering investigations on the structure effect in atomically precise alloy nanoclusters and demonstrates a clear synergistic effect of the AgAu alloy catalysts (Scheme 1).
Table 1. The catalytic performance of CNT-supported metal nanoclusters.
Reaction conditions: 20 mg of catalysis, 2 wt % nanocluster loading, 57 μl (0.5 mmol) of styrene, 144 μl (1.5 mmol) of tert-butyl hydroperoxide (TBHP), 1.5 ml of ethanol, 65°C, 24 hours.
| Selectivity (%)† | |||||
| Entry | Catalyst | Conversion (%)* | Epoxide | Benzaldehyde | Other products |
| 1 | Au25/CNT | 72.8 | 20.3 | 66.4 | 13.3 |
| 2 | Ag44/CNT | 43.6 | 6.1 | 92.6 | 1.3 |
| 3 | Ag46Au24/CNT | 68.2 | 1.2 | 96.3 | 2.5 |
| 4 | Ag32Au12/CNT | 69.7 | 57.6 | 37.6 | 4.8 |
| 5 | CNT | 31.7 | 30.5 | 55.62 | 13.88 |
*Conversion = (styrene converted)/(initial amount of styrene) × 100.
†Determined by gas chromatography.
Scheme 1. Two different kinds of nanocluster catalysts in the styrene oxidization.
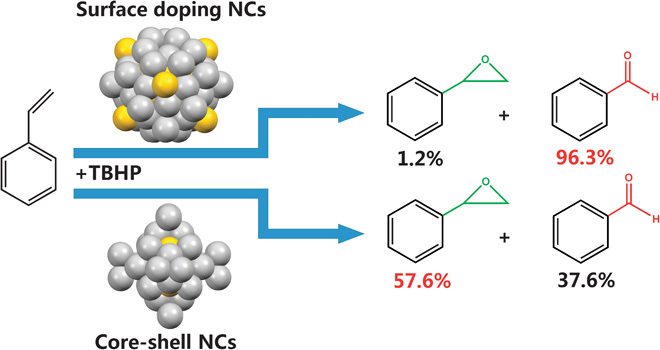
The surface-doped nanocluster (NC) shows high selectivity for benzaldehyde, whereas the core-shell structured nanocluster shows high selectivity for epoxide.
In conclusion, we obtained the x-ray crystal structure of a new magic number bimetallic (Au-Ag) nanocluster formulated as [Ag46Au24(SR)32](BPh4)2 and further investigated its catalytic properties. This nanocluster fills the vacancy between Ag44 and Au102 nanoclusters. The newly found bimetallic shell holds potential in expanding the library of magic-sized nanoclusters as well as the understanding structure-related properties of thiolate-protected alloy nanoclusters at the atomic level by studying styrene oxidization catalysis.
MATERIALS AND METHODS
The detailed information about the synthesis of [Ag46Au24(SR)32](BPh4)2 nanocluster is given in the Supplementary Materials. In summary, AgNO3 and HAuCl4⋅3H2O were dissolved in methanol to give a yellow turbid liquid. Tertiary butyl was added into the solution to obtain the mixture of AuI-SR and AgI-SR complex. Then, NaOH (1 M) was used to adjust the pH value. After that, NaBH4 was used to reduce this mixture complex. The Ag46Au24(SR)322+ nanoclusters were precipitated out from the solution, washed by hexane, extracted by toluene, and redissolved in CH2Cl2 solution. The addition of NaBPh4 (dissolved in methanol) formed [Ag46Au24(SR)32](BPh4)2 nanocluster. The multiwalled CNT was dispersed in toluene, and a calculated amount (0.1 wt %) of cluster was added to the suspension of CNT under vigorous magnetic stirring. After proceeding overnight, the product was separated from the solution by centrifugation and dried under vacuum for 12 hours. Calcination of the Au25:SR/CNT, Ag44:SR/CNT, and Ag46Au24/CNT composites was performed in a quartz-tube oven under vacuum conditions at 200°C for 2 hours to remove the ligands. The detailed method and characterization are available in the Supplementary Materials.
Supplementary Material
Funding
We acknowledge financial support by the National Natural Science Foundation of China (21072001, 21201005, and 21372006), the Ministry of Education and Ministry of Human Resources and Social Security, the Education Department of Anhui Province, Anhui Province International Scientific and Technological Cooperation Project, and 211 Project of Anhui University. Author contributions: S.W. and S.J. conceived and carried out the synthesis and crystallization of the clusters. S.W. and S.Y. conceived and carried out the catalytic reaction. S.C. and Y.S. assisted in the synthesis. J.Z. analyzed the crystal data of the clusters. M.Z. designed the study, supervised the project, analyzed data, and wrote the paper. Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/7/e1500441/DC1
Materials
Fig. S1. 1H NMR spectrum of [Ag46Au24(StBu)32](BPh4)2 nanoclusters (single crystal dissolved in CD2Cl2).
Fig. S2. Typical TEM images and cluster size distributions of (a) Au25/CNT, (b) Ag44/CNT, (c) Ag32Au12/CNT, (d) Ag46Au24/CNT before reaction.
Fig. S3. Typical TEM images and cluster size distributions of (a) Au25/CNT, (b) Ag44/CNT, (c) Ag32Au12/CNT, (d) Ag46Au24/CNT after reaction.
Fig. S4. The digital photo of the [Ag46Au24(StBu)32](BPh4)2 crystals.
Fig. S5. The UV-Vis spectra of (a) Au25(SC2H4Ph)18−(b)Ag44(SPhF2)304−; (c) Ag32Au12(SPhF2)4−; (d) [Ag46Au24(StBu)32](BPh4)2 nanoclusters dissolved in dichloromethane solution.
Table S1. Crystal data and structure refinement for [Ag46Au24(StBu)32](BPh4)2 nanoclusters.
Table S2. Atomic coordinates (× 104) and equivalent isotropic displacement parameters (Å2 × 103) for [Ag46Au24(StBu)32](BPh4)2.
Table S3. Bond lengths (Å) and angles (°) for [Ag46Au24(StBu)32](BPh4)2 nanoclusters.
Table S4. Anisotropic displacement parameters (Å2 × 103) for [Ag46Au24(StBu)32](BPh4)2.
REFERENCES AND NOTES
- 1.Ferrando R., Jellinek J., Johnston R. L., Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Ghosh Chaudhuri R., Paria S., Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 112, 2373–2433 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Link S., Wang Z. L., El-Sayed M. A., Alloy formation of gold–silver nanoparticles and the dependence of the plasmon absorption on their composition. J. Phys. Chem. B 103, 3529–3533 (1999). [Google Scholar]
- 4.Pradeep T., Anshup, Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 517, 6441–6478 (2009). [Google Scholar]
- 5.Sugano Y., Shiraishi Y., Tsukamoto D., Ichikawa S., Tanaka S., Hirai T., Supported Au–Cu bimetallic alloy nanoparticles: An aerobic oxidation catalyst with regenerable activity by visible-light irradiation. Angew. Chem. Int. Ed. 52, 5295–5299 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Wang D., Villa A., Porta F., Su D., Prati L., Single-phase bimetallic system for the selective oxidation of glycerol to glycerate. Chem. Commun. 1956–1958 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Bracey C. L., Ellis P. R., Hutchings G. J., Application of copper–gold alloys in catalysis: Current status and future perspectives. Chem. Soc. Rev. 38, 2231–2243 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Hervés P., Pérez-Lorenzo M., Liz-Marzán L. M., Dzubiella J., Lu Y., Ballauff M., Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 41, 5577–5587 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Xie S., Tsunoyama H., Kurashige W., Negishi Y., Tsukuda T., Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping. ACS Catal. 2, 1519–1523 (2012). [Google Scholar]
- 10.Zhang H., Watanabe T., Okumura M., Haruta M., Toshima N., Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 11, 49–52 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Kesavan L., Tiruvalam R., Ab Rahim M. H., bin Saiman M. I., Enache D. I., Jenkins R. L., Dimitratos N., Lopez-Sanchez J. A., Taylor S. H., Knight D. W., Kiely C. J., Hutchings G. J., Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles. Science 331, 195–199 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Wang D., Li Y., Bimetallic nanocrystals: Liquid-phase synthesis and catalytic applications. Adv. Mater. 23, 1044–1060 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Wang Z. L., Ahmad T. S., El-Sayed M. A., Steps, ledges and kinks on the surfaces of platinum nanoparticles of different shapes. Surf. Sci. 380, 302–310 (1997). [Google Scholar]
- 14.Lee H., Habas S. E., Kweskin S., Butcher D., Somorjai A., Yang P., Morphological control of catalytically active platinum nanocrystals. Angew. Chem. Int. Ed. 45, 7824–7828 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Nilekar A. U., Alayoglu S., Eichhorn B., Mavrikakis M., Preferential CO oxidation in hydrogen: Reactivity of core–shell nanoparticles. J. Am. Chem. Soc. 132, 7418–7428 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Alayoglu S., Nilekar A. U., Mavrikakis M., Eichhorn B., Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 7, 333–338 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Chen M., Kumar D., Yi C., Goodman D. W., The promotional effect of gold in catalysis by palladium-gold. Science 310, 291–293 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Hong J. W., Kim D., Lee Y. W., Kim M., Kang S. W., Han S. W., Atomic-distribution-dependent electrocatalytic activity of Au–Pd bimetallic nanocrystals. Angew. Chem. Int. Ed. 50, 8876–8880 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Njoki P. N., Wu W., Lutz P., Maye M. M., Growth characteristics and optical properties of core/alloy nanoparticles fabricated via the layer-by-layer hydrothermal route. Chem. Mater. 25, 3105–3113 (2013). [Google Scholar]
- 20.Jin R., Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 7, 1549–1565 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Yamazoe S., Koyasu K., Tsukuda T., Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 47, 816–824 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Li G., Jin R., Atomically precise gold nanoparticles as new model catalysts. Acc. Chem. Res. 46, 1749–1758 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Wang S., Meng X., Das A., Li T., Song Y., Cao T., Zhu X., Zhu M., Jin R., A 200-fold quantum yield boost in the photoluminescence of silver-doped AgxAu25–x nanoparticles: The 13th silver atom matters. Angew. Chem. Int. Ed. 53, 2376–2380 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Zhu M., Aikens C. M., Hendrich M. P., Gupta R., Qian H., Schatz G. C., Jin R., Reversible switching of magnetism in thiolate-protected Au25 superatoms. J. Am. Chem. Soc. 131, 2490–2492 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Antonello S., Perera N. V., Ruzzi M., Gascón J. A., Maran F., Interplay of charge state, lability, and magnetism in the molecule-like Au25(SR)18 cluster. J. Am. Chem. Soc. 135, 15585–15594 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Devadas M. S., Kim J., Sinn E., Lee D., Goodson T. III, Ramakrishna G., Unique ultrafast visible luminescence in monolayer-protected Au25 clusters. J. Phys. Chem. C 114, 22417–22423 (2010). [Google Scholar]
- 27.Park S., Lee D., Synthesis and electrochemical and spectroscopic characterization of biicosahedral Au25 clusters. Langmuir 28, 7049–7054 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Walter M., Akola J., Lopez-Acevedo O., Jadzinsky P. D., Calero G., Ackerson C. J., Whetten R. L., Grönbeck H., Häkkinen H., A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl. Acad. Sci. U.S.A. 105, 9157–9162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathew A., Natarajan G., Lehtovaara L., Häkkinen H., Kumar R. M., Subramanian V., Jaleel A., Pradeep T., Supramolecular functionalization and concomitant enhancement in properties of Au25 clusters. ACS Nano 8, 139–152 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Devadas M. S., Thanthirige V. D., Bairu S., Sinn E., Ramakrishna G., Temperature-dependent absorption and ultrafast luminescence dynamics of bi-icosahedral Au25 clusters. J. Phys. Chem. C 117, 23155–23161 (2013). [Google Scholar]
- 31.Li L., Liu H., Shen Y., Zhang J., Zhu J., Electrogenerated chemiluminescence of Au nanoclusters for the detection of dopamine. Anal. Chem. 83, 661–665 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Sfeir M. Y., Qian H., Nobusada K., Jin R., Ultrafast relaxation dynamics of rod-shaped 25-atom gold nanoclusters. J. Phys. Chem. C 115, 6200–6207 (2011). [Google Scholar]
- 33.Dolamic I., Knoppe S., Dass A., Bürgi T., First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. Nat. Commun. 3, 798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H., Wang Y., Huang H., Gell L., Lehtovaara L., Malola S., Häkkinen H., Zheng N., All-thiol-stabilized Ag44 and Au12Ag32 nanoparticles with single-crystal structures. Nat. Commun. 4, 2422 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Kumara C., Aikens C. M., Dass A., X-ray crystal structure and theoretical analysis of Au25–xAgx(SCH2CH2Ph)18– alloy. J. Phys. Chem. Lett. 5, 461–466 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Kumara C., Gagnon K. J., Dass A., X-ray crystal structure of Au38–xAgx(SCH2CH2Ph)24 alloy nanomolecules. J. Phys. Chem. Lett. 6, 1223–1228 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Zhu M., Aikens C. M., Hollander F. J., Schatz G. C., Jin R., Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 130, 5883–5885 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Heaven M. W., Dass A., White P. S., Holt K. M., Murray R. W., Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18]. J. Am. Chem. Soc. 130, 3754–3755 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Qian H., Eckenhoff W. T., Zhu Y., Pintauer T., Jin R., Total structure determination of thiolate-protected Au38 nanoparticles. J. Am. Chem. Soc. 132, 8280− 8281 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Desireddy A., Conn B. E., Guo J., Yoon B., Barnett R. N., Monahan B. M., Kirschbaum K., Griffith W. P., Whetten R. L., Landman U., Bigioni T. P., Ultrastable silver nanoparticles. Nature 501, 399–402 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Teo B. K., Shi X., Zhang H., Pure gold cluster of 1:9:9:1:9:9:1 layered structure: A novel 39-metal-atom cluster [(Ph3P)14Au39Cl6]Cl2 with an interstitial gold atom in a hexagonal antiprismatic cage. J. Am. Chem. Soc. 114, 2743–2745 (1992). [Google Scholar]
- 42.Li G., Lei Z., Wang Q., Luminescent molecular Ag–S nanocluster [Ag62S13(SBut)32](BF4)4. J. Am. Chem. Soc. 132, 17678–17679 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Jin S., Wang S., Song Y., Zhou M., Zhong J., Zhang J., Xia A., Pei Y., Chen M., Li P., Zhu M., Crystal structure and optical properties of the [Ag62S12(SBut)32]2+ nanocluster with a complete face-centered cubic kernel. J. Am. Chem. Soc. 136, 15559–15565 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Das A., Liu C., Byun H. Y., Nobusada K., Zhao S., Rosi N. L., Jin R., Structure determination of [Au18(SR)14]. Angew. Chem. Int. Ed. 54, 3140–3144 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Chen S., Wang S., Zhong J., Song Y., Zhang J., Sheng H., Pei Y., Zhu M., The structure and optical properties of the [Au18(SR)14] nanocluster. Angew. Chem. Int. Ed. 54, 3145–3149 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Das A., Li T., Nobusada K., Zeng C., Rosi N. L., Jin R., Nonsuperatomic [Au23(SC6H11)16]− nanocluster featuring bipyramidal Au15 kernel and trimeric Au3(SR)4 motif. J. Am. Chem. Soc. 135, 18264–18267 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Crasto D., Malola S., Brosofsky G., Dass A., Häkkinen H., Single crystal XRD structure and theoretical analysis of the chiral Au30S(S-t-Bu)18 cluster. J. Am. Chem. Soc. 136, 5000–5005 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Jadzinsky P. D., Calero G., Ackerson C. J., Bushnell D. A., Kornberg R. D., Structure of a thiol monolayer–protected gold nanoparticle at 1.1 Å resolution. Science 318, 430–433 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Heinecke C. L., Ni T. W., Malola S., Mäkinen V., Wong O. A., Häkkinen H., Ackerson C. J., Structural and theoretical basis for ligand exchange on thiolate monolayer protected gold nanoclusters. J. Am. Chem. Soc. 134, 13316–13322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng C., Chen Y., Kirschbaum K., Appavoo K., Sfeir M. Y., Jin R., Structural patterns at all scales in a nonmetallic chiral Au133(SR)52 nanoparticle. Sci. Adv. 1, e1500045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malola S., Häkkinen H., Electronic structure and bonding of icosahedral core-shell gold-silver nanoalloy clusters Au144–xAgx(SR)60. J. Phys. Chem. Lett. 2, 2316–2321 (2011). [Google Scholar]
- 52.Zhu M., Qian H., Meng X., Jin S., Wu Z., Jin R., Chiral Au25 nanospheres and nanorods: Synthesis and insight into the origin of chirality. Nano Lett. 11, 3963–3969 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Zeng C., Li T., Das A., Rosi N. L., Jin R., Chiral structure of thiolate-protected 28-gold-atom nanocluster determined by X-ray crystallography. J. Am. Chem. Soc. 135, 10011–10013 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Zanolli Z., Leghrib R., Felten A., Pireaux J., Llobet E., Charlier J., Gas sensing with Au-decorated carbon nanotubes. ACS Nano 5, 4592–4599 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/7/e1500441/DC1
Materials
Fig. S1. 1H NMR spectrum of [Ag46Au24(StBu)32](BPh4)2 nanoclusters (single crystal dissolved in CD2Cl2).
Fig. S2. Typical TEM images and cluster size distributions of (a) Au25/CNT, (b) Ag44/CNT, (c) Ag32Au12/CNT, (d) Ag46Au24/CNT before reaction.
Fig. S3. Typical TEM images and cluster size distributions of (a) Au25/CNT, (b) Ag44/CNT, (c) Ag32Au12/CNT, (d) Ag46Au24/CNT after reaction.
Fig. S4. The digital photo of the [Ag46Au24(StBu)32](BPh4)2 crystals.
Fig. S5. The UV-Vis spectra of (a) Au25(SC2H4Ph)18−(b)Ag44(SPhF2)304−; (c) Ag32Au12(SPhF2)4−; (d) [Ag46Au24(StBu)32](BPh4)2 nanoclusters dissolved in dichloromethane solution.
Table S1. Crystal data and structure refinement for [Ag46Au24(StBu)32](BPh4)2 nanoclusters.
Table S2. Atomic coordinates (× 104) and equivalent isotropic displacement parameters (Å2 × 103) for [Ag46Au24(StBu)32](BPh4)2.
Table S3. Bond lengths (Å) and angles (°) for [Ag46Au24(StBu)32](BPh4)2 nanoclusters.
Table S4. Anisotropic displacement parameters (Å2 × 103) for [Ag46Au24(StBu)32](BPh4)2.