A novel explanation of why certain pupil shapes are visually advantageous for terrestrial animals in different ecological niches.
Keywords: pupil, eye, aperture, stereopsis, depth of field, blur, chromatic aberration, evolution, anatomy
Abstract
There is a striking correlation between terrestrial species’ pupil shape and ecological niche (that is, foraging mode and time of day they are active). Species with vertically elongated pupils are very likely to be ambush predators and active day and night. Species with horizontally elongated pupils are very likely to be prey and to have laterally placed eyes. Vertically elongated pupils create astigmatic depth of field such that images of vertical contours nearer or farther than the distance to which the eye is focused are sharp, whereas images of horizontal contours at different distances are blurred. This is advantageous for ambush predators to use stereopsis to estimate distances of vertical contours and defocus blur to estimate distances of horizontal contours. Horizontally elongated pupils create sharp images of horizontal contours ahead and behind, creating a horizontally panoramic view that facilitates detection of predators from various directions and forward locomotion across uneven terrain.
INTRODUCTION
Pupils come in a variety of shapes. Why do some animals have vertical pupils, whereas others have round or horizontal? We examined the optical consequences of terrestrial animals’ pupil shape in the context of their ecological niche. We found a striking correlation between pupil shape and ecological niche (Fig. 1). Consider three previous hypotheses about the function of elongated pupils.
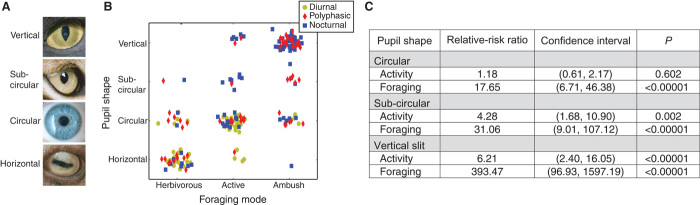
Fig. 1. Activity time, foraging mode, and pupil shape.
(A) Different pupil shapes. From top to bottom: vertical-slit pupil of the domestic cat, vertically elongated (subcircular) pupil of the lynx, circular pupil of man, and horizontal pupil of the domestic sheep. (B) Pupil shape as a function of foraging mode and diel activity. The axes are pupil shape [vertically elongated, subcircular (but elongated vertically), circular, or horizontally elongated] and foraging mode (herbivorous prey, active predator, or ambush predator). Each dot represents a species. Colors represent diel activity: yellow, red, and blue for diurnal, polyphasic, and nocturnal, respectively. The dots in each bin have been randomly offset to avoid overlap. (C) Results of statistical tests on the relationship between foraging, activity, and pupil shape. Multinomial logistic regression tests were conducted with foraging mode, activity time, and pupil shape as factors and genus as a covariate. Relative-risk ratios were computed for having a circular, subcircular, or vertical-slit pupil relative to having a horizontal pupil as a function of foraging mode or diel activity. Activity time proceeded from diurnal to polyphasic to nocturnal. Foraging mode proceeded from herbivorous prey to active predator to ambush predator. When the relative-risk ratio is greater than 1, the directional change in the independent variable (foraging or activity) was associated with a greater probability of having the specified pupil shape than a horizontal pupil.
Control of retinal illumination in different light environments
Retinal illumination is the product of pupil area and incident light intensity. Thus, pupil dilation and constriction respectively increases and decreases retinal illumination affording rudimentary adaptation to different light environments. Constriction of circular pupils is achieved by ring-shaped muscles, whereas closure of slit pupils involves two additional muscles that laterally compress the opening, allowing much greater change in area (1, 2). For example, the vertical-slit pupils of the domestic cat and gecko undergo area changes of 135- and 300-fold (3–5), respectively, whereas humans’ circular pupil changes by ~15-fold (6). Species that are active in night and day need to dilate sufficiently under dim conditions while constricting enough to prevent dazzle in daylight. A slit pupil provides the required dynamic range.
This hypothesis is persuasive. It explains why pupils are elongated in species that require more light regulation than other species. However, the hypothesis only explains why some species evolved elongated pupils, not why they are vertical in some species and horizontal in others.
Increased depth of field for certain contours
Brischoux and colleagues (7) and Heath and colleagues (8) discussed the utility of vertical-slit pupils in some reptiles. They claimed that the image formed by a vertical pupil has a greater depth of field for horizontal contours and thereby ensures sharp focus of horizontals across a range of distances [Fig. 8 in (8)]. This claim is unfortunately false. The depth of field for horizontal contours is determined by the vertical extent of the pupil; thus, with a vertical slit, the depth of field will be greater for vertical contours, not horizontal. Even if the proponents of this hypothesis corrected the error concerning depth of field, it does not explain why vertical elongation is functionally adaptive for some species and horizontal elongation is for others.
Maintain correction for chromatic aberration
Simple lenses focus different wavelengths at different distances: for example, blue at nearer distance than red. This chromatic aberration produces noteworthy blur in images containing a wide range of wavelengths. Kröger and colleagues (9, 10) proposed that some animal eyes minimize blur due to chromatic aberration with a multifocal lens. This lens has concentric zones of different focal lengths, with each zone focusing a different wavelength band onto the retina. They argued that the multifocal arrangement is useful because it allows reasonable image sharpness across a range of wavelengths at the expense of some contrast: “dividing the lens into three zones of equal aperture areas and focusing a specific wavelength improves the functionality of the lens in comparison to a monofocal lens” [(10), p. 1792]. When a circular pupil constricts, the peripheral zones of a multifocal lens are no longer involved in image formation, thus preventing the suggested improvement in image quality. Malmström and Kröger (11) hypothesized that the slit pupil is an adaptation for maintaining image quality because when the pupil constricts to a slit, the peripheral zones of the lens remain involved in image formation.
Even if the proposed benefit for image quality does in fact occur with slit pupils, this hypothesis applies only to species with multifocal lenses and, more importantly, does not explain why slit pupils are elongated vertically in some species and horizontally in others.
RESULTS
Figure 1A provides examples from top to bottom of vertical-slit, subcircular, circular, and horizontal pupils. The vertically elongated pupils in the first category can be adequately described as slits, but the horizontally elongated pupils in the fourth category cannot; horizontally elongated pupils are roughly rectangular and their aspect ratio changes with dilation and constriction (1). Interestingly, there were no terrestrial species for which we could obtain the relevant data that had diagonally elongated pupils.
Figure 1B plots pupil shape as a function of foraging mode and diel activity for our database. There is a clear relationship between ecological niche and the shape of the pupil. For example, herbivorous (prey) animals are very likely to have horizontal pupils, and most diurnal predators have circular pupils. Additionally, nocturnal and polyphasic ambush predators generally have vertical-slit pupils, which was previously documented for snakes (7) and described somewhat informally for other species (1). Figure S1 is an interactive version of Fig. 1B, table S1 is a list of the species.
Figure 1C shows the results of a multinomial logistic regression using foraging mode and diel activity to predict pupil shape. (More detailed tables and descriptions are provided in tables S2 and S3.) The relative-risk ratios in Fig. 1C indicate the increase in the likelihood of having the specified pupil shape, relative to horizontal, when the indicated niche parameter was incremented from the lowest to the highest value and the other niche parameter was held constant. There was a highly significant increase in the probability of vertical-slit pupils as animals moved from being herbivorous (prey) to ambush predators. There were also very significant increases in the probability of subcircular and circular pupils going from prey to ambush predator. Additionally, there were significant increases in the probability of vertical-slit and subcircular pupils when animals moved from diurnal to nocturnal. The overall effect of foraging mode and diel activity in predicting pupil shape was highly significant: χ2 = 219.9; P < 1 × 10−15.
Nearly half the animals in our database are snakes. We asked if the relationship between niche and pupil shape persists when snakes are removed. Indeed, it does: The same trends were statistically reliable, and the overall relationship between foraging mode, diel activity, and pupil shape remained highly significant: χ2 = 102.5; P < 1 × 10−15.
The strong relationship between foraging mode and activity time on the one hand and pupil shape on the other suggests that there are functional advantages for particular pupil types in certain ecological niches. Our goal is to determine what those advantages are. That is, why would a horizontally elongated pupil be advantageous for prey and a vertically elongated pupil be advantageous for ambush predators who are active at night and day? To answer these questions, we analyzed the optical properties of these eyes and visual requirements in different niches.
We describe new hypotheses for the functional advantages of elongated pupils: one for vertical elongation first and then one for horizontal.
Vertical-slit pupils
Consider a viewer fixating and focusing on a point at distance z0. Another point at a distance z1 creates a blurred image. The diameter of the blur circle on the retina for that point is:
| (1) |
where A is the diameter of the pupillary aperture and s0 is the distance from the aperture to the retina (12). Using the small-angle approximation, the eye-length term s0 drops out, yielding blur-circle diameter in radians:
| (2) |
where ΔD is the difference between distances z0 and z1 in diopters (12). Thus, blur is proportional to aperture diameter and to the difference in diopters between the eye’s focal distance and the point of interest. These equations incorporate geometric blur due to defocus and not blur due to the eye’s aberrations including diffraction (13). Incorporating aberrations yields more blur, but only for object distances at or very close to the focal distance: that is, where ΔD ≈ 0 (14). We are most interested in blur caused by significant defocus, so we will ignore aberrations henceforth.
Now consider an elongated pupil with vertical extent Av and horizontal extent Ah. With the eye focused at z0, the retinal images of contours at z1 are blurred differently, depending on their orientation. For example, the blur of the vertical and horizontal limbs of a cross (Fig. 2B) is determined by Ah and Av, respectively:
| (3) |
| (4) |
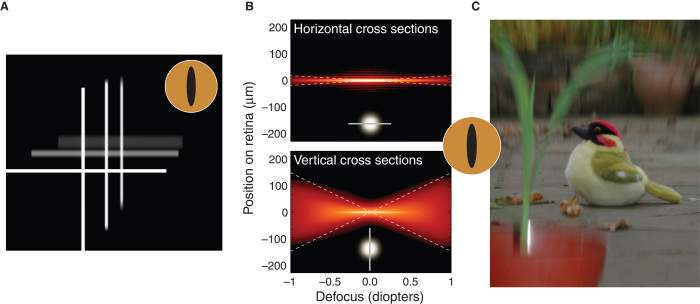
Thus, eyes with vertical-slit pupils have astigmatic depth of field: larger (that is, less blur due to defocus) for vertical than for horizontal contours. Objects in front of and behind the eye’s focal distance are differently blurred such that the retinal images of horizontal contours are more blurred than the images of verticals (Fig. 2A). Figure 2B shows that the equations provide a good approximation of image blur for different pupil orientations and defocus (meaning that diffraction and other aberrations make small contributions to image quality when the eye is defocused). Figure 2C shows astigmatic depth of field for a natural scene (see movie S1 for more details; note that this phenomenon is not the same as astigmatism, a common source of defocus in eyes).
Fig. 2. Image quality for different amounts of defocus and pupil shapes.
(A) Astigmatic depth of field with vertical-slit pupil (12 × 1.5 mm). Three crosses are presented at different distances (0D, 0.4D, and 0.8D). The camera is focused on the nearest cross, so the other two are farther than the focal plane. The vertical limbs of all three crosses are relatively sharp, whereas the horizontal limbs of the two farther crosses are quite blurred. (B) Horizontal and vertical cross sections of point spread functions (PSFs) as a function of focal distance for an eye with a vertical-slit pupil (12 × 1.5 mm). The object was white. The PSFs incorporate diffraction and chromatic aberration. Log intensity in the PSF is represented by brightness (brighter corresponding to higher amplitude). Intensities lower than 10−3 of the peak amplitude have been clipped. The upper panel shows horizontal cross sections (relevant for imaging vertical contours). The icon in the lower middle of the panel represents the cross sections by a nominal PSF with a horizontal cut through it. The lower panel shows vertical cross sections (for imaging horizontals). The icon in the lower middle of the panel represents those cross sections. The dashed white lines are from Eqs. 3 and 4 and show that the equations are a good approximation to the PSF cross sections. (C) Photograph of a depth-varying scene taken with a camera with a vertical-slit aperture. The camera was focused on the toy bird, so objects nearer and farther are blurred, but more vertically than horizontally because of the aperture elongation. Movie S2 shows PSF cross sections and the scene as the aperture rotates from vertical to horizontal and back to vertical.
From Fig. 1, we observe that vertically elongated pupils are much more common in ambush predators than in other species. These animals must estimate the distance to potential prey accurately. Three depth cues, all based on triangulation, can in principle provide the required metric distance estimate: (i) stereopsis (binocular disparity created by two vantage points), (ii) motion parallax (image differences created by moving the vantage point), and (iii) defocus blur (differences created by projecting through different parts of the pupil) (12, 15). Ambush predators cannot use motion parallax because head movements would reveal their position to potential prey. They must rely on stereopsis and defocus blur. Horizontal disparity, the primary depth signal in stereopsis, is proportional to the interocular separation (I) and the difference in dioptric distance between the fixation point and a point of interest (ΔD):
| (5) |
where the disparity δ is in radians (12). From Eq. 2, blur is also proportional to the dioptric difference in distance between the fixated (and presumably focused) point and a point of interest, and to the aperture size (A). The smallest depth intervals ΔDt that can be accurately assessed from disparity and blur are:
| (6) |
where δcrit and βcrit are the smallest discriminable changes in disparity and blur, respectively (16). Thus, as the baseline for triangulation (I or A) increases, the accuracy of depth estimation should increase as well. Stereopsis was classically thought of as a relative distance cue, but is now understood to provide absolute distance information at all but long distances (17). Similarly, blur can provide absolute distance information provided that the fixation (and therefore accommodation) distance is known, which can be estimated from the eyes’ vergence (18).
To use stereopsis, these animals must determine which feature in one eye should be matched with a given feature in the other eye. Horizontal displacements are more readily measured with vertical than with horizontal contours, so stereopsis is understandably most precise for contours that are approximately vertical (19, 20). This is probably why orientation preferences among binocular cortical neurons serving the central visual field tend toward vertical (21, 22). Blur reduces the precision of stereopsis (23). The vertical-slit pupil aligns the orientation of the larger depth of field (that is, less blur) with the vertical contours of potential prey. This is advantageous for frontal-eyed, ambush predators because it facilitates stereopsis while allowing large changes in pupil area and thereby effectively controlling the amount of light striking the retinas (1, 2).
Horizontal contours are commonplace for terrestrial animals. With gaze along the ground, retinal images are foreshortened vertically, so the prevalence of horizontal or nearly horizontal contours in those images increases (24). A vertically elongated pupil provides a short depth of field for horizontals and thus aids the use of defocus blur for estimating distances of horizontal contours along the ground (Eq. 6), providing useful depth information for contour orientations that are problematic for stereopsis.
We conclude that the vertically elongated pupil is a clever adaptation that facilitates stereopsis for estimating distances of objects perched on the ground while simultaneously enabling depth from blur to estimate distances along the ground. The horizontal baseline for depth from disparity is determined by the interocular separation and is unaffected by pupil orientation. The vertical-slit pupil enables a relatively large vertical baseline for depth from blur. Thus, this arrangement of horizontally separated eyes and vertically elongated pupils facilitates depth estimation for contours of any orientation. If instead the pupils were elongated horizontally, the ability to estimate distances of both vertical and horizontal contours would suffer. Thus, many frontal-eyed, ambush predators may use disparity and blur in complementary fashion to perceive three-dimensional layout, much as humans do (16).
The vertical-slit hypothesis predicts that eye height among frontal-eyed, ambush predators might affect the probability of having a vertically elongated pupil. In Fig. 3A, two viewers with different eye heights fixate points along the ground. The eyes are focused at distance z0: nearer for cats than humans. Rays above and below the fixation axis intersect the ground at distances z1+ and z1−, respectively (red and green). The difference in distances (in diopters) between the fixation axis and the axes above and below fixation are plotted in Fig. 3B. Different curves correspond to different eye heights. Except close to the feet, there is essentially no effect of how far along the ground the viewer fixates. Thus, the major determinant of dioptric difference for an eye with fixed pupil size is the height of the eye above the ground.
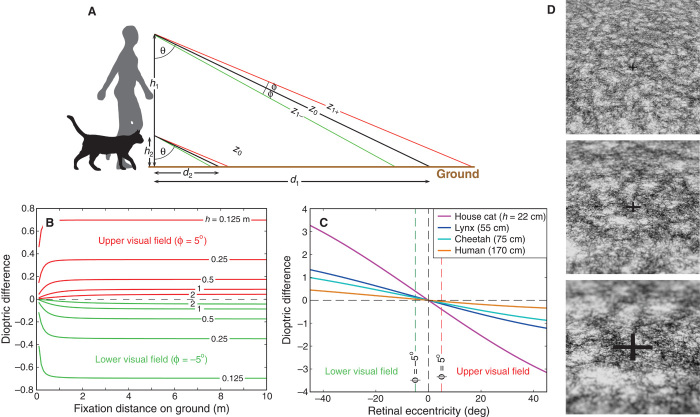
Fig. 3. Height and defocus.
(A) Two viewers—human and domestic cat—with different eye heights, h1 and h2, fixate the ground. Fixation direction relative to earth vertical is θ. Fixation distances along the ground are d1 and d2, and distances along the lines of sight are z0. The eyes are focused at z0, so points above and below the fixation point are defocused. (B) Defocus (difference in dioptric distances: 1/z0 − 1/z1+ and 1/z0 − 1/z1−) as a function of fixation distance along the ground. Red and green curves correspond to the defocus 5° above and below fixation, respectively (ϕ = ±5°). Different curves represent different eye heights. How does pupil size vary with eye height? In vertebrates, A ∝ M0.196, where A is axial length and M is body mass (26). In quadrapeds, L ∝ M0.40, where L is limb length, an excellent proxy for eye height (27). Combining those equations, A ∝ L0.49, which means that axial length is proportional to the square root of eye height. Under the assumption that pupil size is proportional to eye size, the analysis shows that the defocus signal is indeed weaker in taller animals. (C) Defocus (difference in dioptric distances) for different vertical eccentricities. The viewer is fixating the ground. Different curves represent animals of different heights. The eccentricities corresponding to ϕ = ±5° are represented by dashed vertical lines. Because defocus in (B) is nearly independent of fixation distance, we represent the relationship between defocus and retinal eccentricity with one curve for each eye height. (D) Images of the ground for viewers of different heights. A virtual camera with a field of view of 30° and an aperture diameter of 4.5 mm was aimed toward a plane with θ = 56°. The camera was focused on the black cross at distance z0. From top to bottom, z0 was 0.6, 0.2, and 0.1 m (1.7D, 5D, and 10D, respectively).
Figure 3C shows how dioptric difference varies with vertical retinal eccentricity for different eye heights. Shorter animals with their eyes close to the ground will experience much greater change across the retina. Figure 3D illustrates this by showing that the blur gradient is much greater when the camera is close to the surface (bottom panel) than when it is farther away (top panel).
If pupil size were proportional to eye height, the defocus signal would not vary from short to tall animals, and the analysis in Fig. 3 would be invalid. However, eye size (and therefore pupil size) is roughly proportional to the square root of eye height [see figure caption; (25, 26)], so the analysis remains viable.
As we said, ambush predators with frontal eyes use stereopsis to gauge the distance of prey before striking. For precision, they require sufficiently sharp vertical contours (20, 23). Figure 3 suggests that the need to minimize the blur of vertical contours is greater in shorter animals, so selective pressure to restrict the pupil horizontally is greater. In addition, short animals’ viewpoint close to the ground creates a larger blur gradient across the retina, thereby making depth from blur a potentially more effective means for estimating distances along the ground than it is in tall animals. We predict, therefore, that shorter frontal-eyed, ambush predators will be more likely to have a vertical-slit pupil than taller animals in that niche.
We evaluated this prediction by examining the relationship between eye height in these animals and the probability that they have a vertically elongated pupil. There is indeed a striking correlation among frontal-eyed, ambush predators between eye height and the probability of having such a pupil. Among the 65 frontal-eyed, ambush predators in our database, 44 have vertical pupils and 19 have circular. Of those with vertical pupils, 82% have shoulder heights less than 42 cm. Of those with circular pupils, only 17% are shorter than 42 cm.
Nearly all birds have circular pupils (1). The relationship between height and pupil shape offers a potential explanation. A near and foreshortened ground plane is not a prominent part of birds’ visual environment. The only birds known to have a slit pupil (and it is vertically elongated) are skimmers [Rynchopidae; (27)]. The primary foraging method for the black skimmer is to fly close to the water surface with its lower beak in the water, snapping shut when it contacts prey. The black skimmer is crepuscular or nocturnal. This niche is visually somewhat similar to the ones encountered by short terrestrial predators, and they tend to have vertical-slit pupils.
We hypothesize that vertically elongated pupils in frontal-eyed, ambush predators allow complementary use of disparity and blur to estimate the distances of vertical and horizontal contours, respectively. However, some ambush predators, such as crocodiles, alligators, and geckos, have lateral eyes and are therefore unlikely to have useful stereopsis. Their distance estimation presumably has to rely on defocus blur. Their slit pupils again allow more control of aperture area and therefore enable functional vision in dim and bright conditions (1, 2). But why is the elongation vertical? Again the slit pupil creates astigmatic depth of field such that vertical contours that are nearer and farther than the eye’s focal distance remain relatively sharp. This allows the animal to see objects standing on the ground sharply for identification while also facilitating distance estimation from the blur gradient associated with foreshortened horizontal contours in the retinal image of the ground or water surface. Vertical elongation is more advantageous than horizontal elongation because it aligns the axis of short depth of field with the ground or water surface, thereby enabling depth estimation from the accompanying blur gradient, and it aligns the axis of long depth of field with vertical contours that can be used for object identification. Many of these animals may use the blur gradient to adjust accommodation and then estimate distance from an extra-retinal signal associated with the accommodative response (1).
Horizontally elongated pupils
We next describe the hypothesis for horizontally elongated pupils. Figure 1 shows that terrestrial animals with horizontal pupils are very likely to be prey (of the 42 herbivorous prey animals, 36 have horizontal pupils).
The optic axis is the axis of symmetry through the cornea and lens. It is a reasonable proxy for the visual axis, which connects the point being fixated and the area centralis (or fovea). The angle between the optic axes is the laterality angle [orbital convergence is a related quantification; (28, 29)]. The laterality angle is ~0 when the axes point in nearly the same direction as in humans with distant fixation; it is much greater than 0 when the axes point in nearly opposing directions. From our database and some additional species (30), we find that 26 of 27 terrestrial animals categorized as prey have laterality angles greater than 87°. Thus, terrestrial prey are very likely to have both horizontally elongated pupils and laterally placed eyes.
The visual field is the region of space around the head from which an animal can gather visual information. There are two portions to the visual field: the binocular zone, which is the region in front of the animal seen by both eyes, and the monocular zones, the regions seen by one eye or the other. The blind zone is the region seen by neither eye. Laterality angle is a good predictor for the horizontal extents of the binocular, monocular, and blind zones (28, 31).
Large laterality angles (that is, divergent optic axes) yield wide monocular fields with little binocular overlap and thereby minimize the width of the blind zone (1, 29, 31–33). Most terrestrial lateral-eyed animals are prey, so their adaptive strategy is to detect predators approaching along the ground and to flee quickly to avoid capture. The visual requirements for this strategy are striking. On the one hand, these animals must see panoramically to detect predators that could approach from various directions. On the other hand, they must see sufficiently clearly in the forward direction to guide rapid locomotion over potentially rough terrain. In both cases, the regions of greatest importance are centered on or near the ground.
In most eyes, image quality for eccentric objects is quite poor because of astigmatism of oblique incidence (34–36). In humans, for example, objects 70° eccentric from the optic axis create images with more than 10D of astigmatism (37).
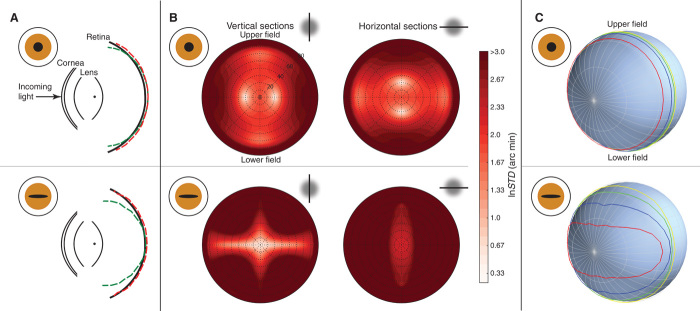
To gain insight into why horizontal pupils are so common among terrestrial prey (the great majority of whom also have lateral eyes), we examined how pupil shape affects image quality and field of view by using a published model of the sheep eye (38). Figure 4A shows in plan view the focal surfaces for line objects at infinite distance and different horizontal eccentricities: red for vertical lines and green for horizontal lines. The difference between the red and green lines is a manifestation of astigmatism of oblique incidence. Horizontal lines are focused more myopically (that is, the focal surface is closer to the front of the eye) relative to vertical lines, particularly when the pupil is horizontally elongated. As an object moves toward a non-accommodating eye, the surface of best focus moves toward the back of the eye, so horizontal contours at nearer distances and displaced from the optic axis are better focused than vertical contours. To investigate image quality, we took vertical or horizontal cross sections through the point spread functions (PSFs) and calculated the spread of those sections. The upper and lower halves of Fig. 4B show the results for circular and horizontal pupils, respectively, and the left and right halves, the results for horizontal and vertical cross sections, respectively. The reduced vertical extent of horizontally elongated pupils increases depth of field for horizontal contours and thereby reduces blur for such contours. Thus, horizontal pupils minimize the blur of horizontal contours caused by astigmatism of oblique incidence. As the distance to imaged contours decreases (for example, nearer points on the ground), the horizontal strip of high image quality in the lower left panel widens, whereas the corresponding region in the upper left panel does not. By reducing blur for horizontals, the horizontally elongated pupil improves image quality for features in the ground ahead of and behind the animal. This is surely advantageous for visual guidance of locomotion across uneven terrain while also yielding greater dynamic range in the amount of light striking the retina. The results for vertically elongated pupils (not shown) are the same as for horizontally elongated pupils but rotated by 90°. Thus, a vertical pupil would reduce the blur of vertical contours above and below the animal’s head.
Fig. 4. Pupil shape and image quality in the model sheep eye.
(A) Schematic sheep eyes viewed from above. The upper plot is for a circular pupil and the lower plot for a horizontally elongated pupil with the same area. The black curves represent, from left to right, the anterior and posterior surfaces of the cornea (radius 11.66 and 13 mm, thickness 0.8 mm, refractive index 1.382), the anterior and posterior surfaces of the lens (radius 9.17 and −8.12 mm, thickness 9 mm, refractive index 1.516), and the retina (radius 12 mm). The red and green dashed curves respectively represent the focal surfaces for vertical and horizontal contours. (B) Widths of sections through the PSF for different pupils and retinal positions. The upper and lower plots were computed with circular (2.8 × 2.8 mm) and horizontally elongated (8 × 1 mm) pupils, respectively. The optic axis is in the center of each circular plot. Black concentric dashed circles represent different eccentricities. Colors correspond to the SD of the PSF (a measure of the spread of the PSF cross section) for vertical (left) and horizontal cross sections (right); lighter red corresponds to the smallest SD (that is, the sharpest image) and darker red corresponds to the largest SD (least sharp image). (C) Throughput for circular and horizontal pupils. The contour lines represent regions of constant throughput: red, blue, green, and yellow for 80, 60, 40, and 20%, respectively.
Figure 4C shows another important optical effect due to pupil shape. The colored contour lines correspond to different amounts of throughput, where throughput is defined as the proportion of incident light that ends up on the retina. With a circular pupil, the iso-throughput contours are circular on the back of the eye. With a horizontally elongated pupil, the iso-throughput contours are horizontally stretched, facilitating visual function in front of and behind the animal. The compression of the contours vertically is also advantageous because it reduces the amount of overhead sunlight that would otherwise strike the retina. Interestingly, many of these animals have comb-like structures called corpora nigra at the top of the pupillary aperture, and those structures also help reduce dazzle to overhead sunlight (1, 39–41). Thus, the horizontally elongated pupil allows the eye to capture light in important directions along the ground while reducing the capture in less important directions from which a great deal of light may be incident. The results for vertically elongated pupils are again identical but rotated 90°.
We conclude that the optimal pupil shape for terrestrial prey is horizontally elongated. Such a pupil improves image quality for horizontal contours in front of and behind the animal and thereby helps solve the fundamental problem of guiding rapid locomotion in a forward direction despite lateral eye placement. It also facilitates a horizontally panoramic view for detecting predators approaching along the ground.
For the hypothesized benefit to occur, the long axis of the pupil in these lateral-eyed animals should maintain alignment with earth horizontal. Specifically, the eyes should rotate about the optic axes in response to changes in head pitch (that is, nose up and nose down). Because the eyes are positioned laterally, the rotation should be opposite in direction in the two eyes: that is, cyclovergence.
Compensatory cyclovergence with head pitch is indeed observed in mammals with lateral eyes. For example, the rabbit exhibits cyclovergence in response to changes in head pitch with a gain (amount of eye rotation divided by amount of head pitch) of ~0.7 (42). However, rabbits have circular pupils. We went to farms and zoos and observed five lateral-eyed species with horizontally elongated pupils: sheep, goat, horse, white-tailed deer, and moose. With changes in head pitch of ~70°, the eyes counter-rolled with a gain of at least 0.7. These observations are documented in photographs and a video in fig. S2.
The response has also been documented in lateral-eyed reptiles with vertical-slit pupils (8, 43–45). In a crocodile, Caiman sclerops, the response gain is ~0.8 for relatively small pitch changes (8). This aligns the pupil’s long axis with earth vertical, which is consistent with our hypothesis for the vertical-slit pupil. However, there are some exceptions: the green vine snake (Dryophis nasutus) does not make compensatory cyclovergence movements with pitch changes (8).
Thus, in animals with lateral eyes, compensatory eye movements in response to changes in head pitch maintain rough alignment of the long axis of the pupil with the ground plane: horizontal pupils parallel to the ground and vertical slits perpendicular to it. These observations confirm our prediction that the functional advantage conferred by elongated pupils is maintained as the head pitches. For grazers like sheep and horse, this means that the pupil maintains rough alignment with the projection of the ground as the animal holds the head upright to scan the environment and pitches the head downward to graze. Many species with lateral eyes have streak retinas with high receptor density centered on the eye’s horizontal meridian (1, 39, 40). Compensatory eye movements with head pitch also help align the streak with the projection of the ground.
DISCUSSION
Multiple apertures
Some species—for example, geckos, rays, skates, flatfish, catfish, and bottle-nosed dolphins—have pupils that constrict to multiple apertures under bright illumination. A single aperture must constrict to a small size to achieve a large depth of field, and this greatly reduces retinal illumination. Walls (1) and Duke-Elder (39) argued that multiple apertures allow for high retinal illumination with large depth of field. However, the concept of depth of field does not really apply to multiple-aperture systems because the image quality of out-of-focus images is badly compromised. For example, with two pinhole apertures, a point object creates two sharp images on the retina whenever the object is in front of or behind the eye’s focal plane (46, 47).
Consider the gecko pupil. When dilated, it is large and circular; when constricted, it creates three or four vertically aligned pinholes. The area change is ~300-fold, so the pupil allows control of retinal illumination in bright and dark environments, consistent with the animal’s polyphasic behavior (5). Geckos are ambush predators, so they must gauge the distance to their prey without revealing their position by moving. Their eyes are lateral, so they presumably cannot use stereopsis for gauging distance. Murphy and Howland (46) proposed that geckos instead use defocus blur to estimate distance, similar to the Scheiner principle used in some clinical eye examinations (47).
We offer an extension to this hypothesis. The depth of field of the gecko eye when the pupil is constricted has two aspects. First, the images formed by each pinhole have a large depth of field and therefore little variation in blur as a function of distance. Second, the sum of images through the pinholes creates multiple images separated in the manner described by Eq. 2. If the baseline A is large, the image separations can be large, creating a small depth of field as previously suggested (46). The gecko is able to accommodate over a large range of distances by altering the shape of the lens (1). Presumably, the sensory signal being monitored is the separation of the images of interest. Thus, the gecko and other animals with multiple-aperture pupils can use image separation to guide accommodation and, once accommodated, use the extra-retinal signal from the musculature controlling the lens to judge distance. The chameleon uses such a mechanism, albeit with a large single-aperture pupil, to estimate distance when catching prey (48). We conclude that the multiple-pinhole pupil is a clever adaptation that provides an effectively large baseline for the purpose of estimating depth from blur while also allowing a large reduction in retinal illumination. A related method is used in computational photography. With a complex aperture, a conventional camera can be used to estimate depth from a single photograph (49).
Convergent and parallel evolution
The striking correlation between ecological niche and pupil shape (Fig. 1 fig. S1 and table S3) implies that selective pressure has determined optimal shape in various lineages. However, many of the species in our database are closely related. Perhaps today’s niche-shape correlations are due to evolution in a handful of common ancestors and therefore do not reflect selective pressure operating independently on a large number of species. We examined phylogenetic relationships to determine whether a few common ancestors or convergent/parallel evolution provides a better account.
A previous study of Elapid snakes reconstructed the most parsimonious ancestral tree for pupil shape, foraging mode, and diel activity for that family (7). The analysis showed that vertical-slit pupils evolved at least twice from a common ancestor with circular pupils and subcircular pupils as many as six times. The results are consistent with independent evolution.
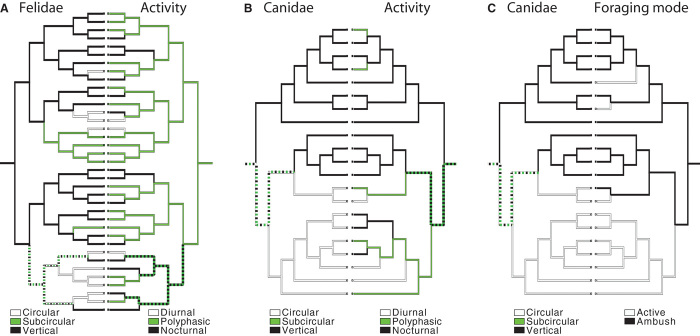
We subjected a subset of our data to a similar analysis. The subset was chosen on the basis of species for which there are published, high-confidence phylogenetic trees: one for Felidae (50) and three for Canidae (51). For both families, we reconstructed ancestral states using parsimony.
The Felid analysis suggests that the last common ancestor for modern Felidae was a nocturnal or polyphasic, ambush predator with vertical-slit pupils. In the estimated tree, subcircular pupils evolved two to four times from that ancestral state, and circular pupils, six times (Fig. 5A). Pupil shape is significantly correlated with diel activity in this analysis, which takes phylogenetic relatedness into account (P < 0.03 or P < 0.001, depending on whether subcircular pupils are grouped with vertical-slit or not). Pupil shape in Felidae is not significantly correlated with foraging mode because there is little variation in foraging strategy across that family. These results are consistent with independent evolution in Felidae of vertical-slit and subcircular pupils linked to activity time.
Fig. 5. Ancestral reconstruction of pupil shape, activity, and foraging mode for Felidae and Canidae using parsimony.
Line colors indicate estimated state at each branch. Dashed lines indicate uncertain states; the two colors composing the dash indicate the two possible states. Pupil shape is indicated by the cladogram on the left in each panel. Activity or foraging mode is indicated by the cladogram on the right in each panel. (A) Changes in pupil shape compared to changes in activity time for Felidae. (B) Changes in pupil shape compared to changes in activity time for Canidae. (C) Changes in pupil shape compared to changes in foraging mode for Canidae. Comparison of changes in pupil shape to those in foraging mode for Felidae was omitted because of the lack of variation in foraging mode among the species.
The analysis of ancestral states of Canidae suggests that the last common ancestor was a polyphasic, ambush predator with subcircular pupils. Vertical-slit and circular pupils evolved two times each (Fig. 5, B and C). Canid pupil shape is significantly related to diel activity and foraging mode (Fig. 5, B and C; P < 0.05 for polyphasic grouped with diurnal, P < 0.006 for polyphasic grouped with nocturnal, and P < 0.001 for foraging mode). These results are consistent with independent evolution in Canidae of vertical-slit and circular pupils linked to activity time and foraging mode (more details in table S4).
Thus, transitions in pupil shape have occurred multiple times within and between lineages. The transitions are typically associated with specific ecological niches: circular pupils with diurnal activity and active foraging, vertically elongated pupils with nocturnal activity and ambush foraging, and horizontal pupils with being prey. The number of times pupil shape has changed in these families implies that the shape of the eye’s aperture has evolved in response to the environment, and not because of emergence in a few common ancestors.
MATERIALS AND METHODS
We categorized 214 terrestrial species according to foraging mode, diel activity (active time of day), and pupil shape. From the classification scheme of Brischoux and colleagues (7), foraging mode describes whether the animal is primarily prey or predator. We divided predators into active foragers—animals that chase down prey—and ambush predators—animals that use a sit-and-wait strategy to catch prey. Diel activity was divided into diurnal, polyphasic (active at day and night), and nocturnal. Pupil shape was determined from the shape when constricted; it was divided into horizontally elongated, circular, and vertically elongated. Vertically elongated was further subdivided into subcircular and vertical slit, the former having an aspect ratio closer to 1.
To create a representative database of terrestrial species, we incorporated Australian snakes (7) and other terrestrial groups for which we could determine foraging mode, diel activity, and pupil shape. We included every species from the Felid and Canid families and also included some species (for example, hyena and fossa) that split from Felidae and Canidae before their modern radiation. We added most of the mongooses from Herpestidae and Eupleridae. Finally, we included as many ungulate families as we could, including Bovidae, Cervidae, and Suidae from Artiodactyla and Equidae, Tapiridae, and Rhinocerotidae from Perissodactyla. We prioritized species for which eye laterality (the amount by which the eyes’ optic axes diverge) has been quantified (30).
We assessed the statistical reliability of the relationship between ecological niche and pupil shape with multinomial logistic regression using foraging mode and diel activity to predict specific pupil shapes (7). We used horizontally elongated pupils as the reference outcome. The regression results summarize how changes in ecological niche are associated with having circular, subcircular, and vertical pupils relative to horizontal pupils.
We constructed our model for horizontal pupils on the basis of a schematic eye of the sheep [(38); Fig. 4A]. Using the Zemax ray tracer, we calculated images formed for distant objects at various positions relative to the optic axis. We did not include image degradation due to chromatic aberration or higher-order aberrations but did include diffraction. We calculated PSFs for vertically elongated, circular, and horizontally elongated pupils of the same area.
To reconstruct ancestral states, we used Pagel’s correlation analysis. We first binarized the data for use with Pagel’s correlation analysis (52). We used the eight-parameter model in Mesquite 2.6 (53, 54).
Supplementary Material
Acknowledgments
The data are presented in Fig. 1, fig. S1, and table S3. The other data presented in this paper are available from http://dx.doi.org/10.15128/736664852. We thank F. Brischoux for providing us the data from (6); J. Read, S. Watt, and P. Fine for comments on an earlier draft; and J. Read and L. Goodwin for assistance in photographing horses and sheep. Funding: This work was supported by NIH and Engineering and Physical Sciences Research Council (EPSRC). Author contributions: M.S.B. wrote most of the manuscript, prepared figures, constructed some of the supplementary material, captured photographic data on animals, and aided in analyses. W.W.S. wrote sections on the data analysis and phylogenetic analyses, constructed some of the supplementary material, and constructed parts of Figs. 1 and 4, and all of Fig. 5. J.S. conducted the analysis in Fig. 4 and prepared parts of that figure. J.A.Q.P. assisted with construction of species list and parts of Fig. 2. G.D.L. helped write the manuscript, constructed Fig. 2 and some of the supplementary material, and captured photographic and video data on animals. Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/7/e1500391/DC1
Fig. S1. Interactive version of database.
Fig. S2. Photographs of eye rotation and head pitch in the horse.
Movie S1. Video of eye rotation with head pitch in sheep.
Table S1. List of species.
Table S2. Number of species in each category.
Table S3. Relative-risk ratios with horizontal pupil as reference.
Table S4. Statistical significance of relationships between ecological niche and pupil shape for Felids and Canids with pylogenetic relatedness taken into account.
Movie S2. Video showing changes in image properties for different amounts of defocus and pupil orientations.
REFERENCES AND NOTES
- 1.G. L. Walls, The Vertebrate Eye and Its Adaptive Radiation (Hafner, New York, 1942). [Google Scholar]
- 2.Detwiler S. R., The eye and its structural adaptations. Proc. Am. Philos. Soc. 99, 224–238 (1955). [Google Scholar]
- 3.Hammond P., Mouat G. S. V., The relationship between feline pupil size and luminance. Exp. Brain Res. 59, 485–490 (1985). [DOI] [PubMed] [Google Scholar]
- 4.Wilcox J. G., Barlow H. B., The size and shape of the pupil in lightly anaesthetized cats as a function of luminance. Vision Res. 15, 1363–1365 (1975). [DOI] [PubMed] [Google Scholar]
- 5.Roth L. S. V., Lundström L., Kelber A., Kröger R. H. H., Unsbo P., The pupils and optical systems of gecko eyes. J. Vis. 9, 27.1–27.11 (2009). [DOI] [PubMed] [Google Scholar]
- 6.De Groot S. G., Gebhard J. W., Pupil size as determined by adapting luminance. J. Opt. Soc. Am. 42, 492–495 (1952). [DOI] [PubMed] [Google Scholar]
- 7.Brischoux F., Pizzato L., Shine R., Insights into the adaptive significance of vertical pupil shape in snakes. J. Evol. Biol. 23, 1878–1885 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Heath J. E., Northcutt R. G., Barber R. P., Rotational optokinesis in reptiles and its bearing on pupillary shape. Z. vergl. Physiol. 62, 75–85 (1969). [Google Scholar]
- 9.Kröger R. H. H., Campbell M. C. W., Fernald R. D., Wagner H. J., Multifocal lenses compensate for chromatic defocus in vertebrate eyes. J. Comp. Physiol. A 184, 361–369 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Gagnon Y., Söderberg B., Kröger R. H. H., Optical advantages and function of multifocal spherical fish lenses. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 29, 1786–1793 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Malmström T., Kröger R. H. H., Pupil shapes and lens optics in the eyes of terrestrial vertebrates. J. Exp. Biol. 209, 18–25 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Held R. T., Cooper E. A., O’Brien J. F., Banks M. S., Using blur to affect perceived distance and size. ACM Trans. Graph. 29, 1–16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson B. J., Decker K. E., Roorda A., Monochromatic aberrations provide an odd-error cue to focus direction. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 19, 833–839 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Jacobs R. J., Smith G., Chan C. D., Effect of defocus on blur thresholds and on thresholds of perceived change in blur: Comparison of source and observer methods. Optom. Vis. Sci. 66, 545–553 (1989). [DOI] [PubMed] [Google Scholar]
- 15.Schechner Y. Y., Kiryati N., Depth from defocus vs. stereo: How different really are they? Int. J. Comput. Vis. 39, 141–162 (2000). [Google Scholar]
- 16.Held R. T., Cooper E. A., Banks M. S., Blur and disparity are complementary cues to depth. Curr. Biol. 22, 426–431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradshaw M. F., Glennerster A., Rogers B. J., The effect of display size on disparity scaling from differential perspective and vergence cues. Vision Res. 36, 1255–1264 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Burge J., Geisler W. S., Optimal defocus estimation in individual natural images. Proc. Natl. Acad. Sci. U.S.A. 108, 16849–16854 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker R. Y., Superiority of binocular over monocular vision in depth perception in respect to the vertical or horizontal position of the object. J. Aviat. Med. 11, 87–95 (1940). [Google Scholar]
- 20.Ebenholtz S. M., Walchli R. M., Stereoscopic thresholds as a function of head- and object-orientation. Vision Res. 5, 455–461 (1965). [DOI] [PubMed] [Google Scholar]
- 21.DeAngelis G. C., Ohzawa I., Freeman R. D., Depth is encoded in the visual cortex by a specialized receptive field structure. Nature 352, 156–159 (1991). [DOI] [PubMed] [Google Scholar]
- 22.Durand J. B., Celebrini S., Trotter Y., Neural bases of stereopsis across visual field of the alert macaque monkey. Cereb. Cortex 17, 1260–1273 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Goodwin R. T., Romano P. E., Stereoacuity degradation by experimental and real monocular and binocular amblyopia. Invest. Ophthalmol. Vis. Sci. 26, 917–923 (1985). [PubMed] [Google Scholar]
- 24.Witkin A. P., Recovering surface shape and orientation from texture. Artif. Intell. 17, 17–45 (1981). [Google Scholar]
- 25.Howland H. C., Merola S., Basarab J. R., The allometry and scaling of the size of vertebrate eyes. Vision Res. 44, 2043–2065 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Kilbourne B. M., Hoffman L. C., Scale effects between body size and limb design in quadrupedal mammals. PLOS One 8, e78392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zusi R. L., Bridges D., On the slit pupil of the black skimmer (Rynchops niger). J. Field Ornithol. 52, 338–340 (1981). [Google Scholar]
- 28.Heesy C. P., On the relationship between orbit orientation and binocular visual field overlap in mammals. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281A, 1104–1110 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Heesy C. P., Ecomorphology of orbit orientation and the adaptive significance of binocular vision in primates and other mammals. Brain Behav. Evol. 71, 54–67 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Heesy C. P., Function of the mammalian postorbital bar. J. Morphol. 264, 363–380 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Ross C. F., Into the light: The origin of Anthropoidea. Annu. Rev. Anthropol. 29, 147–194 (2000). [Google Scholar]
- 32.Cartmill M., Rethinking primate origins. Science 184, 436–443 (1974). [DOI] [PubMed] [Google Scholar]
- 33.A. Hughes, in The Visual System in Vertebrates, F. Crescitelli, Ed. (Springer-Verlag, New York, 1977), pp. 613–756. [Google Scholar]
- 34.Jennings J. A. M., Charman W. N., Off-axis image quality in the human eye. Vision Res. 21, 445–455 (1981). [DOI] [PubMed] [Google Scholar]
- 35.Navarro R., Artal P., Williams D. R., Modulation transfer of the human eye as a function of retinal eccentricity. J. Opt. Soc. Am. A 10, 201–212 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Williams D. R., Artal P., Navarro R., McMahon M. J., Brainard D. H., Off-axis optical quality and retinal sampling in the human eye. Vision Res. 36, 1103–1114 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Ferree C. E., Rand G., Interpretation of refractive conditions in the peripheral field of vision. Arch. Ophthalmol. 9, 925–938 (1933). [Google Scholar]
- 38.Coile D. C., O’Keefe L. P., Schematic eyes for domestic animals. Ophthalmic Physiol. Opt. 8, 215–220 (1988). [DOI] [PubMed] [Google Scholar]
- 39.S. Duke-Elder, The Eye in Evolution, vol. 1 of System of Ophthalmology (Henry Kimpton, London, 1958). [Google Scholar]
- 40.M. F. Land, D.-E. Nilsson, Animal Eyes (Oxford Univ. Press, Oxford, 2012). [Google Scholar]
- 41.I. R. Schwab, Evolution’s Witness: How Eyes Evolved (Oxford Univ. Press, Oxford, 2011). [Google Scholar]
- 42.Van der Steen J., Collewijn H., Ocular stability in the horizontal, frontal and sagittal planes in the rabbit. Exp. Brain Res. 56, 263–274 (1984). [DOI] [PubMed] [Google Scholar]
- 43.Munro D. F., Vertical position of the pupil in the Crotalidae. Herpetologica 5, 106–108 (1949). [Google Scholar]
- 44.Munro D. F., Vertical orientation of the eye in snakes. Herpetologica 6, 84–86 (1950). [Google Scholar]
- 45.Allen E. R., Niell W. T., The vertical position of the pupil in crocodilians and snakes. Herpetologica 6, 95–96 (1950). [Google Scholar]
- 46.Murphy C. J., Howland H. C., On the gekko pupil and Scheiner’s disc. Vision Res. 26, 815–817 (1986). [DOI] [PubMed] [Google Scholar]
- 47.C. Scheiner, Oculus, Hoc Est: Fundamentum Opticum (Agricola, Vienna, 1619). [Google Scholar]
- 48.Harkness L., Chameleons use accommodation cues to judge distance. Nature 267, 346–349 (1977). [DOI] [PubMed] [Google Scholar]
- 49.Levin A., Fergus R., Durand F., Freeman W. T., Image and depth from a conventional camera with a coded aperture. ACM Trans. Graph. 26, 70-1–70-9 (2007). [Google Scholar]
- 50.Johnson W. E., Eizirik E., Pecon-Slattery J., Murphy W. J., Antunes A., Teeling E., O’Brien S. J., The late Miocene radiation of modern Felidae: A genetic assessment. Science 311, 73–77 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Bardeleben C., Moore R. L., Wayne R. K., A molecular phylogeny of the Canidae based on six nuclear loci. Mol. Phylogenet. Evol. 37, 815–31 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Pagel M., Detecting correlated evolution on phylogenies: A general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 (1994). [Google Scholar]
- 53.W. P. Maddison, D. R. Maddison, Mesquite: A modular system for evolutionary analysis, version 2.6 (2009); http://mesquiteproject.org.
- 54.P. Midford, W. P. Maddison, Pagel’s 1994 correlation method. Mesquite Manual (2009); http://mesquiteproject.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/7/e1500391/DC1
Fig. S1. Interactive version of database.
Fig. S2. Photographs of eye rotation and head pitch in the horse.
Movie S1. Video of eye rotation with head pitch in sheep.
Table S1. List of species.
Table S2. Number of species in each category.
Table S3. Relative-risk ratios with horizontal pupil as reference.
Table S4. Statistical significance of relationships between ecological niche and pupil shape for Felids and Canids with pylogenetic relatedness taken into account.
Movie S2. Video showing changes in image properties for different amounts of defocus and pupil orientations.