Investment in play can take ontogenetic priority over unconstrained physical development with consequences for life history.
Keywords: Developmental origins of health and disease, resource allocation, phenotypic plasticity, surplus resource hypothesis, motor training hypothesis, juvenile risk hypothesis, human evolution
Abstract
The developmental costs and benefits of early locomotor play are a puzzling topic in biology, psychology, and health sciences. Evolutionary theory predicts that energy-intensive behavior such as play can only evolve if there are considerable benefits. Prominent theories propose that locomotor play is (i) low cost, using surplus energy remaining after growth and maintenance, and (ii) beneficial because it trains motor skills. However, both theories are largely untested. Studying wild Assamese macaques, we combined behavioral observations of locomotor play and motor skill acquisition with quantitative measures of natural food availability and individual growth rates measured noninvasively via photogrammetry. Our results show that investments in locomotor play were indeed beneficial by accelerating motor skill acquisition but carried sizable costs in terms of reduced growth. Even under moderate natural energy restriction, investment in locomotor play accounted for up to 50% of variance in growth, which strongly contradicts the current theory that locomotor play only uses surplus energy remaining after growth and maintenance. Male immatures played more, acquired motor skills faster, and grew less than female immatures, leading to persisting size differences until the age of female maturity. Hence, depending on skill requirements, investment in play can take ontogenetic priority over physical development unconstrained by costs of play with consequences for life history, which strongly highlights the ontogenetic and evolutionary importance of play.
INTRODUCTION
Growth is a key life history process in animal development with strong consequences for reproduction and survival (1–7). Life history theory proposes that under ecological constraints, resource allocation to growth is traded against concurrent investments in other processes and energy-demanding activities (2–5). One such activity characteristic for many immature vertebrates is play (8, 9). Play is generally assumed to be of minor ontogenetic importance and thus not considered as a growth rate–limiting factor (4–8). However, the developmental costs and benefits and its significance in life history are largely unknown (8). If play is performed at the expense of growth, it must be of key ontogenetic importance and should only evolve in case of considerable benefits. Here, we aim at testing this hypothesis by investigating the relationship between investments in locomotor play and growth, and identifying the benefits of locomotor play, in an ecologically valid setting.
It is a long-standing assumption in biology and psychology that physical development takes strong ontogenetic and evolutionary priority over competence acquisition via play [“surplus resource hypothesis” (8)]. Locomotor play has been shown to involve energy costs in mammals (10, 11). However, the developmental costs of play are thought to be naturally buffered because only surplus resources remaining after maintenance and growth are allocated to play (8, 12–14), and resource allocation to play is developmentally cost-free and negligible for life history trade-offs. This hypothesis is indirectly supported (i) by findings that growth rates [in primates (15, 16)] and play rates [across mammals (17–22)] increase with increasing food availability and (ii) by the phylogenetic distribution of play because play probably originated when animals “had sufficient metabolic resources, and could accumulate [significantly] more energy than required for growth and maintenance” [(8), p. 404]. Although many ontogenetic and evolutionary theories on early development and life history trade-offs build more or less explicitly on the surplus resource hypothesis (5, 6, 8, 12, 19, 23, 24), it has not been directly explored yet.
The main benefit of locomotor play is thought to be an acceleration of motor skill acquisition depending on the rate and/or intensity of play, which in turn may increase flight/fight competence [“motor training hypothesis” (8, 19, 23, 25–29)]. This assumption is the basis of health recommendations and development-stimulating measures (30–32), but it has not been adequately tested (8, 17, 25, 33), and current evidence is indirect and inconclusive. Across mammals, it has been shown that sex differences in play correspond to the diverging needs of adults (8, 22, 23, 34, 35), the peak of play activity matches the sensitive periods of motor brain areas (8, 25), and early social play may predict later dominance relationships (36). However, the causality of correlations between locomotor play rate and motor skill level in mammals, including humans (19, 37), remains unresolved, because enhanced motor skills may also enable higher play rates (33, 38).
Our longitudinal study aims to fill these two fundamental gaps by directly investigating whether locomotor play is (i) developmentally beneficial by accelerating motor skill acquisition and (ii) developmentally costly because resource allocation to locomotor play is traded off against investments in growth. We combine behavioral observations of locomotor play and latencies of motor skill acquisition with quantitative measures of natural food availability and individual growth rates measured noninvasively via photogrammetry. The study was conducted on 17 infants of a wild unprovisioned multi-male–multi-female group of Assamese macaques (Macaca assamensis) living in their natural habitat with a diverse predator community at Phu Khieo Wildlife Sanctuary in Thailand. Body size measures were recorded for all group members to evaluate the complete growth trajectories of both sexes from birth until adulthood.
RESULTS
Controlling for temporal variation in food availability, we found a strong negative correlation between individual growth rates and time spent in locomotor play (Fig. 1A, see also fig. S7). The range in locomotor play time (4.6 to 12.2% activity time; mean ± SD, 7.7 ± 2.3%) accounted for a difference in growth rate of about 30% (Fig. 1A). This energy trade-off was not caused by time constraints on feeding behavior, because resting time, not feeding time, was traded in for locomotor play time (Fig. 1, B to E, and fig. S8). The trade-off between play and growth was also independent of infant sex, and both sexes fit the same regression line (Fig. 1A). All the infants spent time in locomotor play during periods of low food availability even though low food availability also slowed down growth (Fig. 2).
Fig. 1. Energy trade-off between locomotor play and growth.
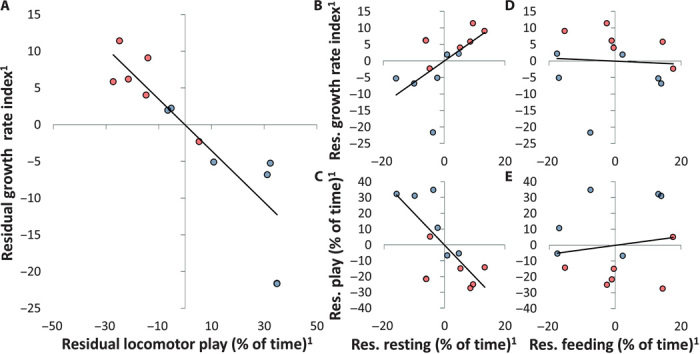
Red, female; blue, male. Residual plots of the individual values for the whole study period (Pearson partial correlations controlled for average food availability and lactation category; n = 12). 1Residuals are translated into deviations from average in percentage. (A) Growth rate over locomotor play (r = −0.889, P < 0.001); additionally controlled for sex (no figure): r = −0.785, P = 0.002; additionally controlled for average play intensity (no figure): r = −0.895, P < 0.001. (B and C) Growth rate (r = 0.612, P = 0.060) and locomotor play (r = −0.759, P = 0.011) over resting time. (D and E) Growth rate (r = −0.037, P = 0.919) and locomotor play (r = 0.155, P = 0.668) over feeding time.
Fig. 2. Sex-specific investment in growth and locomotor play with increasing food availability.
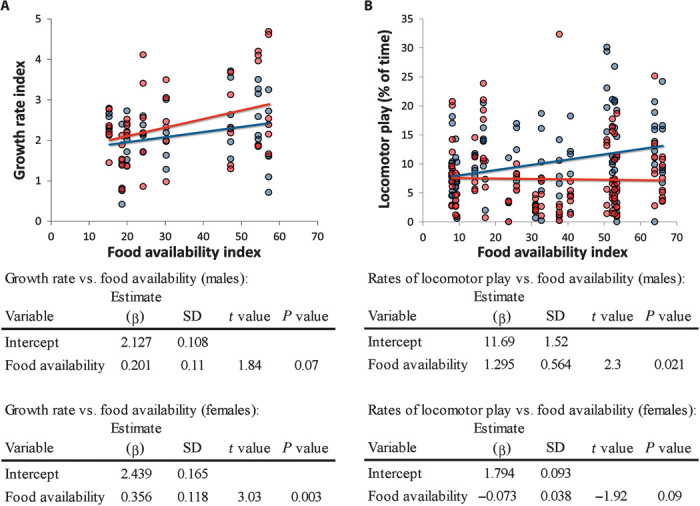
Red, female; blue, male. (A) Growth rate over food availability: with increasing food availability, female immatures invested in increased growth rates (model significance P = 0.003 compared to null model, R2 = 0.251, n = 52), whereas male immatures did not (model significance P = 0.07, R2 = 0.065, n = 48; all: P < 0.001, R2 = 0.171). (B) Locomotor play over food availability: male immatures invested energy from increased food availability in locomotor play (model significance P = 0.021, R2 = 0.360, n = 109), whereas female immatures did not (model significance P = 0.084, R2 = 0.321, n = 119; all: P = 0.95, R2 = 0.032; all model predictor variables were z-transformed).
Play also carried benefits. Across motor skills, skill acquisition latency was predicted by the statistical interaction of the proportion of time spent in locomotor play and the intensity of locomotor play: the higher the proportion of (high-intensity rough-and-tumble) social locomotor play of all locomotor play, the stronger the effect of play duration on skill acquisition latency (Fig. 3 and fig. S9). This interaction also remained the strongest predictor after controlling for variation in overall levels of locomotor play (measured as play time immediately after the acquisition; Fig. 3). Sex of the infant was not significant and was thus excluded from the final model. Because the motor skill cannot influence the amount of play before its acquisition, these results suggest that locomotor play may drive motor skill acquisition (39).
Fig. 3. Latencies of motor skill acquisition are predicted by the interaction between the amount and the intensity of locomotor play before the acquisition.
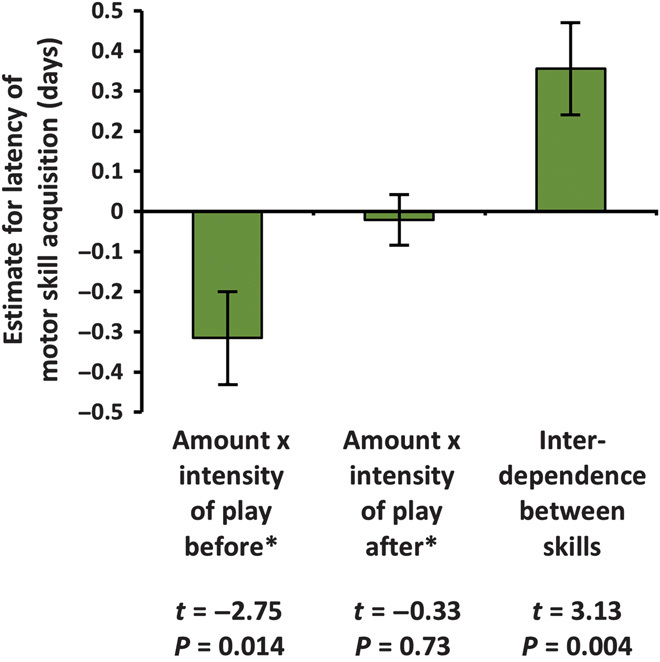
Estimates ± SD of the z-transformed variables predicting latency of motor skill acquisition of 16 skills (LMM, n = 184). Random factor: motor skill labels; model significance: P = 0.014, R2 = 0.715; intercept: estimate 8.2 ± 0.4. *Before/after the respective age of motor skill acquisition. Sex of the infant was not significant and thus excluded from the model.
Although these general patterns were independent of infant sex, we found sex-specific adaptations to the energy trade-off. The more food was available, the more female immatures invested in growth and male immatures in locomotor play (Fig. 2 and fig. S8). Consequently, male immatures grew less (P = 0.017, t = −2.84, n = 12; Fig. 1A), participated more in locomotor play (P = 0.028, t = 2.57, n = 12; Fig. 1A), and acquired motor skills faster than female immatures (P = 0.006, t = 2.78, n = 184, all two-sided t tests). In a cross-sectional analysis across all group members and ages, both sexes accelerated growth after the cessation of the play period (from birth through 4 years of age; Fig. 4). Throughout their first 4 years of life, however, males grew slower than females, leading to persisting size differences until the age of female maturity, with females reaching adult female body size about 5 months earlier than males (Fig. 4D).
Fig. 4. Sex differences in growth rates over age.
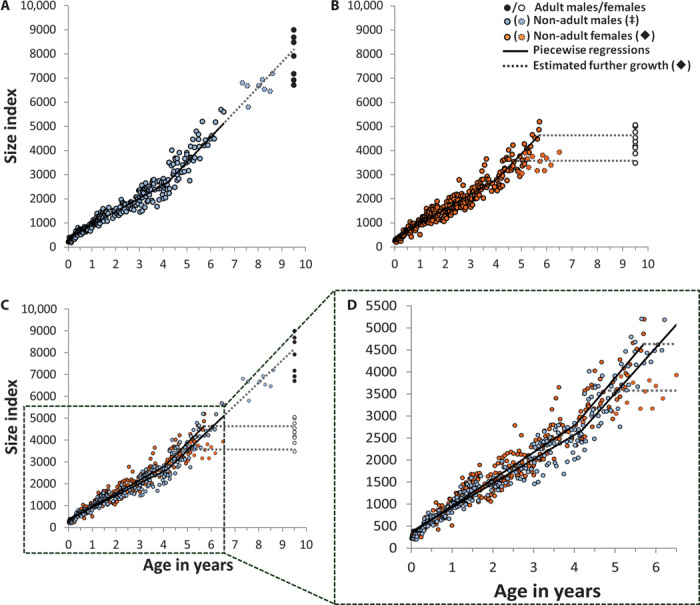
(A) Male body size index over age (break point = 4.2 ± 0.15 years, adjusted R2 = 0.952, P < 0.0001; slopes different at P < 0.0001; n = 278; ‡ open blue circles: exact birth date unknown, excluded from regression). (B) Female body size index over age (break point = 4.0 ± 0.15 years, adjusted R2 = 0.935, P < 0.0001; slopes different at P < 0.0001; n = 331; ♦ open red circles and lower scattered line: female with low age (5.0 years) at first birth, excluded from regression). (C and D) Before growth spurt, female growth rate was 11.1% higher than male growth rate (GLM: interaction age × sex, P < 0.01), resulting in an average body size difference of 13.0% at age 3.6 to 4.1 years. Adult individual averages set to the age of 9.5 years, the estimated full-grown age of males.
DISCUSSION
Using behavioral observation, photogrammetric measurements, and measurements of food availability under natural conditions, we have shown that resource allocation to locomotor play causes sizable developmental costs. Even under moderate natural energy restriction, investments in locomotor play have strong negative effects on growth, accounting for up to 50% of variance in growth with persisting consequences for life history. These results contradict the current theory stating that physical development takes strong ontogenetic priority over play for skill acquisition (8, 9, 12, 13, 17–19, 23, 40). If developmental costs of play are high, then play should be associated with considerable benefits compensating for these costs. In line with this, our results confirm the widespread, but so far unconfirmed, assumption that locomotor play is developmentally beneficial by accelerating motor skill development (8, 19, 23, 25–28, 33, 37). Additionally, we found sex-differential life history strategies with female immatures focusing their investment on growth at the expense of locomotor play and motor skill acquisition, and vice versa in males.
According to the “surplus resource hypothesis,” the energy excess after growth is a precondition for the proximate occurrence as well as the evolution of play in animals (8, 9). Indeed, these links may be rather complex, considering the underlying correlates such as prolonged development, parental care, and large brains, as well as the existence of several structurally and functionally different play types (8, 9). However, even our results of a trade-off under moderate activity levels and ecological conditions contradict predictions that enough energy after growth needs to be available for play to occur and that the developmental or even energy costs of physically active play are negligible (8, 9, 12, 13, 17). Instead, they support findings in humans where extreme physical activity during childhood in athletes of competitive sports affects growth and sexual maturation (41). The surplus resource hypothesis has been so influential that resource allocation to immature play was not even mentioned in recent reviews summarizing ecological and evolutionary factors limiting growth rates (5, 6). Our results thus strongly contribute to current life history theory by identifying resource allocation to physically active play as one crucial factor in the process.
Our results support the motor training hypothesis, which proposes that physically active play accelerates motor skill acquisition (25, 28). Accordingly, play may increase fitness by decreasing immature mortality and increasing future dominance rank across mammals (19, 22, 36, 42) because motor skills enhance flight/fight competence, which can prevent damage of physical integrity caused by predation and fights (23, 28). Previous studies provide correlational evidence of a positive association between rates of play and motor skills in mammals including humans (37, 43). Higher levels of motor skills, however, may also enable higher levels and amounts of play (33, 38), so the causality remained unclear. To our knowledge, our study is the first that shows time series causality supporting the “motor training hypothesis.”
Sex differences in energy allocation as found in our study are unexpected in the light of sexual selection theory predicting polygynous males to invest in body size and weaponry (44). In cercopithecine primates, however, female investment in growth and maturation is important because reproductive life span and thus age at first reproduction are major fitness components for females but not for males (16, 45, 46). Male reproductive success is driven by their dominance rank at prime age (46, 47) and coalitionary activity (48), both of which are likely to also be affected by fight/flight competence and, thus, motor skills (23). Notably, the developmental benefits of play may not only be limited to motor skill acquisition but may also enhance social skill development and train behavioral flexibility to deal with unexpected events (8, 23). The sex differences in energy allocation to growth or locomotor play may thus correspond to sex-differential life history strategies.
One of the most puzzling phenomena in life history theory is prolonged juvenility (from weaning until sexual maturation), which can last several years in primates and large mammals, with individuals being independent but not yet reproductive (1, 2, 5, 35, 49, 50). In addition to the extra energy required for the development of large brains (49–52), it has been proposed that the acquisition of complex skills may require sufficient time, causing prolonged juvenility to be associated with low growth rates [for example, (53)]. Janson and van Schaik (52) argued that this hypothesis lacks causality because it is unclear how skill acquisition may force, or rely on, low growth rates or sexual immaturity. They proposed the alternative (though not mutually exclusive) “juvenile risk aversion” hypothesis, stating that low growth rates evolved to avoid the high risk of starvation and predation during juvenility (5, 52). Our results provide a potential causal link between skill acquisition via energy-demanding locomotor play and low growth rates. We show that growth rates are indeed restricted by resource availability as proposed by the “juvenile risk aversion” hypothesis, but also that the resources available to growth are not only limited by ecology (that is, energy intake) but also by the amount of locomotor play for skill acquisition (that is, individual skill requirements). This indicates that both mechanisms are involved in causing low growth rates and thus extended juvenility.
The strong ontogenetic trade-off between growth and locomotor play for motor skill acquisition suggests that animals may face a health trade-off from birth between unconstrained physical development, their flight/fight competence, and physical integrity challenged by predation and adult fights. This health trade-off may also include disease risk because resource allocation to intense physical activity reduces resource allocation to immune function and increases infection risk in humans (54). This plasticity may be used via pre- and postnatal maternal effects because, under unstable ecological or social conditions, or high predation pressure, mothers could inhibit or encourage infant locomotion and physical activity to accelerate either physical development and health or motor skill acquisition (55). We suggest that future research explores the long-term effects of locomotor play on life history traits such as age at sexual maturity, adult body size, rank acquisition, survival, and fecundity, as well as the impact on the development of social and sexual networks to evaluate the long-term fitness costs.
When suffering from malnutrition, supplemental food provisioning also increases play and motor skill acquisition in human children (21, 56). Our results show that enhancing physical activity as recommended by the World Health Organization and United Nations (30–32) is indeed beneficial in terms of accelerated motor skill acquisition, but is accompanied by constrained physical development even under moderate malnutrition and activity levels. This may add a new aspect to the currently highly debated concept of the developmental origins of health and (noncommunicable) disease [DOHaD (57)], but it also suggests that reduced growth can be adaptive and developmentally beneficial rather than being generally pathological.
Together, our results show that locomotor play entails high developmental benefits but also costs; hence, differential life history strategies may determine the level of acceptable costs. They also show that play behavior and physical growth are of high phenotypic plasticity (1, 6, 57). That investments in play can take ontogenetic priority over unconstrained physical development with persisting consequences for life history strongly highlights the ontogenetic and evolutionary importance of play.
MATERIALS AND METHODS
Experimental design
The study was conducted from May 2011 to December 2012 at a long-term study site (established in 2005) at the Phu Khieo Wildlife Sanctuary (157,300 ha, 16°5′–35′N, 101°20′–55′E, 300 to 1300 masl) in northeastern Thailand (58–61). Female Assamese macaques are philopatric and reach sexual maturity within 5 to 6 years, whereas males disperse from their natal group between late infancy and adulthood and show delayed maturation (fully grown ~9 to 10 years). The timing of reproduction is strictly seasonal, and birth season ranges from April to July (62). Across age-sex classes, Assamese macaques are strongly arboreal, spending about 90% of their activity time off the ground (61), including during locomotor play activity (fig. S1).
Data were collected on a fully habituated social group consisting of 22 adults (9 males, 13 females), 23 juveniles (10 males, 13 females), and 12 infants born in 2011 (6 males, 6 females) and 5 infants born in 2012 (2 males, 3 females). All 17 infants were focal animals. The first 2011 cohort became juvenile after weaning at 12 months of age, but were observed for another 6 months until the end of the study. Therefore, we use the term “immatures” whenever these 6 months are also included in an analysis.
Behavioral data
During the 30-min focal animal protocols, instantaneous activity data were recorded every minute (1385.4 focal hours; mean ± SD, 5.5 ± 0.2 hours per individual and month; 86,518 records). We recorded whether the infant was resting, feeding, travelling, socially interacting (either affiliative such as grooming or agonistic), or engaged in solitary or social play. Solitary play was divided into solitary object and solitary locomotor play, and social play was always locomotor and divided into rough-and-tumble play (including elements of chasing and/or wrestling) and other social play (such as sexual play or the clumsy interactions at the advent of social play; fig. S2). Social play was differentiated from other social behaviors such as sitting in body contact, grooming, or aggression by the use of a play face and/or regular role changes. Independent from this general activity, we additionally recorded every minute the height of the individual in the tree in 5-m steps and its positional behavior (that is, whether the individual was lying, sitting, standing, walking, running, jumping, climbing, hanging, or brachiating).
Motor skills
We recorded all occurrences of 18 different basic motor skills for all 17 focal animals (n = 5333 ad libitum records) to assess the individual latencies of acquisition, that is, the age at first occurrence for each separate motor skill in each individual. The skills were jumping or running (both either on the ground or in a tree), jumping a distance from branch to branch of either more or less than 1 m in more versus less than 5-m height, and hanging from either all extremities or one or two arms or legs either in a solitary context or in an unpredictable social play context where the open skill (63) is played out (see table S1 for details).
Growth rate
Size from the length of the lower arms was measured every month via photogrammetry. We took 1706 pictures of the 17 focal animals (6.4 ± 2.1 pictures per individual and month; mean ± SE) and 1754 of all 23 juveniles, which were 1 to 4 years old at the beginning of the study (4.4 ± 1.9 pictures per individual and month). Picture and object distance were simultaneously recorded using a Nikon D5000 camera and a Bosch PLR 50 laser distance measurement tool (accuracy, ±2 mm) as described in (64). The number of pixels in the picture was determined using ImageJ 1.44p (National Institutes of Health). Length was then calculated by multiplying the object distance with the number of pixels in the picture (64) and applying a correction (fig. S3). Length measurements were highly reliable between observers (n = 179 picture random blind subset; correlation between values generated by two raters, r = 0.950). Outliers (mean ± 2 SD) were excluded for each month and individual separately, and monthly individual average size from the remaining pictures was used for analyses. Because linear growth is expected for increase in volume instead of length, we used the cubic value of our length measure (=size index). Growth rate indices were then calculated as slopes of linear regressions of these monthly values over time.
Intensity of locomotor play
In our study, solitary and social (that is, 83% rough-and-tumble) locomotor play strongly differed in intensity, challenge level, and age curve. The rate of high-intensity locomotion [running, climbing, jumping, hanging, and pendulously travelling; (10, 29)] was much higher during social than during solitary locomotor play. Conversely, the rate of low- and medium-intensive locomotion (that is, standing and walking) was higher during solitary than during social locomotor play (fig. S4). Infants exhibited low-intensity solitary locomotor play first, probably due to neuromuscular immaturity, but soon changed to social locomotor play (figs. S2 and S5 and table S2). Because both the rate and the intensity of play may affect skill acquisition (28), we used a statistical interaction term of locomotor play time and the proportion of social of all locomotor play as predictor of skill acquisition.
Availability of ecological energy resources
Monthly food availability indices were calculated on the basis of the fruit abundance in 650 trees and the density of these tree species, based on 44 botanical plots within the home range of the study group, covering 20.75 ha of forest [for details and seasonal variation of food availability in the study side, see (60)]. The food availability index is correlated to individual energy intake (60). Because differences in lactation may have a strong impact on individual energy intake and behavior (65), we additionally placed the infants into two lactation categories, that is, whether the mother conceived again early in the lactation period (that is, at 8 and 10 months of offspring age, n = 2) or well after weaning (interbirth intervals are bimodally distributed in our group because females can conceive in the subsequent mating season or 1 year after). Because female rank was neither correlated to female energy intake in our group [(60), see also (66)] nor to the energy content or yield of maternal milk in rhesus macaques [Macaca mulatta (67)], we did not control for mother’s rank.
Statistical analyses
All statistical analyses were run with R 3.1.2. All tests were two-tailed with α level set to 0.05. Test assumptions were controlled for by computing variance inflation factors (vif for all LMM and GLM; all vif <2.2) and applying Shapiro-Wilk tests (all P ≥ 0.18) in addition to visual inspection of scatterplots, histograms, and Q-Q plots of residuals to check for normality, linearity, and homogeneity of variance.
Growth versus locomotor play
We ran a partial correlation between individual growth rates and the proportion of time individuals spent in locomotor play and controlled for individual differences in average food availability (the average of the monthly food availability indices an individual was exposed to from birth to the end of the study period) and lactation category. To avoid strong influences of different life spans (that is, different proportions of different life history stages), the infants born in 2012 were excluded from the analysis of the growth-play trade-off. Covariance between growth and locomotor play could be mediated by time budget constraints on independent feeding time; that is, increasing time proportions in locomotor play might be at the expense of independent feeding time and, thus, energy intake, which in turn could affect growth rate. Therefore, we ran additional partial correlations to investigate potential covariance of the time spent feeding or resting with growth rate and/or the time spent in locomotor play.
Growth and locomotor play versus food availability
We applied LMMs (R 3.1.2, packages car, lme4 and MuMIn; individual as random factors) to explore the relationships between food availability and (i) the proportion of activity time the individuals spent in locomotor play and (ii) individual growth rates. For (i), we used monthly values but excluded the first 2 months of age because low play rates were probably due to physical immaturity at this age (fig. S2 and table S2). Test assumptions were met after the response variable was square root– (for LMM on all individuals) or cubic root–transformed (for LMM on females). Monthly values were, however, not applicable for (ii). Because growth rate indices were calculated as linear regression slopes of size over time, which is based on one average value per individual and month (see above), meaningful calculations required 6-month periods (that is, linear regressions over six values). Growth rate calculations of consecutive months were based on strongly overlapping periods and were highly autocorrelated (fig. S6A). No apparent autocorrelation was found if every other month was ignored (fig. S6B), so we treated these data as independent data points.
Growth versus age
We applied sex-specific continuous piecewise regressions with Davies tests for change in slope (R 3.1.2, packages segmented, sm) to explore long-term patterns and age-dependent changes in growth rate. Average monthly size indices of all 40 immatures were used. Average individual adult body size indices were calculated from all pictures taken occasionally throughout the study period, and all adults with fewer than three data points were excluded (n = 7 of 22). The significance of the difference between male and female growth rates before the growth spurt at 4 years was tested using a GLM (R 3.1.2) including an interaction between age and sex.
Locomotor play versus motor skill acquisition
We applied an LMM (R 3.1.2, packages car, lme4, MuMIn and rgl; square root transformation of the response variable to meet test assumptions) to explore the relationship between the time spent in locomotor play from birth to the acquisition of a motor skill and the latencies of this skill’s acquisition, with separate values for each motor skill and individual (n = 184) and the motor skill labels as a random factor. Time spent in locomotor play was calculated as interaction between the proportion of time in and the average intensity of locomotor play, thus accounting for the two dimensions of locomotor play (see above). To control for reversed causality [that is, motor skill level predicts amount of locomotor play (33, 38)] and methodological skew (that is, motor skills may be easier and earlier detectible in more active infants), we also included the time spent in locomotor play after the acquisition of a motor skill (same time span) as a proxy for variation in overall locomotor play times into the model. Sex of the infant was included to control for sex-specific genetic programming. Age at acquisition of the previous motor skill in the acquisition sequence was added as a predictor to control for interdependence between the motor skills. The sequence was generated by applying the I&SI rank order method [Matman1.1 (68)] on a before-after matrix (that is, how often motor skill A was acquired before or after motor skill B over all individuals; table S1).
We only included cases where play duration values were based on at least 400 instantaneous records per individual to control for sampling effort. Because this precondition was not met for most of the infants for the two motor skills acquired first during ontogeny, these were excluded from analyses. Also, five individuals that were born more than 1 week before the start of observations were excluded from analysis. We ran a t test (paired, two-sided) to compare the latencies of the motor skill acquisition (normalized via mean scaling within each motor skill) to explore overall sex differences.
Supplementary Material
Acknowledgments
We thank S. Benhaiem, T. Clutton-Brock, D. Maestripieri, and F. Trillmich for comments on earlier versions of the manuscript. We thank the National Research Council of Thailand and the Department of National Parks, Wildlife and Plant Conservation for the permission to conduct this study (project ID 2008/045). We are grateful to K. Nitaya, T. Wosnak, K. Kreetiyutanont, K. Roongadulpisan, N. Bhumpakphan, and W. Wajjwalku for support; N. Bualeng, N. Juntuch, P. Saisawatdikul, S. Jomhlotwong, W. Nuagchiyos, T. Wisate, J. Wanart, J. Kalbitz, and C. Minge for help with data collection; and M. Heesen for establishing and analyzing the food availability index. Funding: Financial support was granted by the Leibniz Association (Leibniz Graduate School for the Foundations of Primate Social Behavior) and the German Initiative of Excellence to University of Göttingen. Author contributions: A.B. collected and analyzed data. A.B., O.S., and J.O. designed the study and wrote the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The raw data from the study are available upon request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/7/e1500451/DC1
Fig. S1. Percentage of time in locomotor play at different tree heights.
Fig. S2. Percentage of different play patterns at different ages.
Fig. S3. Validation of photogrammetric measurement.
Fig. S4. Differences between solitary and social locomotor play in locomotion intensity.
Fig. S5. The proportion of social on all locomotor play was positively related to age and the number of potential play partners around.
Fig. S6. Period overlap for monthly growth rate calculation.
Fig. S7. Energy trade-off between social locomotor play and growth.
Fig. S8. Time budget analysis: Sex differences in time spent in locomotor play were due to sex differences in resting time, not feeding time.
Fig. S9. Latencies of motor skill acquisition as a function of average intensity and time spent in locomotor play before acquisition.
Table S1. List of the 18 motor skills used in this study.
Table S2. Percentage of time spent in locomotor play (mean ± SD) for each sex and age.
REFERENCES AND NOTES
- 1.P. M. Kappeler, M. E. Pereira, Primate Life Histories and Socioecology (The Univ. of Chicago Press, Chicago, 2003). [Google Scholar]
- 2.Pontzer H., Raichlen D. A., Gordon A. D., Schroepfer-Walker K. K., Hare B., O’Neill M. C., Muldoon K. M., Dunsworth H. M., Wood B. M., Isler K., Burkart J., Irwin M., Shumaker R. W., Lonsdorf E. V., Primate energy expenditure and life history. Proc. Natl. Acad. Sci. U.S.A. 111, 1433–1437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.S. C. Stearns, The Evolution of Life Histories (Oxford Univ. Press, Oxford, 1992). [Google Scholar]
- 4.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B., Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- 5.Jones J. H., Primates and the evolution of long, slow life histories. Curr. Biol. 21, R708–R717 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dmitriew C. M., The evolution of growth trajectories: What limits growth rate? Biol. Rev. Camb. Philos. Soc. 86, 97–116 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Sibly R. M., Grady J. M., Venditti C., Brown J. H., How body mass and lifestyle affect juvenile biomass production in placental mammals. Proc. Biol. Sci. 281, 20132818 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham K. L., Burghardt G. M., Current perspectives on the biological study of play: Signs of progress. Q. Rev. Biol. 85, 393–418 (2010). [DOI] [PubMed] [Google Scholar]
- 9.G. M. Burghardt, The Genesis of Animal Play: Testing the Limits (MIT Press, Cambridge, MA, 2005). [Google Scholar]
- 10.Pellegrini A. D., Horvat M., Huberty P., The relative cost of children’s physical play. Anim. Behav. 55, 1053–1061 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Miller M. N., Byers J. A., Energetic cost of locomotor play in pronghorn fawns. Anim. Behav. 41, 1007–1013 (1991). [Google Scholar]
- 12.Martin P., The energy cost of play: Definition and estimation. Anim. Behav. 30, 294–295 (1982). [Google Scholar]
- 13.Bekoff M., Byers J. A., Time, energy and play. Anim. Behav. 44, 981–982 (1992). [Google Scholar]
- 14.H. Spencer, The Principles of Psychology (D. Appleton and Company, New York, ed. 2, 1872). [Google Scholar]
- 15.Garcia C., Lee P. C., Rosetta L., Growth in colony living anubis baboon infants and its relationship with maternal activity budgets and reproductive status. Am. J. Phys. Anthropol. 138, 123–135 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Onyango P. O., Gesquiere L. R., Altmann J., Alberts S. C., Puberty and dispersal in a wild primate population. Horm. Behav. 64, 240–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin P., Caro T. M., On the functions of play and its role in behavioral development. Adv. Study Behav. 15, 59–103 (1985). [Google Scholar]
- 18.Müller-Schwarze D., Stagge B., Müller-Schwarze C., Play behavior: Persistence, decrease, and energetic compensation during food shortage in deer fawns. Science 215, 85–87 (1982). [DOI] [PubMed] [Google Scholar]
- 19.Nunes S., Muecke E.-M., Lancaster L. T., Miller N. A., Mueller M. A., Muelhaus J., Castro L., Functions and consequences of play behaviour in juvenile Belding’s ground squirrels. Anim. Behav. 68, 27–37 (2004). [Google Scholar]
- 20.Sharpe L. L., Clutton-Brock T. H., Brotherton P. N. M., Cameron E. Z., Cherry M. I., Experimental provisioning increases play in free-ranging meerkats. Anim. Behav. 64, 113–121 (2002). [Google Scholar]
- 21.Espinosa M. P., Sigman M. D., Bwibo N. O., Neumann C. G., McDonald M. A., Playground behaviors of school-age children in relation to nutrition, schooling, and family characteristics. Dev. Psychol. 28, 1188–1195 (1992). [Google Scholar]
- 22.Cameron E. Z., Linklater W. L., Stafford K. J., Minot E. O., Maternal investment results in better foal condition through increased play behaviour in horses. Anim. Behav. 76, 1511–1518 (2008). [Google Scholar]
- 23.A. D. Pellegrini, The Role of Play in Human Development (Oxford Univ. Press, New York, 2009). [Google Scholar]
- 24.Pellegrini A. D., Dupuis D., Smith P. K., Play in evolution and development. Dev. Rev. 27, 261–276 (2007). [Google Scholar]
- 25.Byers J. A., Walker C., Refining the motor training hypothesis for the evolution of play. Am. Nat. 146, 25–40 (1995). [Google Scholar]
- 26.K. Groos, E. L. Baldwin, The Play of Animals. J. Baldwin, Ed. (D. Appleton and Company, New York, 1898). [Google Scholar]
- 27.Maestripieri D., First steps in the macaque world: Do rhesus mothers encourage their infants’ independent locomotion? Anim. Behav. 49, 1541–1549 (1995). [Google Scholar]
- 28.Bekoff M., Motor training and physical fitness: Possible short and long term influences on the development of individual differences in behavior. Dev. Psychobiol. 21, 601–612 (1988). [DOI] [PubMed] [Google Scholar]
- 29.A. D. Pellegrini, F. Symons, J. Hoch, Observing Children in Their Natural Worlds: A Methodological Primer (Psychology Press, New York, ed. 3, 2013). [Google Scholar]
- 30.World Health Organization, Global Recommendations on Physical Activity for Health (WHO Global strategy on diet, physical activity and health, World Health Organization, Geneva, Switzerland, 2011). [Google Scholar]
- 31.UNICEF, The State of the World’s Children 2012 (UNICEF, New York, 2012). [Google Scholar]
- 32.UNOSDP, Sport for Children and Youth: Fostering Development and Strengthening Education (UNOSDP Harnessing the power of sport for development and peace: Recommendations to governments, United Nations Office on Sport for Development and Peace, Geneva, Switzerland, 2008). [Google Scholar]
- 33.Fulton J. E., Burgeson C. R., Perry G. R., Sherry B., Galuska D. A., Alexander M. P., Wechsler H., Caspersen C. J., Assessment of physical activity and sedentary behavior in preschool-age children: Priorities for research. Pediatr. Exerc. Sci. 13, 113–126 (2001). [Google Scholar]
- 34.Maestripieri D., Ross S. R., Sex differences in play among western lowland gorilla (Gorilla gorilla gorilla) infants: Implications for adult behavior and social structure. Am. J. Phys. Anthropol. 123, 52–61 (2004). [DOI] [PubMed] [Google Scholar]
- 35.M. E. Pereira, L. A. Fairbanks, Juvenile Primates: Life History, Development, and Behavior (Oxford Univ. Press, New York, ed. 2, 2002). [Google Scholar]
- 36.Blumstein D. T., Chung L. K., Smith J. E., Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris). Proc. Biol. Sci. 280, 20130485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher A., Reilly J. J., Kelly L. A., Montgomery C., Williamson A., Paton J. Y., Grant S., Fundamental movement skills and habitual physical activity in young children. Med. Sci. Sports Exerc. 37, 684–688 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Wrotniak B. H., Epstein L. H., Dorn J. M., Jones K. E., Kondilis V. A., The relationship between motor proficiency and physical activity in children. Pediatrics 118, e1758–e1765 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Granger C. W. J., Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37, 424–438 (1969). [Google Scholar]
- 40.Kenrick D. T., Griskevicius V., Neuberg S. L., Schaller M., Renovating the pyramid of needs: Contemporary extensions built upon ancient foundations. Perspect. Psychol. Sci. 5, 292–314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malina R. M., Baxter-Jones A. D. G., Armstrong N., Beunen G. P., Caine D., Daly R. M., Lewis R. D., Rogol A. D., Russell K., Role of intensive training in the growth and maturation of artistic gymnasts. Sports Med. 43, 783–802 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagen R., Fagen J., Play behaviour and multi-year juvenile survival in free-ranging brown bears, Ursus arctos. Evol. Ecol. Res. 11, 1053–1067 (2009). [Google Scholar]
- 43.Nunes S., Muecke E. M., Sanchez Z., Hoffmeier R. R., Lancaster L. T., Play behavior and motor development in juvenile Belding’s ground squirrels (Spermophilus beldingi). Behav. Ecol. Sociobiol. 56, 97–105 (2004). [Google Scholar]
- 44.T. H. Clutton-Brock, Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems (The Univ. of Chicago Press, Chicago, 1988). [Google Scholar]
- 45.C. P. van Schaik, in Comparative Socioecology. The Behavioural Ecology of Humans and Other Mammals, V. Standen, R. A. Foley, Eds. (Blackwell Scientific Publications, Oxford, 1989), pp. 195–218. [Google Scholar]
- 46.Dubuc C., Ruiz-Lambides A., Widdig A., Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behav. Ecol. 25, 878–889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostner J., Nunn C. L., Schülke O., Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150–1158 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schülke O., Bhagavatula J., Vigilant L., Ostner J., Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Kuzawa C. W., Chuganic H. T., Grossman L. I., Lipovich L., Muzik O., Hof P. R., Wildman D. E., Sherwood C. C., Leonard W. R., Lange N., Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. U.S.A. 111, 13010–13015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barton R. A., Capellini I., Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl. Acad. Sci. U.S.A. 108, 6169–6174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarrete A., van Schaik C. P., Isler K., Energetics and the evolution of human brain size. Nature 480, 91–93 (2011). [DOI] [PubMed] [Google Scholar]
- 52.C. H. Janson, C. P. van Schaik, in Juvenile Primates: Life History, Development, and Behavior, M. E. Pereira, L. A. Fairbanks, Eds. (The Univ. of Chicago Press, Chicago, 2002), pp. 57–74. [Google Scholar]
- 53.Poirier F. E., Smith E. O., Socializing functions of primate play. Am. Zool. 14, 275–287 (1974). [Google Scholar]
- 54.Romeo J., Wärnberg J., Pozo T., Marcos A., Physical activity, immunity and infection. Proc. Nutr. Soc. 69, 390–399 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Maestripieri D., Maternal encouragement of infant locomotion in pigtail macaques, Macaca nemestrina. Anim. Behav. 51, 603–610 (1996). [Google Scholar]
- 56.Adu-Afarwuah S., Lartey A., Brown K. H., Zlotkin S., Briend A., Dewey K. G., Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: Effects on growth and motor development. Am. J. Clin. Nutr. 86, 412–420 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Hanson M. A., Gluckman P. D., Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 94, 1027–1076 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borries C., Larney E., Kreetiyutanont K., Koenig A., The diurnal primate community in a dry evergreen forest in Phu Khieo Wildlife Sanctuary, Northeast Thailand. Nat. Hist. Bull. Siam Soc. 50, 75–88 (2002). [Google Scholar]
- 59.Ostner J., Vigilant L., Bhagavatula J., Franz M., Schülke O., Stable heterosexual associations in a promiscuous primate. Anim. Behav. 86, 623–631 (2013). [Google Scholar]
- 60.Heesen M., Rogahn S., Ostner J., Schülke O., Food abundance affects energy intake and reproduction in frugivorous female Assamese macaques. Behav. Ecol. Sociobiol. 67, 1053–1066 (2013). [Google Scholar]
- 61.Schülke O., Pesek D., Whitman B. J., Ostner J., Ecology of Assamese macaques (Macaca assamensis) at Phu Khieo Wildlife Sanctuary, Thailand. J. Wildl. Thailand 18, 1–15 (2011). [Google Scholar]
- 62.Fürtbauer I., Schülke O., Heistermann M., Ostner J., Reproductive and life history parameters of wild female Macaca assamensis. Int. J. Primatol. 31, 501–517 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poulton E. C., On prediction in skilled movements. Psychol. Bull. 54, 467–478 (1957). [DOI] [PubMed] [Google Scholar]
- 64.Breuer T., Robbins M. M., Boesch C., Using photogrammetry and color scoring to assess sexual dimorphism in wild western Gorillas (Gorilla gorilla). Am. J. Phys. Anthropol. 134, 369–382 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Leventakou V., Roumeliotaki T., Koutra K., Vassilaki M., Mantzouranis E., Bitsios P., Kogevinas M., Chatzi L., Breastfeeding duration and cognitive, language and motor development at 18 months of age: Rhea mother–child cohort in Crete, Greece. J. Epidemiol. Community Health 69, 232–239 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Majolo B., Lehmann J., Vizioli A. D., Schino G., Fitness-related benefits of dominance in primates. Am. J. Phys. Anthropol. 147, 652–660 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Hinde K., Power M. L., Oftedal L. T., Rhesus macaque milk: Magnitude, sources, and consequences of individual variation over lactation. Am. J. Phys. Anthropol. 138, 148–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Vries H., Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim. Behav. 55, 827–843 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/7/e1500451/DC1
Fig. S1. Percentage of time in locomotor play at different tree heights.
Fig. S2. Percentage of different play patterns at different ages.
Fig. S3. Validation of photogrammetric measurement.
Fig. S4. Differences between solitary and social locomotor play in locomotion intensity.
Fig. S5. The proportion of social on all locomotor play was positively related to age and the number of potential play partners around.
Fig. S6. Period overlap for monthly growth rate calculation.
Fig. S7. Energy trade-off between social locomotor play and growth.
Fig. S8. Time budget analysis: Sex differences in time spent in locomotor play were due to sex differences in resting time, not feeding time.
Fig. S9. Latencies of motor skill acquisition as a function of average intensity and time spent in locomotor play before acquisition.
Table S1. List of the 18 motor skills used in this study.
Table S2. Percentage of time spent in locomotor play (mean ± SD) for each sex and age.


