Trace amounts of the simplest Criegee intermediate in the gas-phase ozonolysis of ethylene are detected by high-resolution spectroscopy.
Keywords: Criegee intermediates, ozonolysis, atmospheric chemistry, microwave spectroscopy, tropospheric chemistry, kinetics, reaction dynamics
Abstract
Ozonolysis is one of the dominant oxidation pathways for tropospheric alkenes. Although numerous studies have confirmed a 1,3-cycloaddition mechanism that generates a Criegee intermediate (CI) with form R1R2COO, no small CIs have ever been directly observed in the ozonolysis of alkenes because of their high reactivity. We present the first experimental detection of CH2OO in the gas-phase ozonolysis of ethylene, using Fourier transform microwave spectroscopy and a modified pulsed nozzle, which combines high reactant concentrations with rapid sampling and sensitive detection. Nine other product species of the O3 + C2H4 reaction were also detected, including formaldehyde, formic acid, dioxirane, and ethylene ozonide. The presence of all these species can be attributed to the unimolecular and bimolecular reactions of CH2OO, and their abundances are in qualitative agreement with published mechanisms and rate constants.
INTRODUCTION
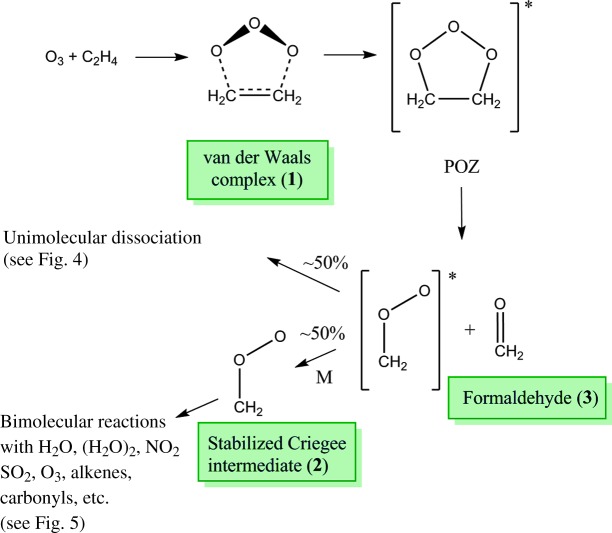
Ozonolysis is one of the most common oxidation pathways for unsaturated hydrocarbons in the troposphere. It is the predominant sink for biogenic and anthropogenic alkenes and is a significant source of OH radicals (1), but many aspects of this complex reaction pathway remain poorly understood even after decades of study (1–4). It is generally understood, however, that the reaction proceeds via a concerted 1,3-cycloaddition of O3 across the alkene double bond, through a van der Waals complex, to form a 1,2,3-trioxolane primary ozonide (denoted as a POZ, and shown in Fig. 1). This initial step is highly exothermic; consequently, the POZ is formed with high vibrational energy and promptly decomposes to form a carbonyl and a carbonyl oxide, which is commonly referred to as a Criegee intermediate (CI) (2). Although a portion of the internal energy in the POZ is lost to translational and rotational degrees of freedom of the two fragments, the CI is formed with a considerable amount of vibrational energy (5).
Fig. 1. The initial reactions in the ozonolysis of ethylene.
The reaction proceeds through a van der Waals complex and then forms a high-energy primary ozonide, which immediately decomposes into a CI and formaldehyde. Depending on the energy available, the CI may undergo unimolecular dissociation or bimolecular reactions with other atmospheric species. Shaded text boxes indicate species detected in our experiment.
Nascent CIs are generally categorized as either “excited” CIs, which have sufficient vibrational energy to isomerize or dissociate rapidly, or “stabilized” CIs (SCIs), which had been initially formed with high vibrational energy but have been stabilized by collisional energy transfer. However, SCIs remain quite reactive owing to their zwitterionic character (6, 7) and undergo rapid bimolecular reaction with other atmospheric species; as such, only one instance of a SCI resulting from gas-phase ozonolysis has been reported, albeit at low resolution (8). Theoretical and indirect experimental studies (9) have determined the branching fraction to SCI to be as high as 0.54 for CH2OO formed in the ozonolysis of ethylene (10). The branching fraction for larger SCIs is highly dependent on a number of factors, including temperature, pressure, and the nature of its substituents (11). A schematic of the initial reaction pathways for the ozonolysis of ethylene, the simplest alkene, is shown in Fig. 1.
Although the Criegee mechanism is now widely accepted (12), CIs eluded direct detection in the gas phase until 2008, when Taatjes et al. (13) measured the simplest CI, CH2OO (2), by photolysis of dimethyl sulfoxide and photoionization mass spectroscopy. In 2012, the same group showed that the photolysis of diiodomethane (CH2I2) in the presence of excess molecular oxygen was a more efficient method for selectively generating CH2OO (14). This production method has since been used by many groups to measure the vibrational (15), rotational (16–18), and electronic (19, 20) spectra of small CIs, as well as to study their reactivity with common atmospheric molecules (7, 8, 21–23). Although this method has been enormously valuable in characterizing the spectroscopy and kinetics of CH2OO, a number of questions remain about the product branching of the nascent CIs formed directly from ozonolysis. Furthermore, the generation of larger CIs with the photolysis method is contingent on the availability of analogously larger diiodo-substituted precursors. For these reasons, we have studied the ozonolysis of ethylene at atmospheric pressure and temperature, using Fourier transform microwave (FTMW) spectroscopy and a modified pulsed nozzle. This work has resulted in the detection of species ranging from the pre-reactive complex to secondary reaction products. Most significantly, the simplest CI, CH2OO, was detected in trace amounts.
RESULTS AND DISCUSSION
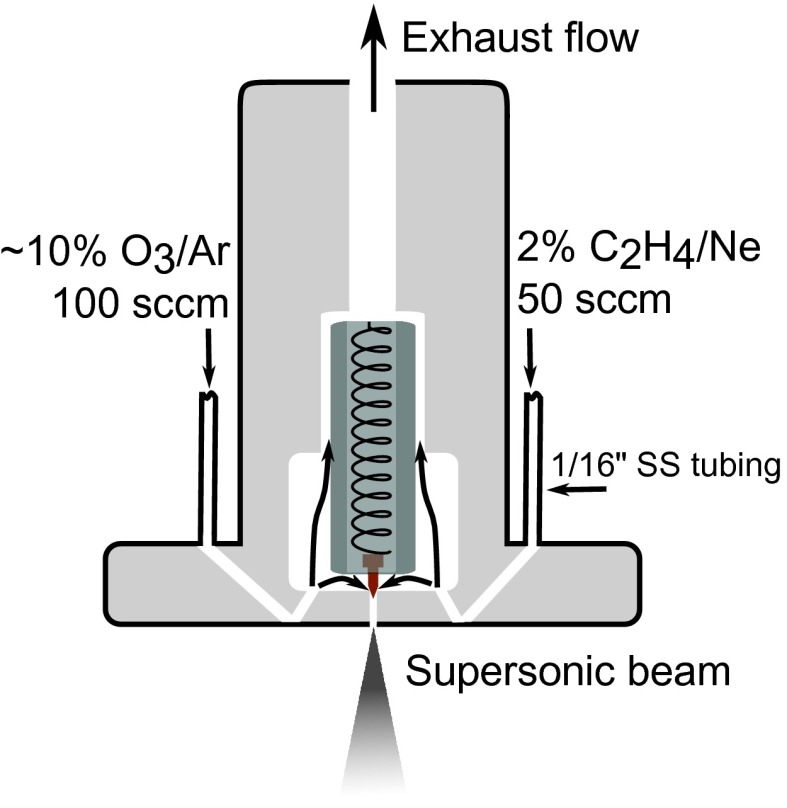
The first step of the O3 + C2H4 reaction is highly exothermic (ΔH ≅ −50 kcal/mol) (24, 25), but the overall reaction rate coefficient is relatively small (1.45 × 10−18 cm3 molecule−1 s−1) (10). Nascent CH2OO is highly reactive with many species, including the O3 and C2H4 reactants. For these reasons, detection of CH2OO likely requires (i) large concentrations of O3 and C2H4 to ensure a high reaction rate, (ii) rapid sampling after the two reactants are mixed to minimize secondary chemistry, and (iii) an exquisitely sensitive and selective detection method capable of measuring many different species at parts per billion concentrations (that is, ~109 molecules/pulse). The Balle-Flygare type cavity FTMW spectrometer used in these experiments (16, 26), described in Materials and Methods fulfills this last requirement. To address the second requirement, we constructed a nozzle source (see Fig. 2) based on the design of Lovas et al. (27, 28), who studied the O3···C2H4 complex by “freezing” the products mid-reaction through supersonic expansion into a vacuum chamber.
Fig. 2. A schematic of a fast-flow reactor to rapidly sample products of the O3 + C2H4 reaction.
The reactants were passed through 1/16-inch inlets into the inner body of the nozzle. The armature, poppet, and spring in the center of the nozzle controlled the flow of 3.5% of the reacting mixture into the detector, while the remainder of the sample was exhausted to a fume hood. Further details may be found in Materials and Methods.
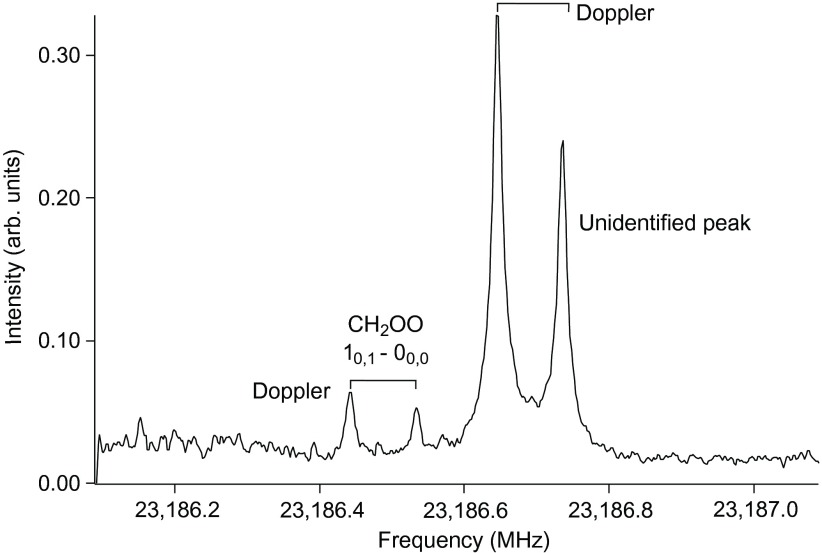
In total, nine product species were detected in our experiment at room temperature and pressure. All required the presence of both O3 and C2H4 and were sensitive to the flow rates and relative O3 and C2H4 concentrations. The signal attributed to CH2OO, shown in Fig. 3, was scrutinized carefully. Although weak, the frequency of this line matches the published high-resolution data (16, 17) to within 3 kHz, and we ensured that it was not an oscilloscope artifact or image by shifting the probe frequency. Additionally, the line vanished when a second microwave source, aligned perpendicularly to the cavity axis in a double resonance scheme, excited the 20,2–10,1 transition (16, 17). For these reasons, the line can be unequivocally attributed to CH2OO. Its signal intensity corresponds to about 5.9 × 109 molecules/pulse, which is close to the detection limit of the microwave spectrometer. Other detected species are listed in Table 1. All can be attributed to established secondary chemistry of the O3 + C2H4 reaction, as illustrated in Figs. 4 and 5, and discussed below. A small number of the product species in the figures were not detected, because they either could not be detected in our spectrometer, were too reactive, or do not have published rotational lines.
Fig. 3. The fundamental rotational line (10,1–00,0) of the simplest CI, CH2OO, detected in the O3 + C2H4 reaction, acquired after 4.3 hours of integration, or 93,000 sample injections.
The peak is split into two Doppler components, a result of the alignment of the molecular beam with the two traveling waves of the Fabry-Pèrot cavity. A more intense peak is visible, about 200 kHz higher in energy, the carrier of which remains unidentified.
Table 1. Products species detected in the O3 + C2H4 reaction.
Bold parenthetical numbers refer to species in Figs. 4 and 5. Rotational transitions of each product were monitored at the frequencies listed in the third column, which are obtained from the references in the fourth column. The absolute abundances in each pulse are estimated using a calibrated OCS sample and have a margin of error of about an order of magnitude owing to uncertainties in the rotational temperature and instrument response function.
| Molecule | Rotational transition | Frequency (MHz) | Reference | Absolute abundance (molecules/pulse) | Relative abundance | |
| 1,2,3-Trioxolane (POZ) | 11,1–00,0 | 12,591.52 | (29) | Not observed | — | |
| O3 ⋯C2 H4 | (1) | 21,1–10,1 | 15,800.7834 | (30) | 3 × 1012 | 500 |
| CH2OO | (2) | 10,1–00,0 | 23,186.4873 | (16, 17) | 6 × 109 | 1 |
| Formaldehyde* | (3) | 21,2–21,1 | 14,488.4803 | (31) | 2 × 1016 | 3.3 × 106 |
| Dioxirane | (4) | 21,1–20,2 | 31,752.8794 | (32) | 3 × 1011 | 50 |
| Formic acid | (6) | 10,1–00,0 | 22,471.1795 | (33) | 2 × 1013 | 3333 |
| Ethylene ozonide | (8) | 11,1–00,0 | 12,828.6233 | (34) | 5 × 1011 | 83 |
| Ethylene oxide | (11) | 11,0–10,1 | 11,385.9111 | (35) | 8 × 1011 | 133 |
| Acetaldehyde | (12) | 11,1–20,2 | 8,243.4683 | (36) | 7 × 1012 | 1166 |
| Formic anhydride | (13) | 21,2–11,1 | 11,734.1252 | (37) | 2 × 1012 | 333 |
*The only accessible transition of formaldehyde in the 5 to 42 GHz range of our spectrometer is a low-frequency K -type doublet, which makes the molecular abundance calculation less certain.
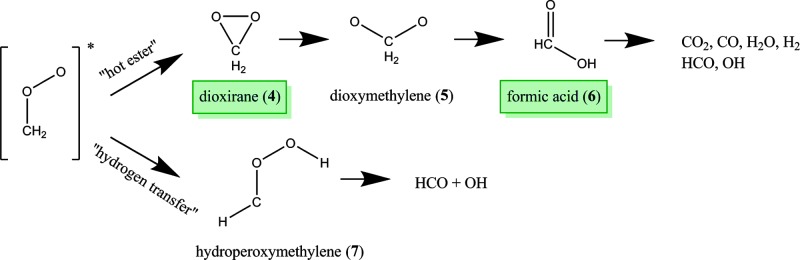
Fig. 4. The two unimolecular reactions available to vibrationally excited CH2OO.
The mechanism for each is discussed in the text. Shaded text boxes indicate the observed species.
Fig. 5. The most likely bimolecular reactions of CH2OO formed under our laboratory conditions.
The mechanisms and rate coefficients for each are discussed in the text. Shaded text boxes indicate species detected in our experiment.
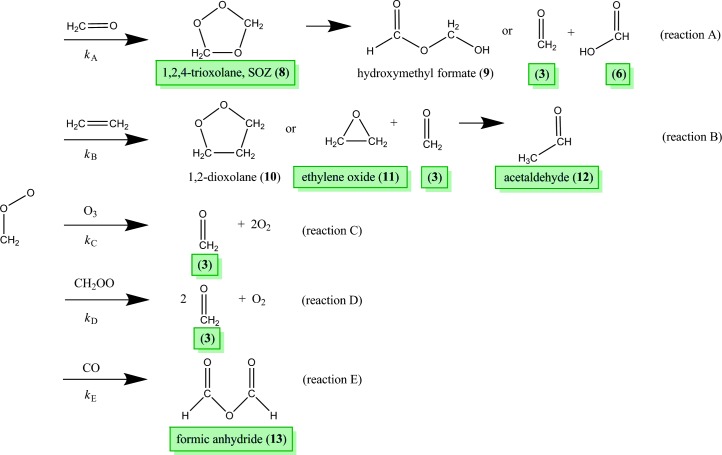
Figure 4 illustrates the two unimolecular pathways available for excited CH2OO, denoted as the “hot ester” and “hydrogen transfer” mechanisms. The hot ester pathway involves a ring-closing mechanism to form dioxirane (4), then O-O bond cleavage through an unstable dioxymethylene intermediate (5) to make formic acid (6). This pathway was experimentally verified by the matrix isolation FTIR experiments of Lovas et al. (32), and we detected dioxirane and formic acid in high relative concentrations: 50 and 3300 times that of CH2OO, respectively. Dioxymethylene is very reactive and has not been studied in the microwave region and, consequently, was not measured here. Some fraction of formic acid was presumably formed with sufficient vibrational energy to isomerize to CO2 + H2 and CO + H2O, but these two- and three-atom species do not have rotational frequencies in the 5 to 42 GHz range of our spectrometer. There is also some evidence of HCO + OH formation (38), but HCO cannot be measured in our spectrometer, and OH was not observed, likely because of its reactivity.
The second pathway available is the hydrogen transfer mechanism. In CIs with hydrocarbon substituents in the syn position, this pathway is a 1,4-hydrogen transfer and is highly favored, leading to either stabilized vinyl hydroperoxides or OH radicals (1, 3, 5). Indeed, this pathway is thought to be the dominant source of nighttime hydroxyl radicals in the troposphere (4). Because of the absence of β-hydrogens, CH2OO may instead undergo a 1,3-hydrogen transfer to the unstable hydroperoxymethylene (7), which immediately decomposes to HCO + OH. The microwave spectrum of 7 has not been previously measured and was therefore not measured here. However, this pathway is believed to have a barrier height more than 13 kcal/mol higher than the hot ester pathway (39) and is thus expected to be a minor reaction.
Figure 5 shows the major bimolecular reaction pathways of CH2OO in our experiment. Under true atmospheric conditions, the reaction of CH2OO with water is believed to be its dominant pathway (1) because of the prevalence of water in the troposphere (40). Other key co-reactants include SO2, NO2, and the water dimer; the reactivity of CH2OO with each has been well studied (23, 40–42). Reactions with ozone, alkenes, and carbonyl oxides have also been studied (43), but are less important in the atmosphere because of the relative scarcity of these molecules. Under our experimental conditions, however, O3, C2H4, and CH2O are present in high abundance, and thus, the bimolecular chemistry with CH2OO is dominated by the reactions of these three species.
CH2OO is highly reactive with carbonyl groups, and formaldehyde (3) is the most prevalent carbonyl in our system, not only because it is the cofragment of POZ decomposition but also because many bimolecular reactions of CH2OO yield formaldehyde. Indeed, Table 1 shows that formaldehyde is present in concentrations three orders of magnitude greater than any other product. The reaction with formaldehyde (reaction A; Fig. 5) proceeds through ethylene ozonide (8, also called 1,2,4-trioxolane or the secondary ozonide), which immediately decomposes to hydroxymethyl formate (9) or formaldehyde and formic acid. Our experimental results are in good agreement with this mechanism, as evidenced by the detection of ethylene ozonide, but in trace amounts relative to formic acid. Because the rotational spectrum of hydroxymethyl formate is unknown, its abundance could not be determined. Taatjes and co-workers (22) measured the reaction rate of CH2OO with acetaldehyde and found no evidence of secondary ozonide, but their experiments were done under low-pressure (4 torr) conditions, and secondary ozonide stabilization is likely pressure-dependent. The rate coefficient was recently estimated as kA = 6.0 × 10−13 cm3 molecule−1 s−1 (22, 43), but older studies (42) have reported values between 10−12 and 10−17 cm3 molecule−1 s−1.
The reaction of CH2OO with C2H4 (reaction B) was studied both experimentally (21) and theoretically (43, 44) and found to yield 1,2-dioxolane (10) through a barrierless pathway, although Crehuet et al. (44) suggest that a minor pathway, leading to ethylene oxide (11) and formaldehyde, is also possible. A portion of the ethylene oxide may isomerize to the more stable acetaldehyde isomer (12). We detected both 11 and 12, but the microwave spectrum of 10 has yet to be measured. Vereecken et al. (43) suggest an overall rate coefficient of kB = 5.45 × 10−15 cm3 molecule−1 s−1 for this reaction, but the experimental work of Buras et al. (21) indicates a rate coefficient of kB = 7 × 10−16 cm3 molecule−1 s−1, nearly an order of magnitude smaller.
The reactions of CH2OO with ozone (reaction C) and with itself (reaction D) both yield formaldehyde and molecular oxygen. The former reacts with a rate coefficient of kC = 1 × 10−12 cm3 molecule−1 s−1 (43), although there is some disagreement about whether the reaction proceeds through a biradical (43) or a cyclic (45, 46) intermediate. Neither intermediate would likely be sufficiently stable for detection in our system, nor are there microwave spectral data available to test that hypothesis. Vereecken et al. (43) report that the CH2OO self-reaction proceeds exothermically through a cyclic biperoxide before decomposing to 2 CH2O + O2.
Buras and co-workers (47) have measured this rate coefficient as kD = 6.0 × 10−11 cm3 molecule−1 s−1. Finally, CH2OO may react with CO to make formic anhydride (reaction E), as suggested by Kühne et al. (48). As CO is a dissociation product of vibrationally excited formic acid, this reaction is certainly possible, and we see evidence of formic anhydride (13). However, no experimental or theoretical rate constants have been published for this reaction, and we cannot measure the concentration of CO; thus, the importance of this reaction in our experiments remains unclear. Formic anhydride has also been suggested to be a product of the reaction of CH2OO with formic acid (49).
A simple kinetic model of the experimental CH2OO abundance is in qualitative agreement with the theoretically published rate constants. In this model, [CH2OO] was treated as a steady-state concentration, with its formation resulting from O3 + C2H4 alone [kform = 1.45 × 10−18 cm3 molecule−1 s−1 (10)], and its destruction occurring through unimolecular reaction [kuni = 75 s−1 (22, 47)], and the bimolecular reactions listed in Fig. 5 involving species with large abundances (kA through kD) only.
| 1 |
The concentrations of O3, C2H4, and CH2O are large and were thus treated as constant. Equation 1 was solved for [CH2OO] using the initial concentrations of [O3] = 0.005 M and [C2H4] = 0.0003 M, and the experimentally measured [CH2O] ≲ 0.002 M. Although several wide-ranging values of kA and kB have been published, neither has a large effect on [CH2OO], and thus, the most recently published value was selected. Other bimolecular reactions, including reaction E, were not included in this model because their rate coefficients have not been measured. The margins of error of all rate constants were assumed to be no smaller than an order of magnitude. This zeroth-order model, while rudimentary, predicts (3 ± 1) × 109 CH2OO molecules/pulse, in very good agreement with the about 6 × 109 molecules/pulse detected. The estimated CH2O abundance is uncertain because the only observable transition in the range of our spectrometer is a low-frequency K-type doublet, and consequently, its value has been derived solely on the basis of a population difference between two closely spaced K levels rather than two rotational levels. The derived value is therefore larger than expected given the initial concentration of C2H4, but it is likely due to the organic mixture rich in formaldehyde that builds up over time inside the nozzle body (see Materials and Methods). Regardless, it should be emphasized that the results of the kinetic model are largely insensitive to the CH2O concentration; an order of magnitude decrease in [CH2O] results in a predicted steady-state value of [CH2OO] = 3.7 × 109 molecules/pulse, which is well within the margin of error. We find instead that the reaction with ozone is the dominant destruction mechanism for CH2OO in our model.
As demonstrated by the numerous observed species, the mechanism of the ozonolysis of ethylene is quite complex. Although the experimental results agree qualitatively with the simple kinetic model, an as-of-yet undiscovered mechanism may conceivably be responsible for some fraction of the steady-state CH2OO abundance. For example, Wang et al. (50) calculated the energetic barriers and rate constants for the CH2O + O3 reaction, and found CH2OO + O2 to be a possible product channel. However, the barrier is higher than 60 kcal/mol, and the rate coefficient at 298 K was found to be on the order of 10−33 cm3 molecule−1 s−1. Given the concentration of ozone and formaldehyde found in these experiments, we would expect the formation of only 102 CH2OO molecules/pulse from this mechanism. Similarly, the CH2OO signal in our previous discharge studies of CH4/O2 (16) was tentatively attributed to a secondary reaction of excited CH3OO (51). However, only trace amounts of CH3OO were detected in the present discharge-free experiments, and thus, this reaction is not expected to significantly contribute to the CH2OO signal.
Experiments are currently under way to detect the monosubstituted CI, CH3CHOO, via the reaction of O3 with C3H6. Under similar experimental conditions, many of the analogs to the products in the O3 + C2H4 reaction have been observed, including propylene ozonide, propylene oxide, syn-vinyl alcohol, propionaldehyde, acetic acid, and acetone, in addition to the formaldehyde, acetaldehyde, and formic acid found in the ethylene reaction. However, neither the O3···C3H6 van der Waals complex nor methyldioxirane, c-(OOCH)-CH3, has been studied in the microwave region. Because detection of O3···C2H4 (1) and dioxirane (4) was a critical step in optimizing the experimental conditions in this study, our inability to optimize the experimental conditions in the O3 + C3H6 system using these analogs has made it difficult to definitively assign any signal to CH3CHOO.
CONCLUSIONS
We have reported the first detection of CH2OO in the gas-phase ozonolysis of ethylene at atmospheric temperature and pressure, using FTMW spectroscopy and a modified nozzle designed for rapid sampling. Although the signal was weak, it can be unequivocally attributed to CH2OO. Other products were detected in relative amounts that qualitatively support the established reaction pathways of the nascent excited and stabilized CIs in the literature. Although it is conceivable that an alternative mechanism is responsible for the CH2OO signal, the detected signals match very well with the kinetic model, and no other plausible mechanism has been suggested in the literature. Our results appear to confirm this long postulated oxidation mechanism. With improvements to the experimental methods described here, including developing a more consistent ozone source and increasing the steady-state concentration of CH2OO, this direct ozonolysis method may allow for the formation and characterization of larger CIs under atmospheric conditions. Direct formation from O3 + alkenes greatly simplifies the formation kinetic analysis and can yield important information about branching and OH radical formation, which are key questions for understanding the critical role of ozonolysis in atmospheric chemistry.
MATERIALS AND METHODS
In our nozzle design, two 1/16-inch-diameter holes were drilled into the inner body of a Parker-Hannifin Series 9 pulsed nozzle in a V-shape (Fig. 2). Stainless steel tubing (inner diameter 1/16 inch) was welded to each inlet, and O3 and ethylene were separately passed into the nozzle, using mass flow controllers to regulate carefully their flow. In one inlet, 2% C2H4 in neon flows at a rate of 50 sccm (cm3/min at STP), whereas a ~10% mixture of O3 in argon flows through the second inlet at 100 sccm. The O3 is obtained by trapping the ozone from a Welsbach ozonator on a silica gel trap maintained at −60°C and then flowing argon through the trap. Ozone is explosive in high concentrations, so for safety we collected very small samples of ozone on the trap (<500 mg) and refilled it every 2 hours. The rate of desorption of O3 is roughly proportional to the trapped concentration; thus, a high initial ozone concentration is observed (quantified by sodium thiosulfate titration), followed by a steady decrease over 2 hours. The concentration of O3 inside the nozzle can therefore only be estimated.
The reactants mix at 1 atm and 298 K inside the 1.6-cm3 body of the nozzle, and the pulsed nozzle injects ~3.5% of the resulting mixture into the FTMW spectrometer; the remainder of the gas vents through the rear of the nozzle. We estimate that the products are sampled within the first 0.5 s of the reaction because there is a high steady gas flow and sampling occurs at a rate of 6 Hz. The signal levels began to diminish after about 2 hours, ostensibly owing to the buildup of a thin film of organic material on the inside of the nozzle, which required manual cleaning. The microwave spectrum obtained by flowing an inert gas over this material indicates the presence of formic acid and formaldehyde, but the low volatility of the material suggests the additional presence of larger oligomers.
A portion of the reaction mixture is supersonically expanded (Trot ≈ 3 K) into a vacuum chamber maintained at 10−6 torr, and probed by a 5 to 42 GHz cavity FTMW spectrometer. A tunable microwave synthesizer generates ~1 μs microwave pulses, which excites all rotational transitions that fall within the bandwidth of the pulse. Coherently rotating molecules undergo free induction decay, which is detected using a sensitive microwave receiver; a Fourier transform of this signal yields a power spectrum. Products in the O3 + C2H4 reaction are detected by monitoring known low-J rotational transitions of each. No attempt was made to detect species whose microwave transitions have yet to be measured. The species detected are shown in Table 1, along with their molecular abundances, estimated by calibrating with a stable molecule of known abundance [0.04% carbonyl sulfide (OCS) in Ar] and corrected for differences in dipole moments, rotational partition functions, and the spectrometer instrument response function.
Acknowledgments
We gratefully acknowledge F. Lovas, J. Stanton, T. Lam Nguyen, W. Green, Z. Buras, and J. Kroll for helpful discussions. We would also like to thank W. Pringle for the loan of an ozonator, E. S. Palmer and P. Antonucci for technical assistance, and K. Crabtree for developing the data acquisition software. Funding: This work was supported by the National Science Foundation under grant no. CHE-1058063. C.C.W. acknowledges the support of the Camille and Henry Dreyfus Foundation Postdoctoral Program in Environmental Chemistry. Author contributions: C.C.W., M.-A.M.-D., and G.G.B. collected the data; C.C.W., R.W.F., and M.C.M. designed the study; and C.C.W. and M.C.M. wrote the manuscript. Competing interests: The authors declare that they have no competing financial interests.
REFERENCES
- 1.Johnson D., Marston G., The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem. Soc. Rev. 37, 699–716 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Taatjes C. A., Shallcross D. E., Percival C. J., Research frontiers in the chemistry of Criegee intermediates and tropospheric ozonolysis. Phys. Chem. Chem. Phys. 16, 1704–1718 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Donahue N. M., Drozd G. T., Epstein S. A., Presto A. A., Kroll J. H., Adventures in ozoneland: Down the rabbit-hole. Phys. Chem. Chem. Phys. 13, 10848–10857 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Vereecken L., Francisco J. S., Theoretical studies of atmospheric reaction mechanisms in the troposphere. Chem. Soc. Rev. 41, 6259–6293 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Olzmann M., Kraka E., Cremer D., Gutbrod R., Andersson S., Energetics, kinetics, and product distributions of the reactions of ozone with ethene and 2,3-dimethyl-2-butene. J. Phys. Chem. A 101, 9421–9429 (1997). [Google Scholar]
- 6.Li J., Carter S., Bowman J. M., Dawes R., Xie D., Guo H., High-level, first-principles, full-dimensional quantum calculation of the rovibrational spectrum of the simplest Criegee intermediate (CH2OO). J. Phys. Chem. Lett. 5, 2364–2369 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Su Y.-T., Lin H.-Y., Putikam R., Matsui H., Lin M. C., Lee Y.-P., Extremely rapid self-reaction of the simplest Criegee intermediate CH2OO and its implications in atmospheric chemistry. Nat. Chem. 6, 477–483 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Ahrens J., Carlsson P. T. M., Hertl N., Olzmann M., Pfeifle M., Wolf J. L., Zeuch T., Infrared detection of Criegee intermediates formed during the ozonolysis of β-pinene and their reactivity towards sulfur dioxide. Angew. Chem. Int. Ed. Engl. 53, 715–719 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Atkinson R., Gas-phase tropospheric chemistry of volatile organic compounds: 1. Alkanes and alkenes. J. Phys. Chem. Ref. Data 26, 215–290 (1997). [Google Scholar]
- 10.Alam M. S., Camredon M., Rickard A. R., Carr T., Wyche K. P., Hornsby K. E., Monks P. S., Bloss W. J., Total radical yields from tropospheric ethene ozonolysis. Phys. Chem. Chem. Phys. 13, 11002–11015 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Drozd G. T., Donahue N. M., Pressure dependence of stabilized Criegee intermediate formation from a sequence of alkenes. J. Phys. Chem. A 115, 4381–4387 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Geletneky C., Berger S., The mechanism of ozonolysis revisited by 17O-NMR spectroscopy. Eur. J. Org. Chem. 1998, 1625–1627 (1998). [Google Scholar]
- 13.Taatjes C. A., Meloni G., Selby T. M., Trevitt A. J., Osborn D. L., Percival C. J., Shallcross D. E., Direct observation of the gas-phase Criegee intermediate (CH2OO). J. Am. Chem. Soc. 130, 11883–11885 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Welz O., Savee J. D., Osborn D. L., Vasu S. S., Percival C. J., Shallcross D. E., Taatjes C. A., Direct kinetic measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2. Science 335, 204–207 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Su Y.-T., Huang Y.-H., Witek H. A., Lee Y.-P., Infrared absorption spectrum of the simplest Criegee intermediate CH2OO. Science 340, 174–176 (2013). [DOI] [PubMed] [Google Scholar]
- 16.McCarthy M. C., Cheng L., Crabtree K. N., Martinez O. Jr, Nguyen T. L., Womack C. C., Stanton J. F., The simplest Criegee intermediate (H2COO): Isotopic spectroscopy, equilibrium structure, and possible formation from atmospheric lightning. J. Phys. Chem. Lett. 4, 4133–4139 (2013). [Google Scholar]
- 17.Nakajima M., Endo Y., Determination of the molecular structure of the simplest Criegee intermediate CH2OO. J. Chem. Phys. 139, 101103 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Nakajima M., Endo Y., Spectroscopic characterization of an alkyl substituted Criegee intermediate syn-CH3CHOO through pure rotational transitions. J. Chem. Phys. 140, 011101 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Beames J. M., Liu F., Lu L., Lester M. I., Ultraviolet spectrum and photochemistry of the simplest Criegee intermediate CH2OO. J. Am. Chem. Soc. 134, 20045–20048 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Lehman J. H., Li H., Beames J. M., Lester M. I., Ultraviolet photodissociation dynamics of the simplest Criegee intermediate CH2OO. J. Chem. Phys. 139, 141103 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Buras Z. J., Elsamra R. M. I., Jalan A., Middaugh J. E., Green W. H., Direct kinetic measurements of reactions between the simplest Criegee intermediate CH2OO and alkenes. J. Phys. Chem. A 118, 1997–2006 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Taatjes C. A., Welz O., Eskola A. J., Savee J. D., Osborn D. L., Lee E. P., Dyke J. M., Mok D. W., Shallcross D. E., Percival C. J., Direct measurement of Criegee intermediate (CH2OO) reactions with acetone, acetaldehyde, and hexafluoroacetone. Phys. Chem. Chem. Phys. 14, 10391–10400 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Taatjes C. A., Welz O., Eskola A. J., Savee J. D., Scheer A. M., Shallcross D. E., Rotavera B., Lee E. P., Dyke J. M., Mok D. K., Osborn D. L., Percival C. J., Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science 340, 177–180 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Anglada J. M., Crehuet R., Bofill J. M., The ozonolysis of ethylene: A theoretical study of the gas-phase reaction mechanism. Chem. Eur. J. 5, 1809–1822 (1999). [Google Scholar]
- 25.Ponec R., Roithová J., Haas Y., Mechanism of gas phase ethene-ozone reaction and concomitant processes. Theoretical study. Croat. Chem. Acta 74, 251–264 (2001). [Google Scholar]
- 26.Balle T. J., Flygare W. H., Fabry-Pèrot cavity pulsed Fourier transform microwave spectrometer with a pulsed nozzle particle source. Rev. Sci. Instrum. 52, 33–45 (1981). [Google Scholar]
- 27.Gillies C. W., Gillies J. Z., Suenram R. D., Lovas F. J., Kraka E., Cremer D., Van der Waals complexes in 1,3-dipolar cycloaddition reactions: Ozone-ethylene. J. Am. Chem. Soc. 113, 2412–2421 (1991). [Google Scholar]
- 28.Gillies J. Z., Gillies C. W., Lovas F. J., Matsumura K., Suenram R. D., Kraka E., Cremer D., Van der Waals complexes of chemically reactive gases: Ozone-acetylene. J. Am. Chem. Soc. 113, 6408–6415 (1991). [Google Scholar]
- 29.Zozom J., Gillies C. W., Suenram R. D., Lovas F. J., Microwave detection of the primary ozonide of ethylene in the gas phase. Chem. Phys. Lett. 140, 64–70 (1987). [Google Scholar]
- 30.Gillies J. Z., Gillies C. W., Suenram R. D., Lovas F. J., Stahl W., The microwave spectrum and molecular structure of the ethylene-ozone van der Waals complex. J. Am. Chem. Soc. 111, 3073–3074 (1989). [Google Scholar]
- 31.Bocquet R., Demaison J., Poteau L., Liedtke M., Belov S., Yamada K. M. T., Winnewisser G., Gerke C., Gripp J., Köhlerc T., The ground state rotational spectrum of formaldehyde. J. Mol. Spectrosc. 177, 154–159 (1996). [Google Scholar]
- 32.Suenram R. D., Lovas F. J., Dioxirane. Its synthesis, microwave spectrum, structure, and dipole moment. J. Am. Chem. Soc. 100, 5117–5122 (1978). [Google Scholar]
- 33.Winnewisser M., Winnewisser B. P., Stein M., Birk M., Wagner G., Winnewisser G., Yamada K. M. T., Belov S. P., Baskakov O. I., Rotational spectra of cis-HCOOH, trans-HCOOH, and trans-H13COOH. J. Mol. Spectrosc. 216, 259–265 (2002). [Google Scholar]
- 34.Gillies C. W., Kuczkowski R. L., Mechanism of ozonolysis. Microwave spectrum, structure, and dipole moment of ethylene ozonide. J. Am. Chem. Soc. 94, 6337–6343 (1972). [Google Scholar]
- 35.Pan J., Sieghard A., Sastry K. V. L. N., Herbst E., De Lucia F. C., The millimeter- and submillimeter-wave spectrum of ethylene oxide (c-C2H4O). Astrophys. J. 499, 517–519 (1998). [Google Scholar]
- 36.Kleiner I., Lovas F. J., Godefroid M., Microwave spectra of molecules of astrophysical interest. XXIII. Acetaldehyde. J. Phys. Chem. Ref. Data 25, 1113–1210 (1996). [Google Scholar]
- 37.Vaccani S., Bauder A., Günthard Hs. H., Microwave spectrum, dipole moment and conformation of formic anhydride. Chem. Phys. Lett. 35, 457–460 (1975). [Google Scholar]
- 38.Kroll J. H., Donahue N. M., Cee V. J., Demerjian K. L., Anderson J. G., Gas-phase ozonolysis of alkenes: Formation of OH from anti carbonyl oxides. J. Am. Chem. Soc. 124, 8518–8519 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Gutbrod R., Schindler R. N., Kraka E., Cremer D., Formation of OH radicals in the gas phase ozonolysis of alkenes: The unexpected role of carbonyl oxides. Chem. Phys. Lett. 252, 221–229 (1996). [Google Scholar]
- 40.Anglada J. M., González J., Torrent-Sucarrat M., Effects of the substituents on the reactivity of carbonyl oxides. A theoretical study on the reaction of substituted carbonyl oxides with water. Phys. Chem. Chem. Phys. 13, 13034–13045 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Vereecken L., Harder H., Novelli A., The reaction of Criegee intermediates with NO, RO2, and SO2, and their fate in the atmosphere. Phys. Chem. Chem. Phys. 14, 14682–14695 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Fenske J. D., Hasson A. S., Ho A. W., Paulson S. E., Measurement of absolute unimolecular and bimolecular rate constants for CH3CHOO generated by the trans-2-butene reaction with ozone in the gas phase. J. Phys. Chem. A 104, 9921–9932 (2000). [Google Scholar]
- 43.Vereecke L., Harder H., Novelli A., The reactions of Criegee intermediates with alkenes, ozone, and carbonyl oxides. Phys. Chem. Chem. Phys. 16, 4039–4049 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Crehuet R., Anglada J. M., Cremer D., Bofill J. M., Reaction modes of carbonyl oxide, dioxirane, and methylenebis(oxy) with ethylene: A new reaction mechanism. J. Phys. Chem. A 106, 3917–3929 (2002). [Google Scholar]
- 45.Kjaergaard H. G., Kurtén T., Nielsen L. B., Jørgensen S., Wennberg P. O., Criegee intermediates react with ozone. J. Phys. Chem. Lett. 4, 2525–2529 (2013). [Google Scholar]
- 46.Wei W. M., Zheng R. H., Pan Y. L., Wu Y. K., Yang F., Hong S., Ozone dissociation to oxygen affected by Criegee intermediate. J. Phys. Chem. A 118, 1644–1650 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Buras Z. J., Elsamra R. M. I., Green W. H., Direct determination of the simplest Criegee intermediate (CH2OO) self reaction rate. J. Phys. Chem. Lett. 5, 2224–2228 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Kühne H., Vaccani S., Ha T. K., Bauder A., Günthard H. H., Infrared-matrix and microwave spectroscopy of the ethylene-ozone gas-phase reaction. Chem. Phys. Lett. 38, 449–455 (1976). [Google Scholar]
- 49.Osada H., Morikawa Y., Nishiwaki T., Sekiya S., Proof of the formation of hydroperoxymethyl formate in the ozonolysis of ethene: Synthesis and FT-IR spectra of the authentic compound. Chem. Phys. Lett. 258, 155–158 (1996). [Google Scholar]
- 50.Wang F., Sun H., Sun J., Jia X., Zhang Y., Tang Y., Pan X., Su Z., Hao L., Wang R., Mechanistic and kinetic study of CH2O+O3 reaction. J. Phys. Chem. A 114, 3516–3522 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Nguyen T. L., McCarthy M. C., Stanton J. F., Relatively selective production of the simplest Criegee intermediate in a CH4/O2 electric discharge: Kinetic analysis of a plausible mechanism. J. Phys. Chem. A (2014). [DOI] [PubMed] [Google Scholar]