Sexual selection has acted on sexual differences in bird plumage color, while natural selection has acted on the color of both sexes.
Keywords: comparative analysis, dimorphism, plumage, sexual selection, spectrometry
Abstract
The bright colors of birds are often attributed to sexual selection on males, but in many species both sexes are colorful and it has been long debated whether sexual selection can also explain this variation. We show that most evolutionary transitions in color have been toward similar plumage in both sexes, and the color of both sexes (for example, bright or dull) was associated with indices of natural selection (for example, habitat type), whereas sexual differences in color were primarily associated with indices of sexual selection on males (for example, polygyny and large testes size). Debate about the evolution of bird coloration can be resolved by recognizing that both natural and sexual selection have been influential, but they have generally acted on two different axes: sexual selection on an axis of sexual differences and natural selection on both sexes for the type of color (for example, bright or dull).
INTRODUCTION
Sexual dichromatism in birds has provided a model system for understanding sexual (1) and natural (2) selection and their roles in speciation (3). For example, Darwin’s (4) theory of sexual selection was based on his observations of the bright colors of males, which he thought were preferred by females and led to a mating advantage for more colorful males. Wallace, on the other hand, pointed out that in many species, females are as “gay and brilliant” as the male, and he suggested that dichromatism evolved as a consequence of nest predation favoring more cryptic females (5). Debate over the evolution of plumage color continues to this day with evidence for both natural (6, 7) and sexual (8, 9) selection acting on plumage color. Part of the controversy may be related to two main limitations of our understanding of plumage color evolution.
First, most studies have examined differences in color between the sexes without quantifying the color of males and females separately. As the debate between Darwin and Wallace illustrates, it is necessary to know if males are becoming brighter or females duller to determine how evolution has produced dichromatism (9, 10). Second, the focus on sexual dichromatism limits our ability to determine how and why monochromatism arises. For example, why are both sexes colorful or both dull? Evolutionary transitions to monochromatism may actually be more common than transitions to dichromatism (11, 12), but it is not known what factors produce these changes in plumage.
Both dichromatism and monochromatism can be produced by natural and sexual selection. For example, dichromatism is often greater in species with stronger sexual selection, as indexed by mating system (for example, polygyny) (9); however, natural selection could also favor dichromatism, if the risk of nest predation favors duller plumage in females than males (Wallace’s hypothesis). Similarly, monochromatism may be favored by sexual (or social) selection if bright plumage in both sexes helps them choose mates or compete intrasexually for territories or other resources (13). Natural selection could also favor monochromatism if both sexes provide parental care and dull plumage in both sexes increases crypsis and, consequently, reduces nest predation. Thus, the extent of dichromatism could be correlated with indices of sexual or natural selection, but we might expect the color (brightness and hue) of both sexes to be primarily correlated with indices of natural selection, such as predation risk, because they are more likely to affect both sexes.
Thus, a comprehensive understanding of plumage color will require analysis of all types of plumage change in each sex. Here, we examined both male and female plumage color in relation to 10 indices of natural and sexual selection to test whether dichromatism was primarily due to sexual selection, as Darwin (4) proposed, whereas the color of both sexes (for example, whether both sexes were dull or bright) was primarily due to natural selection.
RESULTS
Color variation within and between the sexes
We used museum specimens to measure the reflectance spectra (320 to 700 nm) of male and female breeding plumage in a worldwide sample of 977 species (~10% of all species) representing at least 79% of avian orders (data file S1). Most (97%) of the variation in plumage reflectance were described by the first (PC1; 91%) and second (PC2; 6%) principal components, which correspond to brightness and hue, respectively (table S1). Across species, males increased in brightness (Fig. 1A) and hue (Fig. 1C) at a greater rate than did females in phylogenetic regressions. However, there was often more variation in plumage color within a sex than between them (that is, dichromatism; N = 977 species). For example, variation in male brightness (PC1 scores; SD = 13.2; variance ratio test, F976,976 = 5.26, P < 0.001) and female brightness (SD = 12.1; F976,976 = 4.4, P < 0.001) were both greater than variation in brightness between the sexes (that is, dichromatism; SD = 5.8). There was also greater variation in male hue (SD = 4.2) than in sexual dichromatism in hue (SD = 3.1; F976,976 = 1.86, P < 0.001). On the other hand, female hue (SD = 2.8) was not more variable than sexual dichromatism in hue (SD = 3.1; F976,976 = 0.86, P = 0.99).
Fig. 1. Brightness (A) and hue (C) of males and females are strongly correlated in phylogenetic reduced major axis (RMA) regressions of PC scores (red lines).
Males increased in brightness (r2 = 0.74, slope: 1.13, t715 = 7.4, P < 0.001) and hue (r2 = 0.40, slope: 1.52, t816 = 16.9, P < 0.001) at a greater rate than females did (equal rate of change is indicated by the blue dashed line). (B and D) Most evolutionary transitions in brightness (B) and hue (D) were from sexually dichromatic to monochromatic for both males (blue arrows) and females (red arrows). For clarity, arrows are only shown where at least 3% of transitions occurred [median (range) for both PC1 and PC2: 1.0% (0 to 3.7%); n = 72 possible transitions from nine states]. The percentage of evolutionary time in each of the nine states is indicated inside each box. Analysis was based on stochastic character mapping of three categories of brightness and hue for each sex.
Evolutionary transitions to monochromatism
To examine the evolutionary changes that led to these positive correlations between male and female color, we divided the color (PC) scores for each sex into three equal categories of brightness (dull, medium, and bright) or hue {low [orange/red], medium [green/yellow], and high [ultraviolet (UV)/blue]}. Using these categories, we found that there were more evolutionary transitions in brightness (PC1) to monochromatism (that is, both sexes were dull, medium, or bright; median = 43.7 transitions per phylogeny) than to dichromatism (27.9 transitions; Wilcoxon test Z = 3.2, P = 0.001; Fig. 1B) when we analyzed a random sample of 100 phylogenies with stochastic character mapping (14). Evolutionary transitions were equally likely to result in increases (median = 62.5 per phylogeny) or decreases (63.2 transitions) in brightness by one or both sexes (Wilcoxon test Z = 0.12, P = 0.90). Transitions to monochromatism were also equally likely to come from changes in males (median = 88.5 transitions per phylogeny) or females (91.4 transitions; Wilcoxon test Z = 1.52, P = 0.13). Evolutionary changes in hue (using three equal PC2 categories as above) were also more frequent toward monochromatism (median = 39.1 transitions per phylogeny) than dichromatism (24.8 transitions; Wilcoxon test Z = 3.23, P = 0.001; Fig. 1D), and again, transitions were equally likely to result in increases (57.2 transitions per phylogeny) or decreases (56.0 transitions) in hue by one or both sexes (Wilcoxon test Z = 0.74, P = 0.45). Transitions toward monochromatic hue were also equally likely to come from changes in males (median = 83.3 transitions per phylogeny) or females (84.8 transitions; Wilcoxon test Z = 0.24, P = 0.81).
Selection on monochromatism
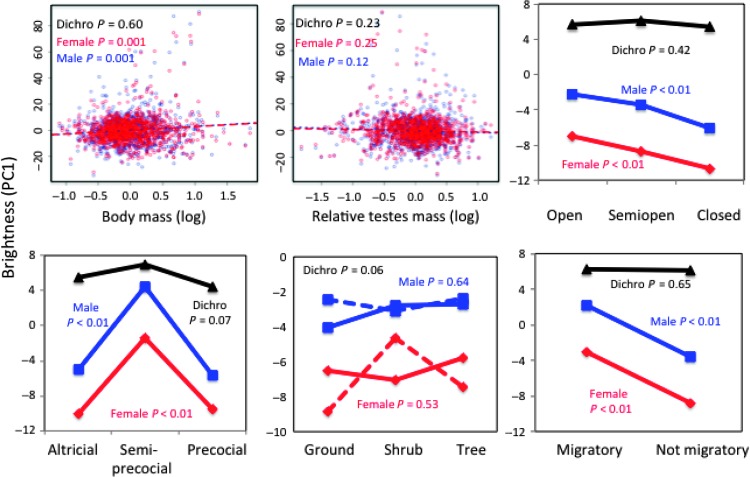
When we examined plumage color in relation to 10 indices of sexual and natural selection, we found that similar changes in the color of both sexes (that is, toward both dull or both bright) were primarily related to indices of natural selection, whereas changes in one sex (leading to sexual dichromatism) were related to indices of both sexual and natural selection (Figs. 2 to 4 and tables S2 and S3). To examine the factors associated with color changes in both sexes, we restricted the analysis to the middle 50% of species (n = 489) in which both sexes had relatively similar plumage (that is, species in the interquartile range of sexual dichromatism). This allowed us to focus on the factors that influence changes in brightness and hue in both sexes without the potentially confounding effects of large changes in dichromatism. In these 489 monochromatic species, brighter plumage was associated with migratory behavior, breeding in the subtropics, semiprecocial young, male parental care, and open (noncavity) nests (table S2). Duller plumage in both sexes was associated with sedentary behavior, breeding in the tropics, altricial young, lack of male parental care, and cavity nesting. Note that these are general characteristics, because some of these variables rarely co-occur (for example, only 5% of species had both altricial young and no male care). Plumage with more UV/blue/green reflectance (higher PC2) in both sexes was associated with larger body mass, sedentary behavior, semiprecocial young (that is, gulls with white UV-reflecting plumage), male parental care, and nesting in trees (table S2). More red/orange reflectance (lower PC2) in the plumage was associated with smaller body mass, migratory behavior, altricial young, lack of male parental care, and nesting on the ground.
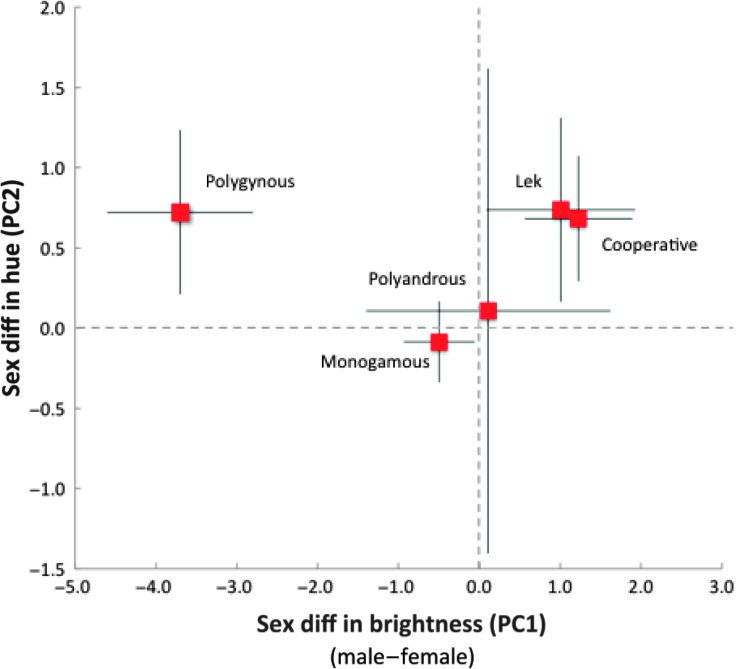
Fig. 2. Sexual dichromatism in brightness (PC1) and hue (PC2) in relation to mating system categories.
Mean (squares) and SE (lines) values are based on full phylogenetic generalized least squares (PGLS) models (table S3). Note that polygynous males were duller than females because many species had extensive black plumage (fig. S3).
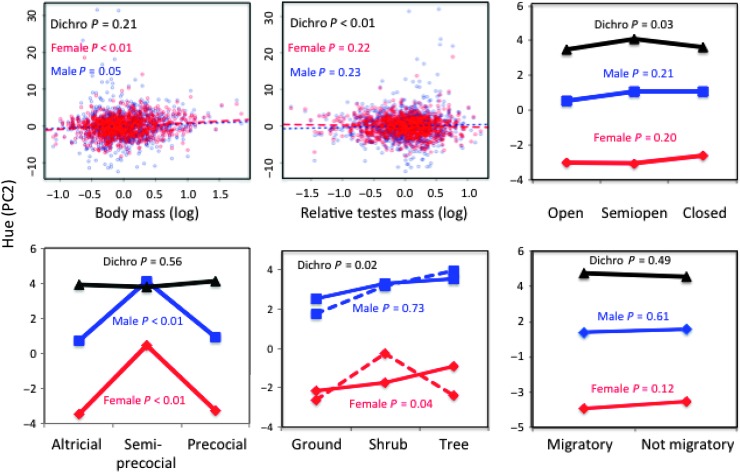
Fig. 4. Plumage hue (PC2) for each sex in relation to morphological, ecological, and behavioral traits.
Mean and P values are based on full PGLS models (table S3). See Fig. 3 legend for more details.
Selection on dichromatism
In contrast, sexual dichromatism was associated with indices of both sexual and natural selection (these analyses used all 977 species; table S3). Sexual differences in brightness (P = 0.021) and hue (P = 0.011) were associated with changes in male plumage across mating systems, and not to changes in female plumage (P = 0.574 for PC1 and P = 0.459 for PC2; tables S3 to S15). In terms of sexual selection, dichromatism in brightness and hue was strongest in lekking, other polygynous, and cooperatively-breeding species (Fig. 2). Furthermore, dichromatism in hue (PC2) was greater in species with relatively larger testes (P = 0.009; Fig. 4 and table S3), a correlate of sperm competition (15).
Sexual selection produced dichromatism in different ways. In lekking species, males were brighter and had greater reflectance in the UV/blue portion of the spectrum than females. However, in other polygynous species, males were generally duller than females (Fig. 2) because many of them had extensive black plumage, which has low reflectance (fig. S3). These patterns were complicated by variation in dichromatism across breeding latitudes (fig. S1 and tables S1 and S3). For example, although males in lekking species had brighter plumage than females on average, lekking species in the subtropics had the dullest males relative to females of any category. Also, in cooperatively-breeding species, males and females were relatively similar in hue in tropical and subtropical regions, but in temperate locations, males had more UV/blue reflectance than females, which was partly due to the strong blue reflectance of fairy-wren males (Maluridae).
In terms of natural selection, dichromatism was also related to nest height and male parental care (table S3). Species nesting at shrub level are generally at greatest risk of nest predation (16), and thus, we might expect changes in female plumage, as well as that of males if they provide parental care, to occur at this level. Indeed, the main changes in plumage in relation to nest height occurred among females nesting at shrub level (Figs. 3 and 4). However, these changes occurred primarily in species in which females lack male parental assistance (for example, polygynous and lekking species). Males did not vary in brightness or hue in relation to nest height, regardless of their participation in parental care (Figs. 3 and 4). Last, dichromatism in hue (PC2) was greater in semi-open (for example, edge or woodland) than open or closed habitats, because male plumage had more UV/blue reflectance than females (Fig. 4).
Fig. 3. Plumage brightness (PC1) for each sex in relation to morphological, ecological, and behavioral traits.
Mean and P values are based on full PGLS models (table S3). Regression lines from PGLS models are shown for each sex (males, blue; females, red) plotted against the original body and testes mass data. P values for nest height refer to interactions between nest height and male parental care [coded yes (solid line) or no (dashed line)]. Dichromatism is the sum of PC scores for males minus the sum for females.
DISCUSSION
As predicted by Darwin, sexual differences in plumage color were strongly related to indices of sexual selection such as the type of mating system and, in the case of hue, sperm competition (testes mass). However, the focus on dichromatism for the past 120 years might be a bit misplaced because most evolutionary transitions have been to monochromatism, and the direction of changes in color in both sexes was related primarily to indices of natural selection (table S2). This dichotomy in selection only became obvious after analyzing the color of each sex separately. Researchers have called for separate analyses of each sex for over a decade (17), but this is the first large-scale study to examine the color of each sex in relation to indices of both natural and sexual selection.
As found in several previous studies (1, 9), sexual dichromatism was greater among polygynous species, which have a high variance in the number of social mates attracted by males (apparent mating success) (18, 19), as well as some cooperatively breeding species, such as fairy-wrens, which can have strong sexual selection as a consequence of extrapair mating (20, 21). However, dichromatism varied with latitude even after controlling for migratory behavior (tables S1 and S3). Several previous studies have suggested that migratory behavior has a major influence on dichromatism because it increases sexual competition for breeding territories or facilitates more rapid mate choice when females are constrained by short breeding seasons at higher latitudes (6, 22, 23). However, these studies did not control for breeding latitude, which covaries with both migration and ecology. By controlling for migration, our results suggest that particular ecological conditions in combination with certain types of mating systems (for example, tropical lekking or temperate cooperative breeding) result in greater competition for mates and increased opportunity for sexual selection on plumage. However, it is important to note that migration was associated with brighter plumage in both sexes, despite the lack of association with sexual dichromatism. Monochromatic bright plumage could be favored in migratory species if it is used in competition to gain access to resources or it facilitates assessment of mates in both sexes.
Dichromatism was also related to nest height and habitat openness, although the patterns were not easily attributable to selection from nest predation, as predicted by Wallace. Changes in dichromatism with nest height occurred primarily because of changes in female plumage and specifically in species without male parental care. The lack of change in female plumage among species with biparental care suggests that nest predation—at least as indexed by nest height—may be a less important selective force when there are two parents that can potentially defend the nest and alert each other to predators.
Although most studies of bird plumage focus on dichromatism, evolutionary change has most often led to similar, rather than different, plumage in males and females. Trends toward sexual monochromatism have been found in some smaller-scale studies and have been attributed to both gains (17, 24) and losses of elaborate plumage in females (25). Our study indicates that monochromatism in both brightness (PC1) and hue (PC2) was equally likely to arise from changes in either sex (Fig. 1), and among monochromatic species, changes in color were associated with ecological rather than sexual variables (table S2). This suggests that natural selection has been the most important source of selection on plumage color in monochromatic species, and thus, bright colors in both sexes are unlikely to be due to a correlated response in females to sexual selection on male plumage. Overall, both natural and sexual selection have influenced the evolution of bird coloration, but in many respects, they have acted on two different axes: sexual selection on an axis of sexual differences and natural selection on an axis of color (for example, dull or bright) in both sexes. Thus, debate about the causes of variation in bird coloration may be resolved by recognizing that natural and sexual selection have generally acted on two different axes.
MATERIALS AND METHODS
Experimental design
We measured the spectral reflectance of plumage colors from museum specimens of 977 species of birds (data file S1). We sampled three adult specimens of each sex for each species. These sample sizes are adequate to minimize type I errors (26) because more than 90% of the variation in color characteristics was found between, rather than within, species (27). For each specimen, we measured reflectance across the bird-visible spectrum (320 to 700 nm) (28, 29) at six body regions: crown, back, tail, throat, belly, and wing coverts using a spectrometer (Ocean Optics USB2000).
Analysis of color
For each reflectance spectrum, we averaged the reflectance data into bins 20 nm wide and then performed a principal components analysis (PCA) on the “binned” (mean reflectance) values from each reflectance spectrum (n = 175,860 spectra used in PCA). Although there are multiple methods of measuring color and dichromatism (30), we chose PCA for its simplicity and because our focus was on the reflectance patterns of birds without making assumptions about the visual systems of potential receivers. See Supplementary Materials and Methods for more details.
Similar to previous studies (29, 31, 32), we found that PC1 loaded evenly across all wavelengths, and PC2 loaded positively in the shorter (UV/blue/green) wavelengths and negatively in the longer (orange/red) wavelengths (table S1; see fig. S2 for examples). We summed these PC scores across all six body regions for each sex and axis (PC1 or PC2) to gain an overall index of brightness (PC1) or hue (PC2). These PC1 and PC2 scores were analyzed to examine how the plumage reflectance of each sex changed in response to the other using RMA regression with corrections for phylogeny as implemented in the R package phytools (33).
For analyses of sexual dichromatism, we calculated differences between the sexes in PC1 and PC2 for each body region for males and females of each species and then summed these differences from all six body regions to produce a dichromatism score for each PC axis and species. We used the sum of the differences of all body regions, rather than the sum of the absolute differences, so that the total score reflected the directionality of the dichromatism across all body regions (see Supplementary Materials and Methods for more details).
Ecological and life history variables
We analyzed 10 ecological and life history variables thought to be associated with plumage color or dimorphism in birds (data file S1). Most of these data were compiled in our previous study that analyzed dichromatism using human visual estimates (9).
As indices of sexual selection, we used the social mating system and testes size relative to total body mass because they are related to variation in social (18) and extrapair (15) mating success, respectively. Mating systems were coded as follows: monogamous, cooperative or group living, lekking or promiscuous, polygynous (but not lekking), or polyandrous (9). We also examined a variety of ecological variables that may influence natural selection on plumage, either directly through their effects on predation or indirectly through trade-offs with reproductive investment (for example, parental care may reduce the opportunity for gaining mates). These variables included total body mass (log-transformed), breeding latitude (tropical, subtropical, temperate to polar), habitat cover (open, semi-open, or closed), migratory behavior (yes/no), development (precocial, semiprecocial, or altricial), nest height (ground, shrub, or tree level), cavity nesting (yes/no), and male participation in parental care (yes/no). Models were examined with these 10 variables, as well as interactions between parental care and latitude, nest height, and cavity nesting to test for differential effects of ecology on male plumage. See Supplementary Materials and Methods for more details and justification.
Phylogenetic analyses
In analyses of continuous dependent variables, such as dichromatism scores, we controlled for similarity between species due to shared ancestry in PGLS models implemented in the R packages ape (34) and nlme (35). The phylogeny used in these analyses was based on a recent analysis of all 9993 species of birds (36) using the topology of orders from Hackett et al. (37). To account for phylogenetic uncertainty in the analyses, we downloaded a randomly selected set of 100 of these trees from the website birdtree.org. A nexus file with the 100 trees is provided in the Supplementary Materials and Methods (data file S2).
We analyzed our data using evolutionary models that incorporated the Ornstein-Uhlenbeck (OU) (38) process, because it provided a better fit than models based on Brownian motion [ΔAIC (Akaike information criterion) was >10 for Brownian models compared to OU models]. OU models assume that there is a trait optimum and that the strength of selection (α) is proportional to the distance of the current trait from the optimum. When α is near zero, the OU model is similar to a Brownian motion model; however, large values of α imply stronger stabilizing selection (38), and thus, phenotypic differences between species will be less affected by their divergence times.
To study the direction and frequency of evolutionary changes in plumage brightness (PC1) and hue (PC2) in each sex, we mapped plumage onto samples of the 100 trees (data files S3 and S4) using stochastic character mapping in SIMMAP v. 1.5 (39), which implements Bayesian methods in Huelsenbeck et al. (14). For this study, the main advantages of this method are the following: (i) we can use more than two categories of color [we used three (low, medium, and high) for each PC axis], (ii) it assumes that traits are more likely to change on longer branches, and (iii) it incorporates phylogenetic uncertainty by sampling ancestral states conditional on the state at the tips of trees. Analyses were based on 500 mutational maps (5 draws from each of 100 trees) using equal (1/k) priors for the bias parameter and a gamma distribution for the rate parameter.
Supplementary Material
Acknowledgments
We thank the curators and collection managers of the following museums for access to specimens: American Museum of Natural History (New York), Australian National Wildlife Collection (Canberra), Field Museum of Natural History (Chicago), Louisiana State University Museum of Natural Science (Baton Rouge), Museum Victoria (Melbourne), and National Museum of Natural History (Washington, DC). We thank G. Hoebel, R. Montgomerie, T. Pizzari, T. Price, R. Rodriguez, and K. Yasukawa for helpful comments on the manuscript. Funding: This work was supported by the National Science Foundation (NSF) grant DEB-0215560 to P.O.D. and L.A.W., and by the NSF and University of Wisconsin–Milwaukee graduate research fellowships to J.K.A. Author contributions: All authors designed the study, J.K.A. collected the data, P.O.D. and J.K.A. analyzed the data, and all authors contributed to the writing of the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data files are available online on the Science Advances website.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/2/e1400155/DC1
Materials and Methods
Fig. S1. Sexual dichromatism in brightness (PC1) and hue (PC2) in relation to mating system and breeding latitude.
Fig. S2. Examples of reflectance spectra for males of species with high (splendid fairy-wren, Malurus splendens) and low (American goldfinch, Carduelis tristis) hue (PC2 scores were 35.7 and −9.9, respectively).
Fig. S3. An example of duller plumage in males of polygynous species.
Table S1. PCA of reflectance data for 977 species of birds.
Table S2. Monochromatism in relation to morphological, ecological, and behavioral variables.
Table S3. Sexual dichromatism in plumage brightness (PC1 dichro) and hue (PC2 dichro), and variation in brightness (PC1) and hue (PC2) for each sex in relation to life history and ecological variables associated with natural and sexual selection (N = 977 species).
Table S4. Sexual dichromatism in plumage brightness (PC1) in relation to life history and ecological variables in PGLS models.
Table S5. Sexual dichromatism in plumage brightness (PC1) in relation to life history and ecological variables in the full PGLS model (see table S4).
Table S6. Sexual dichromatism in plumage hue (PC2) in relation to life history and ecological variables in PGLS models.
Table S7. Sexual dichromatism in plumage hue (PC2) in relation to life history and ecological variables in the full PGLS model (see table S6).
Table S8. Female plumage brightness (PC1) in relation to life history and ecological variables in PGLS models.
Table S9. Female plumage brightness (PC1) in relation to life history and ecological variables in the full PGLS model (see table S8).
Table S10. Male plumage brightness (PC1) in relation to life history and ecological variables in PGLS models.
Table S11. Male plumage brightness (PC1) in relation to life history and ecological variables in the full PGLS model (see table S10).
Table S12. Female plumage hue (PC2) in relation to life history and ecological variables in PGLS models.
Table S13. Female plumage hue (PC2) in relation to life history and ecological variables in the full PGLS model (see table S12).
Table S14. Male plumage hue (PC2) in relation to life history and ecological variables in PGLS models.
Table S15. Male plumage hue (PC2) in relation to life history and ecological variables in the full PGLS model (see table S14).
Data file S1. Plumage color and ecological data for 977 species (Data977.csv).
Data file S2. Nexus file of 100 phylogenetic trees for the 977 species (trees100spp977.tre).
Data file S3. Plumage brightness (PC1) in three categories and 100 phylogenetic trees (PC1_LoMedHi_100trees.xml; Simmap file).
Data file S4. Plumage hue (PC2) in three categories and 100 phylogenetic trees (PC2_LoMedHi_100trees.xml; Simmap file).
REFERENCES AND NOTES
- 1.M. Andersson, Sexual Selection Monographs in Behavior and Ecology (Princeton Univ. Press, Princeton, NJ, 1994). [Google Scholar]
- 2.G. R. Bortolotti, in Bird Coloration, G. E. Hill, K. J. McGraw, Eds. (Harvard Univ. Press, Cambridge, MA, 2006), vol. 2, pp. 3–35.
- 3.T. Price, Speciation in Birds (Roberts and Co., Greenwood Village, CO, 2008). [Google Scholar]
- 4.C. Darwin, The Descent of Man, and Selection in Relation to Sex (J. Murray, London, 1871). [Google Scholar]
- 5.A. R. Wallace, Natural Selection and Tropical Nature (Macmillan, London, 1891). [Google Scholar]
- 6.Friedman N. R., Hofmann C. M., Kondo B., Omland K. E., Correlated evolution of migration and sexual dichromatism in the New World orioles (Icterus). Evolution 63, 3269–3274 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Soler J. J., Moreno J., Evolution of sexual dichromatism in relation to nesting habits in European passerines: A test of Wallace’s hypothesis. J. Evol. Biol. 25, 1614–1622 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Rubenstein D. R., Lovette I. J., Reproductive skew and selection on female ornamentation in social species. Nature 462, 786–789 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Dunn P. O., Whittingham L. A., Pitcher T. E., Mating systems, sperm competition and the evolution of sexual dimorphism in birds. Evolution 55, 161–175 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Owens I. P. F., Hartley I. R., Sexual dimorphism in birds: Why are there so many different forms of dimorphism? Proc. Biol. Sci. 265, 397–407 (1998). [Google Scholar]
- 11.Amundsen T., Why are female birds ornamented? Trends Ecol. Evol. 15, 149–155 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Price T., Birch G. L., Repeated evolution of sexual color dimorphism in passerine birds. Auk 113, 842–848 (1996). [Google Scholar]
- 13.Tobias J. A., Montgomerie R., Lyon B. E., The evolution of female ornaments and weaponry: Social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. B Biol. Sci. 367, 2274–2293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huelsenbeck J. P., Nielsen R., Bollback J. P., Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Møller A. P., Briskie J. V., Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav. Ecol. Sociobiol. 36, 357–365 (1995). [Google Scholar]
- 16.Martin T. E., Badyaev A. V., Sexual dichromatism in birds: Importance of nest predation and nest location for females versus males. Evolution 50, 2454–2460 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Burns K. J., A phylogenetic perspective on the evolution of sexual dichromatism in tanagers (Thraupidae): The role of female versus male plumage. Evolution 52, 1219–1224 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Whittingham L. A., Dunn P. O., Effects of extra-pair and within-pair reproductive success on the opportunity for selection in birds. Behav. Ecol. 16, 138–144 (2005). [Google Scholar]
- 19.Emlen S. T., Oring L. W., Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 (1977). [DOI] [PubMed] [Google Scholar]
- 20.Dunn P. O., Cockburn A., Extrapair mate choice and honest signaling in cooperatively breeding superb fairy-wrens. Evolution 53, 938–946 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Webster M. S., Tarvin K. A., Tuttle E. M., Pruett-Jones S., Promiscuity drives sexual selection in a socially monogamous bird. Evolution 61, 2205–2211 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Hamilton T. H., On the functions and causes of sexual dimorphism in breeding plumage characteristics of North American species of warblers and orioles. Am. Nat. 95, 121–123 (1961). [Google Scholar]
- 23.Fitzpatrick S., Colourful migratory birds: Evidence for a mechanism other than parasite resistance for the maintenance of ‘good genes’ sexual selection. Proc. R. Soc. Lond. B 257, 155–160 (1994). [Google Scholar]
- 24.Price J. J., Eaton M. D., Reconstructing the evolution of sexual dichromatism: Current color diversity does not reflect past rates of male and female change. Evolution 68, 2026–2037 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Hoffman C. M., Cronin T. W., Omland K. E., Evolution of sexual dichromatism. 1. Convergent losses of elaborate female coloration in New World orioles (Icterus spp.). Auk 125, 778–789 (2008). [Google Scholar]
- 26.Harmon L. J., Losos J. B., The effect of intraspecific sample size on type I and type II error rates in comparative studies. Evolution 59, 2705–2710 (2005). [PubMed] [Google Scholar]
- 27.Armenta J. K., Dunn P. O., Whittingham L. A., Quantifying avian sexual dichromatism: A comparison of methods. J. Exp. Biol. 211, 2423–2430 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Bennett A., Cuthill I., Norris K., Sexual selection and the mismeasure of color. Am. Nat. 144, 848–860 (1994). [Google Scholar]
- 29.Cuthill I., Bennett A., Partridge J., Maier E., Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 153, 183–200 (1999). [DOI] [PubMed] [Google Scholar]
- 30.R. Montgomerie, in Bird Coloration, G. E. Hill, K. J. McGraw, Eds. (Harvard Univ. Press, Cambridge, MA, 2006), vol. 1, pp. 90–147.
- 31.Endler J. A., On the measure and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 41, 315–352 (1990). [Google Scholar]
- 32.Grill C. P., Rush V. N., Analysing spectral data: Comparison and application of two techniques. Biol. J. Linn. Soc. 69, 121–138 (2000). [Google Scholar]
- 33.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Meth. Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 34.Paradis E., Claude J., Strimmer K., APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- 35.J. Pinheiro, D. Bates, S. DebRoy, D. Sarkar, R Core (2013).
- 36.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Hackett S. J., Kimball R. T., Reddy S., Bowie R. C., Braun E. L., Braun M. J., Chojnowski J. L., Cox W. A., Han K. L., Harshman J., Huddleston C. J., Marks B. D., Miglia K. J., Moore W. S., Sheldon F. H., Steadman D. W., Witt C. C., Yuri T., A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Butler M. A., King A. A., Phylogenetic comparative analysis: A modeling approach for adaptive evolution. Am. Nat. 164, 683–695 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Bollback J. P., SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics 7, 88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.C. G. Sibley, B. L. Monroe, Distribution and Taxonomy of Birds of the World (Yale Univ. Press, New Haven, CT, 1991). [Google Scholar]
- 41.J. F. Clements, T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, C. L. Wood, The eBird/Clements Checklist of Birds of the World: Version 6.8 (Cornell University Laboratory of Ornithology, Ithaca, NY, 2013). [Google Scholar]
- 42.Armenta J. K., Dunn P. O., Whittingham L. A., Effects of specimen age on plumage color. Auk 125, 803–808 (2008). [Google Scholar]
- 43.Vorobyev M., Osorio D., Bennett A. T. D., Marshall N. J., Cuthill I. C., Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Endler J. A., Mielke P. W., Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 (2005). [Google Scholar]
- 45.Stoddard M. C., Prum R. O., Evolution of avian plumage color in a tetrahedral color space: A phylogenetic analysis of New World buntings. Am. Nat. 171, 755–776 (2008). [DOI] [PubMed] [Google Scholar]
- 46.P. R. Ehrlich, D. S. Dobkin, D. Wheye, The Birder’s Handbook (Fireside, New York, 1988). [Google Scholar]
- 47.P. R. Ehrlich, D. S. Dobkin, D. Wheye, S. L. Pimm, The Birdwatcher’s Handbook (Oxford Univ. Press, Oxford, UK, 1994). [Google Scholar]
- 48.S. Cramp, C. M. Perrins, The Birds of the Western Palearctic (Oxford Univ. Press, Oxford, UK, 1977–1994). [Google Scholar]
- 49.P. J. Higgins, S. J. J. F. Davies, Handbook of Australian, New Zealand and Antarctic birds (Oxford Univ. Press, Melbourne, 1996), vol. 3. [Google Scholar]
- 50.J. del Hoyo, A. Elliott, J. Sargatal, Handbook of the Birds of the World (Lynx Editions, Barcelona, Spain, 1992–2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/2/e1400155/DC1
Materials and Methods
Fig. S1. Sexual dichromatism in brightness (PC1) and hue (PC2) in relation to mating system and breeding latitude.
Fig. S2. Examples of reflectance spectra for males of species with high (splendid fairy-wren, Malurus splendens) and low (American goldfinch, Carduelis tristis) hue (PC2 scores were 35.7 and −9.9, respectively).
Fig. S3. An example of duller plumage in males of polygynous species.
Table S1. PCA of reflectance data for 977 species of birds.
Table S2. Monochromatism in relation to morphological, ecological, and behavioral variables.
Table S3. Sexual dichromatism in plumage brightness (PC1 dichro) and hue (PC2 dichro), and variation in brightness (PC1) and hue (PC2) for each sex in relation to life history and ecological variables associated with natural and sexual selection (N = 977 species).
Table S4. Sexual dichromatism in plumage brightness (PC1) in relation to life history and ecological variables in PGLS models.
Table S5. Sexual dichromatism in plumage brightness (PC1) in relation to life history and ecological variables in the full PGLS model (see table S4).
Table S6. Sexual dichromatism in plumage hue (PC2) in relation to life history and ecological variables in PGLS models.
Table S7. Sexual dichromatism in plumage hue (PC2) in relation to life history and ecological variables in the full PGLS model (see table S6).
Table S8. Female plumage brightness (PC1) in relation to life history and ecological variables in PGLS models.
Table S9. Female plumage brightness (PC1) in relation to life history and ecological variables in the full PGLS model (see table S8).
Table S10. Male plumage brightness (PC1) in relation to life history and ecological variables in PGLS models.
Table S11. Male plumage brightness (PC1) in relation to life history and ecological variables in the full PGLS model (see table S10).
Table S12. Female plumage hue (PC2) in relation to life history and ecological variables in PGLS models.
Table S13. Female plumage hue (PC2) in relation to life history and ecological variables in the full PGLS model (see table S12).
Table S14. Male plumage hue (PC2) in relation to life history and ecological variables in PGLS models.
Table S15. Male plumage hue (PC2) in relation to life history and ecological variables in the full PGLS model (see table S14).
Data file S1. Plumage color and ecological data for 977 species (Data977.csv).
Data file S2. Nexus file of 100 phylogenetic trees for the 977 species (trees100spp977.tre).
Data file S3. Plumage brightness (PC1) in three categories and 100 phylogenetic trees (PC1_LoMedHi_100trees.xml; Simmap file).
Data file S4. Plumage hue (PC2) in three categories and 100 phylogenetic trees (PC2_LoMedHi_100trees.xml; Simmap file).