Isolation and playback experiments demonstrate vocal learning in bats and reveal the learning mechanism by continuous recordings from birth.
Keywords: animal communication, vocal learning, language evolution, acoustic communication, bats
Abstract
The evolution of human language is shrouded in mystery as it is unparalleled in the animal kingdom. Whereas vocal learning is crucial for the development of speech in humans, it seems rare among nonhuman animals. Songbirds often serve as a model for vocal learning, but the lack of a mammalian model hinders our quest for the origin of this capability. We report the influence of both isolation and playback experiments on the vocal development of a mammal, the Egyptian fruit bat. We continuously recorded pups from birth to adulthood and found that, when raised in a colony, pups acquired the adult repertoire, whereas when acoustically isolated, they exhibited underdeveloped vocalizations. Isolated pups that heard bat recordings exhibited a repertoire that replicated the playbacks they were exposed to. These findings demonstrate vocal learning in a social mammal, and suggest bats as a model for language acquisition.
Language is one of the cornerstones of human culture (1–3). Yet, its origin and evolution are still unknown. One of the elemental aspects of human language is its remarkable spontaneous acquisition by children, which relies on the ability to modify vocalizations according to auditory inputs (2). This skill, usually termed vocal learning, has been shown only in a handful of nonhuman animals (4)—mostly birds (5, 6) and a few mammals (7–11). Isolation experiments offer a direct and informative way to test vocal learning, but in contrast to studies on birdsong, such experiments have hardly ever been conducted in mammals and are impractical in humans. Here, we report both isolation and playback experiments in a mammal, the Egyptian fruit bat (Rousettus aegyptiacus). By continuously recording pups from birth and until the age of 9 months, and generating a database of more than 1 million calls, we reveal the ontogeny of a complex vocal repertoire and show the role of vocal learning in its acquisition.
The Egyptian fruit bat is a long-lived social mammal that can live to the age of at least 25 years in captivity (12). Bats of this species congregate in colonies of hundreds to thousands of individuals. Their social interactions are extremely vocal. The vocalizations are composed of sequences of short calls, which come from a rich acoustic repertoire [Fig. 1, A (right) and B]. We hypothesized that the acquisition of the bat vocal repertoire requires exposure to adult vocalizations; hence, it involves vocal learning. Therefore, we predicted that pups reared in vocal isolation will not develop normal adult vocalizations, and that exposing pups to playbacks of an abnormal repertoire will draw their vocalizations toward the playback. To study the vocal ontogeny of these bats, we raised five pups in isolation from any adult vocalization. Heavily pregnant females were caught in a wild colony and kept separately in five private chambers, in which each of them gave birth to a single pup. The chambers were acoustically isolated and were constantly monitored with video cameras and microphones (fig. S1). In the absence of other adults, the mother will remain silent and will not vocally communicate with her pup; thus, pups that were raised under these conditions were not exposed to adult vocalizations (this premise was confirmed using our continuous monitoring; see Supplementary Methods). After weaning, at the age of ca. 80 days, the five pups were grouped into one chamber, without any adult, to examine their vocal communication as exhibited during social interactions. Their vocal development was then monitored for another 5 months (fig. S2). As a control, another five pups were reared with their mothers and one male all together in one colony chamber. These pups were exposed to intensive adult vocalizations until the age of ca. 80 days, when they were transferred into a chamber of their own, without the adults (figs. S1 and S2).
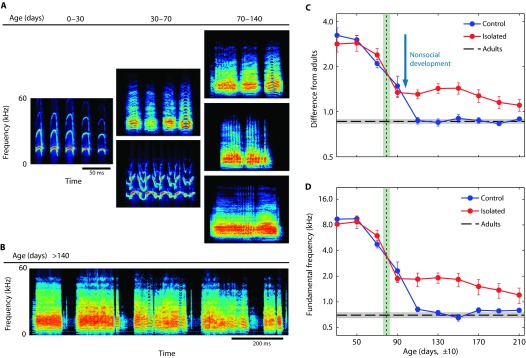
Fig. 1. Ontogeny of bat vocal communication.
(A) Examples (spectrograms) of bat calls from different developmental stages of control pups. The left-most call is an innate isolation call. (B) Typical sequence of adult-like vocalizations. (C) Acoustic difference between pup and adult calls as measured using Euclidean distance in an acoustic feature space (see Supplementary Methods). (D) Average fundamental frequency of the pup calls. In (C) and (D), quantities are measured in bins of 20 days; the ordinate is log-scaled. Blue line, control group (n = 5); red line, isolated group (n = 5; n = 4 for the first two bins); dashed black line, the same measurement when applied to adults (n = 10); green dotted line, average of pups’ age on the assemblage day of pups-only groups. Error bars and shades, SEM.
In both the isolated and the control groups, the pups’ first vocalizations were isolation calls [Fig. 1A (left) and fig. S3 (left)]. These high fundamental multiharmonic calls are naturally induced when a pup fears it may detach from its mother or when it is left alone in the roost. These calls are innate and appear at postnatal day 1. We followed the modification of these calls and observed their gradual transformation into a rich adult repertoire of calls (Fig. 1A). In the control pups, during the second month of the bat’s life, some variability appeared among the isolation calls, and toward the third month, adult-like features, such as low fundamental segments, were incorporated into the calls forming unripe social calls [Fig. 1A (middle) and fig. S3 (middle)]. Around the age of 100 days, adult-like calls dominate the vocal repertoire, whereas the unripe calls gradually disappear [Fig. 1A (right) and fig. S3 (middle and right)].
We quantified this process by measuring the similarity between the vocalizations of pups at different ages and the vocalizations of adults (see Supplementary Methods) and comparing the two experimental groups (control and isolation). At a young age, both the isolation and the control groups greatly differed from the adults. As time passed, pups in both groups began to incorporate adult-like segments into their calls, such that the acoustic characteristics of their calls converged toward those of the adults in what seemed to be, at least partially, an innate process (see bar in Fig. 1C, titled “nonsocial development”). This process in the isolation pups was initiated even before they were grouped, which further emphasizes its innate nature. However, whereas the vocalizations of the control pups quickly matured and became similar to adult calls, the vocal development of the isolated pups lagged far behind, and their vocalizations remained significantly different [Fig. 1C, mixed analysis of variance (ANOVA): F(1,8) = 13.96, P = 0.006, for the difference between the two groups]. One of the most delayed acoustic features in the isolated pups was the fundamental frequency, which was significantly higher in this group [Fig. 1D, mixed ANOVA: F(1,8) = 9.09, P = 0.017, for the difference between the two groups]. The fundamental frequency governs the harmonic content of the vocalizations and has been shown to play an important role in the socially learned vocal behavior of songbirds (13) and in human vocal ontogeny (14) (for examples of the ontogeny of other acoustic features, see fig. S4).
We repeated the full isolation experiment twice in two consecutive breeding seasons, 4 months apart, and observed the same difference between control and isolation pups (Fig. 1D and fig. S5). Because of high mortality rate in the first trial, where only four pups (two of each group) survived, we focus this report only on the second experiment (the results of the first experiment are fully provided in fig. S5).
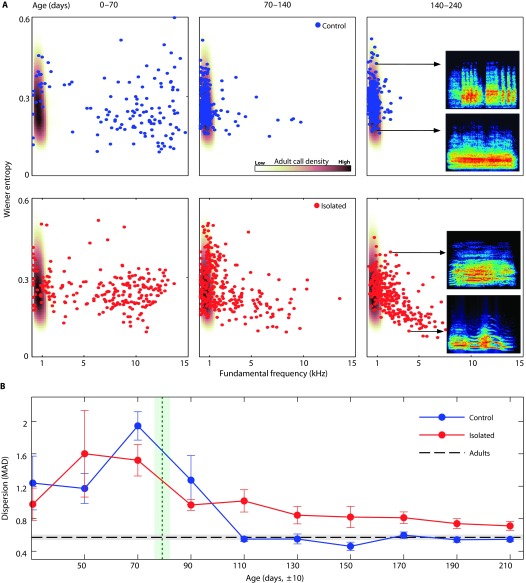
To further illuminate the process in which bat vocal communication crystallizes, we examined how the variability among pup calls changes over time. Both pup groups assumed a highly diverse acoustical repertoire at a young age, much more diverse than that observed in the adults [Fig. 2, A (left panels) and B, and fig. S6]. As the control pups matured in the presence of vocalizing adults, their calls acoustically converged toward the adult vocal repertoire, and the huge diversity quickly disappeared (within ca. 3 months). However, the isolated pups kept their infantile diversity deep into their adolescence [Fig. 2, A (right panels) and B, and fig. S6]. Such extended vocal diversity among sub-adults has also been described in songbirds (15). These results demonstrate that isolated pups were capable of producing adult vocalizations, as they often did, but that they lacked the discriminatory mechanism (for example, adult guidance) that favors the adult-like acoustics.
Fig. 2. Development of vocal diversity.
(A) Three developmental stages of one pup from the control group (upper panels, blue) and one pup from the isolated group (lower panels, red), presented as a scatter plot of two acoustic features. Brown shades indicate these features’ distribution among adult calls. Insets in the right panels display representative spectrograms of vocalizations with different acoustic features. Note how the isolated pup is still using multiharmonic calls with a high fundamental even after the age of 140 days (undermost spectrogram). (B) Dispersion of the developing vocal repertoire, measured as the median absolute deviation (MAD) of the calls at every age in respect to these two features (see Supplementary Methods). Blue line, control group (n = 5); red line, isolated group (n = 5; n = 4 for the first two bins); dashed black line, the same measurement when applied to adults (n = 10); green dotted line, average of pups’ age on the assemblage day of pups-only groups. Error bars and shades, SEM.
Even though the isolated group was clearly lagging behind the control group, careful inspection of the vocal development of the isolated pups, between the ages of ~100 and ~200 days, reveals a slow convergence toward the adult baseline (Fig. 1C) and a creeping reduction in the fundamental frequency (Fig. 1D and fig. S7). Slow acoustic maturation toward adult-like vocalizations, without proper adult guidance, has also been recently demonstrated in isolated zebra finches (16), and suggests a nonsocial development toward the correct acoustic direction. Still, 5 months after the control group already reached the adult repertoire, isolated pups were wandering behind, emphasizing their need of an external stimulus to serve them as a vocal beacon.
The continuous video monitoring enabled the identification of the emitter of a vocalization, its addressee, and the context in which it was emitted. We used these data to exclude the possibility that the isolated pups lacked some behavioral skills, and therefore did not exhibit the full adult repertoire. To this end, we repeated the analysis described above, focusing each time only on one of the most common behavioral contexts (for example, squabbling over sleeping spots, feeding interactions, etc.). This analysis provided similar results, revealing a significant difference between the isolation and control groups in all contexts (fig. S8; a common aggressive behavior is displayed in movies S1 and S2 for the control and isolation groups, respectively).
The two groups of pups were extremely vocal after their assembly and exhibited similar vocal capacity (mean calls per day: control, 244, n = 5; isolated, 211, n = 5; Mann-Whitney U test, P = 0.55). Because the control pups were involved in vocal interactions in their birth colony about 2 to 3 weeks earlier than the isolated pups, we verified that there is no correlation between the number of emitted calls and the produced fundamental frequency (Spearman correlation: ρ = 0.1, P = 0.95; ρ = 0.3, P = 0.68, control and isolated groups, respectively). This thus excluded lack of vocal practice as a possible cause for the isolated pups’ developmental delay. Although pups in both groups emitted dozens of thousands of calls during the research period, the gap between the two groups remained dramatic even after 5 months—long after the control pups reached the adult vocalization repertoire (Fig. 1D and fig. S7). Furthermore, it is also unlikely that the bats suffered from an auditory deficit (which could result from sensory deprivation) because both the isolated pups and their mothers used echolocation consisting of broadband clicks [which cover their hearing frequency range (17)]. There was also no significant difference between the body weights of the pups of the two groups (Mann-Whitney U test, P > 0.1, at four separate time points), suggesting that a physiological difference could not explain the observed acoustic difference. Moreover, we found no significant correlation between the body weight and the similarity to adults (Spearman correlation, P > 0.15; see Supplementary Methods).
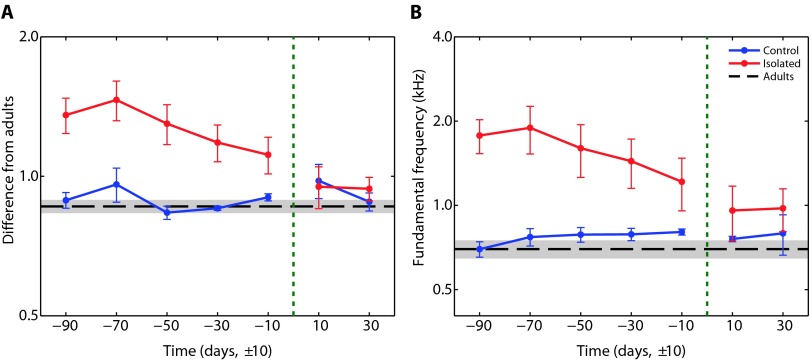
After spending nearly 5 months in two separate chambers, the isolation and control groups were mixed by swapping the locations of three pups from each group, thus creating two mixed groups. One month after this merge, the isolated pups could not be statistically distinguished from the control pups or the adults (Fig. 3). However, because of the slow acoustic maturation described above, we could not verify that the merge itself drove the observed convergence. This finding proves that the isolated pups are able to gain an adult-like vocal behavior, suggesting the absence of a short critical period for vocal learning. This differs from the sensitive period described for several species of songbirds (18, 19), although plasticity in pitch acquisition has been shown in adult songbirds (20).
Fig. 3. Effects of mixing the experimental groups.
The two groups of pups were mixed 5 months after their assembly (when the pups were ca. 225 days old). (A and B) The distance of their calls from adult calls (A), as described in Fig. 1C, and their fundamental frequency (B) are presented against time relative to the mixing day (green dotted line). After the groups were mixed, there was no significant difference between the two groups in both measurements. There was also no significant difference between the adults and any of the groups.
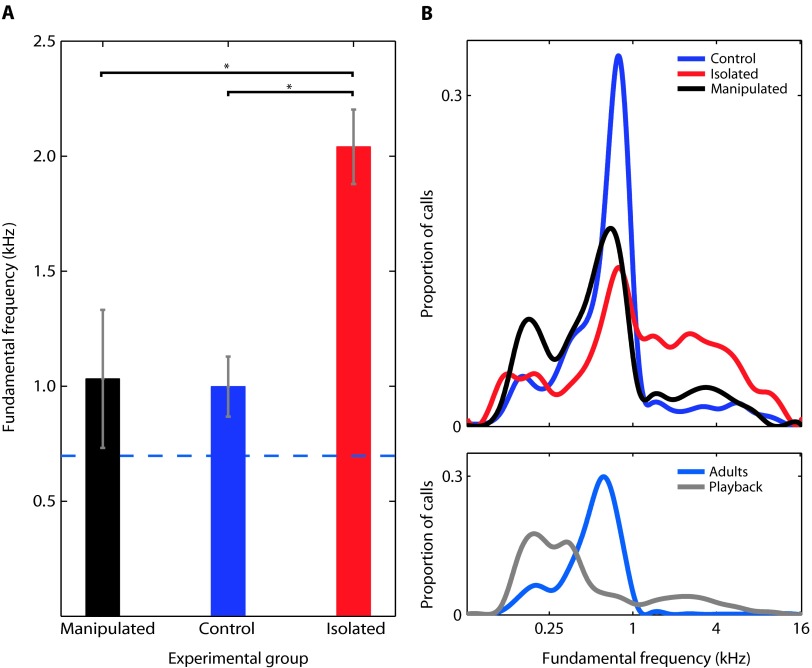
To test if hearing adult vocalizations (without observing social interactions) is sufficient for vocal learning, we conducted a playback experiment. In this experiment, pups were raised under conditions that were similar to those of the isolated group. However, during the entire isolation period, the pups were exposed to an intensive playback of adult bat vocalizations. We chose a subset of the adult repertoire including mostly low-frequency adult calls, aiming to test if we could drive the pups’ vocalizations in the opposite direction of the isolation group (which emitted high-frequency calls). Three pups were weaned in this experiment and were then assembled into a “manipulated” group. In the first month after this group’s assembly, the average fundamental frequencies used by these pups were similar to those of the control group (which were exposed to adult vocalizations) and much lower than those used by the isolated group (Fig. 4A; Mann-Whitney U test, P = 0.035). Furthermore, the manipulated pups often used low frequencies that were abundant in the playbacks but rare in the overall adult repertoire and were seldom used by the control group (Fig. 4B).
Fig. 4. Vocal development under exposure to playback of low-frequency calls.
(A) Average fundamental frequency used by pups from the manipulated (black, n = 3), control (blue, n = 5), and isolated (red, n = 5) groups in the first month after the group assembly. *P < 0.05 (Mann-Whitney U test). Dashed line, the adult average. (B) Detailed distribution of the fundamental frequencies of bat calls. Lower panel: The vocalizations heard by the control group (adult vocalizations, light blue) and the manipulated group (playback, gray). Upper panel: The vocal production of the different groups: control (blue), isolated (red), and manipulated (black). Note the frequent use of low frequencies in the manipulated group, mimicking the distribution of the playback calls.
Our findings indicate that exposure to adult vocalizations is both necessary and sufficient to induce vocal learning in this bat. These results also illuminate the learning mechanism: young pups first exhibit a highly diverse repertoire and require an appropriate auditory reference to converge to the adult acoustics. The rich repertoire of unripe calls that are emitted by pups and are absent in the adult vocal repertoire is reminiscent of the babbling period observed in human babies, songbirds (19), and another species of bat (21). Whereas vocal learning research in birds and in certain mammals focuses on the specific context of advertising songs, which are broadcasted by males, sometimes even in the absence of an audience, this study demonstrates vocal learning in a pairwise communication expressed in everyday social activities, as is the case with human language. There is still much to discover on mammalian vocal learning mechanisms. Yet, even a rudimentary vocal learning process in the bat brain may disclose the evolutionary basis of the refined language acquisition abilities of humans.
Supplementary Material
Acknowledgments
We would like to thank A. Lotem and M. Yartsev for commenting on the manuscript and for many fruitful discussions. We also thank E. Pratt for her high-quality diligent work in annotating the videos. Funding: Y.P. is supported by The Colton Foundation. Author contributions: Y.P., M.T., and Y.Y. conceived and designed the experiment. Y.P. designed and constructed the setup. Y.P. and M.T. conducted the experiments. M.T. analyzed the videos. Y.P. created the processing and analysis tools and performed the analysis. Y.Y. supervised the study. Y.P. and Y.Y. wrote the manuscript and M.T. reviewed it.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/2/e1500019/DC1
Methods
Fig. S1. Scheme of experimental acoustic chambers.
Fig. S2. Experiment outline.
Fig. S3. The developing repertoire of bat calls.
Fig. S4. Vocal ontogeny of different acoustic features.
Fig. S5. The development of fundamental frequency among pups of the first experiment.
Fig. S6. Development of vocal diversity.
Fig. S7. The development of fundamental frequency of individual pups.
Fig. S8. The fundamental frequency of calls produced in different behavioral contexts.
Movie S1. A typical intense face-to-face fight as displayed by the control group.
Movie S2. A typical intense face-to-face fight as displayed by the isolation group.
Reference (22)
REFERENCES AND NOTES
- 1.Fitch W. T., The evolution of speech: A comparative review. Trends Cogn. Sci. 4, 258–267 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Hauser M. D., Chomsky N., Fitch W. T., The faculty of language: What is it, who has it, and how did it evolve? Science 298, 1569–1579 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Morgan T. J., Uomini N. T., Rendell L. E., Chouinard-Thuly L., Street S. E., Lewis H. M., Cross C. P., Evans C., Kearney R., de la Torre I., Whiten A., Laland K. N., Experimental evidence for the co-evolution of hominin tool-making teaching and language. Nat. Commun. 6, 6029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janik V. M., Slater P. J. B., The different roles of social learning in vocal communication. Anim. Behav. 60, 1–11 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield T., Gentner T., Margoliash D., What birds have to say about language. Nat. Neurosci. 14, 947–948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipkind D., Marcus G. F., Bemis D. K., Sasahara K., Jacoby N., Takahasi M., Suzuki K., Feher O., Ravbar P., Okanoya K., Tchernichovski O., Stepwise acquisition of vocal combinatorial capacity in songbirds and human infants. Nature 498, 104–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knörnschild M., Nagy M., Metz M., Mayer F., von Helversen O., Complex vocal imitation during ontogeny in a bat. Biol. Lett. 6, 156–159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janik V. M., Cetacean vocal learning and communication. Curr. Opin. Neurobiol. 28, 60–65 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Stoeger A. S., Manger P., Vocal learning in elephants: Neural bases and adaptive context. Curr. Opin. Neurobiol. 28, 101–107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boughman J. W., Vocal learning by greater spear–nosed bats. Proc. Biol. Sci. 265, 227–233 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyack P. L., Convergence of calls as animals form social bonds, active compensation for noisy communication channels, and the evolution of vocal learning in mammals. J. Comp. Psychol. 122, 319–331 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Kwiecinski G. G., Griffiths T. A., Rousettus egyptiacus. Mamm. Species 611, 1–9 (1999). [Google Scholar]
- 13.Tchernichovski O., Mitra P. P., Lints T., Nottebohm F., Dynamics of the vocal imitation process: How a zebra finch learns its song. Science 291, 2564–2569 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Lee S., Potamianos A., Narayanan S., Acoustics of children’s speech: Developmental changes of temporal and spectral parameters. J. Acoust. Soc. Am. 105, 1455–1468 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Ravbar P., Lipkind D., Parra L. C., Tchernichovski O., Vocal exploration is locally regulated during song learning. J. Neurosci. 32, 3422–3432 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feher O., Wang H., Saar S., Mitra P. P., Tchernichovski O., De novo establishment of wild-type song culture in the zebra finch. Nature 459, 564–568 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yovel Y., Geva-Sagiv M., Ulanovsky N., Click-based echolocation in bats: Not so primitive after all. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 197, 515–530 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Brainard M. S., Doupe A. J., What songbirds teach us about learning. Nature 417, 351–358 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Marler P., A comparative approach to vocal learning: Song development in white-crowned Sparrows. J. Comp. Physiol. Psychol. 71, 1 (1970). [Google Scholar]
- 20.Tumer E. C., Brainard M. S., Performance variability enables adaptive plasticity of “crystallized” adult birdsong. Nature 450, 1240–1244 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Knörnschild M., Behr O., von Helversen O., Babbling behavior in the sac-winged bat (Saccopteryx bilineata). Naturwissenschaften 93, 451–454 (2006). [DOI] [PubMed] [Google Scholar]
- 22.De Cheveigné A., Kawahara H., YIN, a fundamental frequency estimator for speech and music. J. Acoust. Soc. Am. 111, 1917–1930 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/2/e1500019/DC1
Methods
Fig. S1. Scheme of experimental acoustic chambers.
Fig. S2. Experiment outline.
Fig. S3. The developing repertoire of bat calls.
Fig. S4. Vocal ontogeny of different acoustic features.
Fig. S5. The development of fundamental frequency among pups of the first experiment.
Fig. S6. Development of vocal diversity.
Fig. S7. The development of fundamental frequency of individual pups.
Fig. S8. The fundamental frequency of calls produced in different behavioral contexts.
Movie S1. A typical intense face-to-face fight as displayed by the control group.
Movie S2. A typical intense face-to-face fight as displayed by the isolation group.
Reference (22)