Summary
Background
Recommendations have differed nationally and internationally with respect to the best time to start antiretroviral therapy (cART). We compared effectiveness of three strategies for initiation of cART in high-income countries for HIV-positive individuals who do not have AIDS: immediate universal cART initiation, cART initiation at a CD4 cell count below 500, and cART initiation at a CD4 cell count below 350 cells/mm3.
Methods
We used data from the HIV-CAUSAL collaboration of cohorts in Europe and the United States. We included 55,826 individuals diagnosed with HIV between 2000-2013, ART naïve, AIDS-free, aged≥18 years and within 6 months of HIV diagnosis. We used the parametric g-formula to adjust for baseline and time-varying confounders to estimate the following quantities as would have been observed under each cART initiation strategy after 7 years of HIV diagnosis: relative risks of both death and of death or AIDS-defining illness, mean survival time, proportion in need of cART, and proportion with HIV RNA<50 copies/mL.
Findings
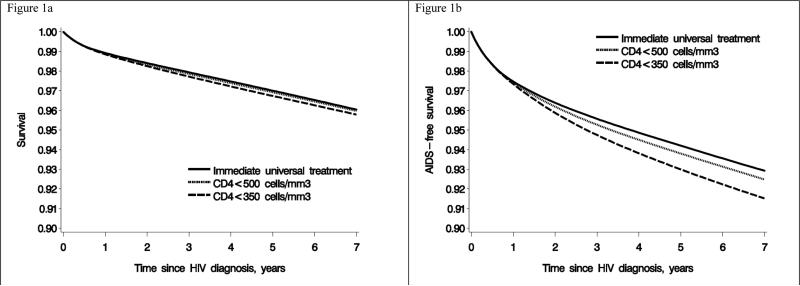
Median [interquantile range] CD4 at HIV diagnosis was 376 [222,551] cells/mm3. Compared with immediate initiation, the mortality risk ratio estimate (95% CI) was 1.02 (1.01,1.02) under initiation at CD4<500 cells/mm3, and 1.06 (1.04,1.08) under initiation at CD4<350 cells/mm3. The corresponding estimates were 1.06 (1.06,1.07) and 1.20 (1.17,1.23) for the combined endpoint. Compared with immediate initiation, the mean survival time at 7 years under initiation at CD4<500 cells/mm3 and at CD4<350 cells/mm3 was 2 (1,2) and 5 (4,6) days shorter. Seven years after HIV diagnosis, 100%, 99%, and 93% of individuals would have been in need of cART, and 87%, 87% and 84% would have HIV-RNA<50 copies/mL, under immediate initiation, initiation at CD4<500 and <350 cells/mm3, respectively.
Interpretation
The benefits of immediate initiation of cART, such as prolonged survival and AIDS-free survival and increased virological suppression, were small in this high-income setting with relatively low CD4 count at HIV diagnosis. The estimated beneficial effect on AIDS is less than in recently reported randomised trials. Increasing rates of HIV testing might be as important as a policy of early initiation of cART.
Keywords: when to start, CD4 cell count, guidelines, mathematical models, causal inference, parametric g-formula, comparative effectiveness
Introduction
Recommendations have differed nationally and internationally with respect to the best time to start combined antiretroviral therapy (cART) in HIV-positive patients who do not have AIDS. In the most recent US guidelines,1,2 initiation of cART is recommended for all individuals who have been newly diagnosed with HIV infection, irrespective of their CD4 count. By contrast with this guidance, WHO recommends initiation of cART when the patient’s CD4 count has fallen below 500 cells/mm3 Moreover, in Europe, treatment initiation is recommended for all patients with a CD4 count less than 350 cells/mm3 and should be considered for individuals with a CD4 count of 350–500 cells/mm3.
These discrepancies are attributable partly to different interpretations of available evidence. Over the past decade, findings of observational studies and clinical trials have shown that starting cART at CD4 counts of 350–500 cells/mm3 is associated with reduced mortality and AIDS morbidity5–12 and decreased transmission of HIV to other people.13 The benefits of such early initiation on survival and transmission might be off set by development of toxicity and drug resistance.14 Moreover, because treatment must be used for the rest of a patient’s life without interruption,15 immediate initiation would increase substantially the proportion of individuals in need of cART and the burden on available resources.
The results from the Temprano trial suggest that immediate universal initiation is the best cART initiation strategy in low-income countries with high risk of opportunistic infections12. Preliminary results from the Strategic Timing of Anti-Retroviral Treatment (START) trial also support the benefit of early initiation (http://www.ctu.mrc.ac.uk/news/2015/start_results_28052015). However, there are no estimates of the relative effectiveness of immediate universal initiation versus initiation according to CD4 thresholds on the mortality and morbidity of populations representative of the clinical HIV practice in high-income countries. These estimates are important not only to patients and clinicians but also, to service providers and policy makers who require estimates of the proportions of individuals in need of treatment and with suppressed viral load to estimate the cost-effectiveness of current or future guidelines on cART initiation and to allocate resources between HIV treatment and other health priorities.
Here, we aimed to compare immediate initiation of cART with strategies for starting treatment based on CD4 thresholds of 350 cells/mm3 and 500 cells/mm3 in cohorts of HIV-positive individuals from Europe and the USA. We aimed to compare clinical outcomes and the proportions of patients in need of treatment and with suppressed virological replication under each initiation strategy up to 7 years after HIV diagnosis.
Methods
Study population
The HIV-CAUSAL collaboration is a consortium of prospective cohort studies from 6 European countries and the United States. All cohorts record routinely collected data in clinical practice within healthcare systems with universal access to care. Data collected include that related to patient characteristics (age, sex, geographical origin, and transmission category), use of cART (type of regimes and dates of start and discontinuation), CD4 cell counts, and plasma HIV-RNA, AIDS-defining illnesses, and deaths. Each cohort submits data in a standardized format (http://www.hicdep.org/) to the coordinating center. The analyses presented here are based on data pooled in September 2013. Ethics approval was granted by the ethic committees of each of the participating cohorts according to country-specific regulations.
Selection of patients
Analyses were restricted to individuals who met the following inclusion criteria: age ≥18 years; HIV diagnosis on or after January 1, 2000; AIDS-free; antiretroviral therapy naïve; CD4 cell count and HIV-RNA measurements within 3 months of each other and within 6 months of the date of HIV diagnosis. Follow-up started at baseline, defined as the date when all criteria were met, and ended at death (or AIDS when considering AIDS-free survival), 12 months after the most recent laboratory measurement, or cohort-specific administrative censoring (ranging from 2011 to 2013), whichever occurred first. Individuals with no CD4 cell count and HIV-RNA measurements after baseline were excluded. Since the relative effectiveness of initiation strategies will depend on CD4 count at HIV diagnosis, we also conducted two sensitivity analyses restricted to individuals with CD4>350 cells/mm3 and CD4>500 cells/mm3 at HIV diagnosis.
cART initiation strategies
cART was defined as a combination of antiretroviral drugs including at least two nucleoside reverse transcriptase inhibitors plus either one or more protease inhibitors, one nonnucleoside reverse transcriptase inhibitor, one entry/fusion inhibitor, or one integrase inhibitor.
We compared the following three cART initiation strategies: i) immediate universal treatment, defined as initiation within 6 months of HIV diagnosis regardless of the CD4 count, ii) initiation within 6 months of a CD4 count <500 cells/mm3 or an AIDS diagnosis, and iii) initiation within 6 months of a CD4 cell count <350 cells/mm3 or an AIDS diagnosis.
Outcomes
We considered the following clinical outcomes: all-cause mortality and a combined endpoint of AIDS-defining illness16 or death. For each cART initiation strategy and outcome, we estimated the 7-year risk and the average restricted survival time17. In sensitivity analyses, we also considered a combined endpoint of death or severe or moderate AIDS-defining illness18 (non-Hodgkin's lymphoma, progressive multifocal leukoencephalopathy, cryptococcosis, cerebral toxoplasmosis, AIDS dementia complex and disseminated Mycobacterium avium complex), and the outcome tuberculosis as later treatment initiation has been associated with increased risk of tuberculosis10,13.
We also estimated the proportion of individuals considered in need of treatment and with suppressed virological replication, defined as HIV-RNA<50 copies/mL, up to 7 years after baseline, assuming that cART is continued once it is started.
Statistical methods
Our estimates need to be adjusted for the time-dependent confounders CD4 cell count, HIVRNA level and AIDS, as well as for confounders measured at baseline. Because standard statistical methods cannot appropriately adjust for time-dependent confounders affected by prior treatment 19,20, we applied the parametric g-formula to obtain adjusted estimates for each treatment strategy under the assumptions of no residual confounding, no measurement error, and no model misspecification21.
The parametric g-formula is a generalization of standardization for time-varying treatments and confounders5,20,21. The estimation procedure for the HIV-CAUSAL Collaboration has been described elsewhere 5. Briefly, the procedure has two steps. First, parametric regression models are used to estimate the probability density functions of the time-varying variables conditional on previous treatment and covariate history. Second, a Monte Carlo simulation using the above estimates is run to simulate the distribution of the post-baseline outcomes and time-varying covariates separately under each cART initiation strategy.
For the first step, we fit separate logistic regression models for time-varying indicators of death, AIDS-defining illness, cART initiation, measurement of CD4 cell count, measurement of HIV RNA, and linear regression models for CD4 cell count and HIV-RNA on the natural logarithm scale. All regression models included as covariates the two most recent values of these time-varying variables, time since last CD4 count and HIV-RNA measurements, and the following baseline variables: CD4 cell count (<50,50-99,100-199,200-349,350-499, ≥500 cells/mm3), HIV-RNA level (<4,4-5, >5 log10 copies/mL), sex, transmission group (heterosexual, homo/bisexual, injecting drug users, or other/unknown), calendar year (2000-2004, 2005-2010, 2011-2013), age (<35,35-50, >50 years), geographical origin (Western countries, sub-Saharan Africa, other, unknown), and cohort. Models for CD4 cell count and HIV-RNA also included an interaction term for number of months since cART initiation. Like all regression-based methods, the parametric g-formula relies on correct model specification. To explore the validity of our parametric assumptions, we compared the observed means of the outcome and time-varying covariates with those predicted by our models (Appendix Figure 1).
We used a nonparametric bootstrap procedure based on 200 samples to obtain percentile-based 95% confidence intervals (CIs). All analyses were conducted with the publicly available SAS macro GFORMULA (http://www.hsph.harvard.edu/causal/software/).
Sensitivity analyses
We conducted several sensitivity analyses in which we (i) adjusted for a time-fixed indicator of hepatitis C co-infection, (ii) included individuals with no CD4 cell count and HIV-RNA measurements after baseline, (iii) excluded the cohorts of HIV seroconverters (GEMES, PRIMO, SEROCO, UK Register of Seroconverters) who might have been treated with short-course antiretroviral treatment during primary HIV infection (shown to be have immunological and virological benefits after treatment is stopped22-24), and (iv) computed the proportion of individuals with suppressed virological replication defined as HIV-RNA<400 copies/mL rather than <50 copies/mL.
Role of funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
The HIV-CAUSAL collaboration included 70,488 HIV positive individuals with known date of HIV diagnosis between 2000-2013. We excluded 5,027 who had no CD4 count or HIV-RNA after baseline and 9,635 whose baseline date was >6 months after HIV diagnosis.
Table 1 shows the baseline characteristics of the 55,826 individuals eligible for our analyses: 77% were men, 73% started follow-up after 2004; their median [IQR] CD4 cell count, HIV-RNA and age at baseline were 376 [222,551] cells/mm3, 4.6 [4.0,5.1] log10 copies/mL and 36 [30,44] years, respectively. During the follow-up 71% initiated cART. The median [IQR] CD4 cell count and HIV-RNA at cART initiation were 259 [161,355] cells/mm3 and 4.8 [4.2,5.3] log10 copies/mL, respectively. The median [IQR] time between HIV diagnosis and cART initiation was 2 [1,14] months, and the proportion of initiators with CD4 cell count >500 cells/mm3 was 9%. Compared with individuals who initiated cART with CD4 cell count ≤500 cells/mm3, those who initiated cART with CD4 count>500 cells/mm3 were less likely to be immigrants and more likely to be in the homosexual/bisexual transmission group (Appendix Table 1).
Table 1.
Baseline characteristics and incidence rates of mortality and of AIDS or mortality of 55,826 eligible individuals, HIV-CAUSAL Collaboration 2000-2013.
| No. individuals | Person-years | % cART initiators during follow-up | Median [IQR] follow-up time, months | No. deaths | Incidence rate of death, per 1000 person-years | No. AIDS events or deaths | Incidence rate of AIDS or death, per 1000 person-years | ||
|---|---|---|---|---|---|---|---|---|---|
| CD4 cell count, cells/mm3 | <50 | 3080 | 12960 | 95 | 43 [21.74] | 323 | 24.9 | 521 | 49.4 |
| 50-100 | 2573 | 10906 | 96 | 44 [21,75] | 197 | 18.1 | 330 | 34 | |
| 100 - 200 | 6398 | 26321 | 95 | 41 [20,73] | 291 | 11.1 | 534 | 21.6 | |
| 200 - 350 | 13217 | 52259 | 86 | 39 [19,69] | 351 | 6.7 | 757 | 15.1 | |
| 350 - 500 | 12927 | 48826 | 67 | 36 [18,66] | 272 | 5.6 | 608 | 12.9 | |
| >500 | 17631 | 64248 | 46 | 34 [18,63] | 303 | 4.7 | 722 | 11.5 | |
| HIV-RNA, copies/mL | <10,000 | 13631 | 49891 | 54 | 35 [18,62] | 287 | 5.8 | 532 | 10.9 |
| 10,000 - 100,000 | 24702 | 95501 | 72 | 37 [19,68] | 691 | 7.2 | 1469 | 16.1 | |
| >100,000 | 17493 | 70129 | 84 | 39 [19,71] | 759 | 10.8 | 1471 | 22.7 | |
| Sex | Male | 42940 | 166625 | 70 | 38 [19,68] | 1509 | 9.1 | 2787 | 17.6 |
| Female | 12886 | 48896 | 74 | 36 [18,67] | 228 | 4.7 | 685 | 14.9 | |
| Age, years | < 35 | 25359 | 93346 | 65 | 34 [18,64] | 246 | 2.6 | 945 | 10.5 |
| 35 - 50 | 22722 | 92025 | 75 | 40 [20,71 | 713 | 7.7 | 1535 | 17.7 | |
| >50 | 7745 | 30150 | 82 | 38 [19,69] | 778 | 25.8 | 992 | 35 | |
| Transmission group | Heterosexual | 20567 | 80626 | 75 | 38 [19,69] | 459 | 5.7 | 1243 | 16.4 |
| Homo/bisexual | 26547 | 102663 | 67 | 38 [19,67] | 371 | 3.6 | 1093 | 11.1 | |
| Injection drug-use | 1514 | 5530 | 66 | 32 [16,63] | 119 | 21.5 | 171 | 32.9 | |
| Other/unknown | 7198 | 26703 | 76 | 35 [18,65] | 788 | 29.5 | 965 | 38.2 | |
| Geographical origin | Western Countries | 38122 | 149012 | 71 | 38 [19,68] | 1414 | 9.5 | 2450 | 17.2 |
| Sub-Saharan Africa | 9713 | 37410 | 77 | 37 [19,68] | 154 | 4.1 | 624 | 18 | |
| Rest of World | 5235 | 18147 | 68 | 33 [17, 59] | 97 | 5.3 | 263 | 15.3 | |
| Unknown | 2756 | 10951 | 65 | 35 [17,69] | 72 | 6.6 | 135 | 12.9 | |
| Calendar year | 2000 - 2004 | 15180 | 90114 | 76 | 71 [31,109] | 889 | 9.9 | 1644 | 19.5 |
| 2005 - 2010 | 35124 | 12517 | 73 | 37 [22,58] | 826 | 6.8 | 1785 | 15.3 | |
| 2011- 2013 | 5522 | 3890 | 48 | 8 [4,12] | 22 | 5.7 | 43 | 11.2 | |
| Total | 55826 | 215521 | 71 | 37 [19,68] | 1737 | 8.1 | 3472 | 16.9 | |
During 215,521 person-years of follow-up 1,737 individuals died and 3,472 developed an AIDS-defining illness or died. The estimated 7-year risk (95% CI) of death was 4.0% (3.8, 4.2) for immediate cART initiation, 4.0% (3.8, 4.3) for initiation at CD4<500 cells/mm3, and 4.2% (4.0, 4.5) for initiation at CD4<350 cells/mm3. Compared with immediate initiation, the risk ratio (95% CI) was 1.02 (1.01, 1.03) under initiation at CD4<500 cell/mm3, and 1.06 (1.03, 1.10) under initiation at CD4<350 cell/mm3 (Table 2). Compared with immediate initiation, the mean survival time at 7 years under initiation at CD4<500 cells/mm3 and at CD4<350 cells/mm3 was 2 (1,2) and 5 (4,6) days shorter. The estimates did not materially change in sensitivity analyses (Appendix Table 2).
Table 2.
Seven-year risks, risk ratios, and risk differences by cART initiation strategy, HIV-CAUSAL Collaboration 2000-2013.*
| Clinical Outcome | cART initiation strategy | Risk at 7 years, % (95% CI) | Risk Ratio (95% CI) | Risk difference,** % (95% CI) | Difference in restricted mean survival time,** days (95% CI) |
|---|---|---|---|---|---|
| All-cause mortality | Immediate universal | 4.0 (3.8, 4.2) | 1 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| <500 cells/mm3 | 4.0 (3.8, 4.3) | 1.02 (1.01,1.03) | 0.06 (0.02,0.11) | −2 (−2,−1) | |
| <350 cells/mm3 | 4.2 (4.0, 4.5) | 1.06 (1.03,1.10) | 0.25 (0.14,0.37) | −5 (−6,−4) | |
| AIDS or death | Immediate universal | 7.1 ( 6.8, 7.3) | 1 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| <500 cells/mm3 | 7.5 (7.2, 7.8) | 1.06 (1.06,1.07) | 0.44 (0.37,0.51) | −7 (−8,−6) | |
| <350 cells/mm3 | 8.5 (8.2, 8.8) | 1.20 (1.17,1.23) | 1.41 (1.24,1.59) | −21 (−23,−19) | |
Estimates based on the parametric g-formula adjusted for measured time-varying confounders (CD4 count, HIV-RNA and AIDS) and baseline characteristics (calendar period and age of HIV diagnosis, risk group, gender, geographical origin, ethnicity and cohort).
The risk difference is the 7-year risk of death under each strategy minus the 7-year risk of death under immediate universal initiation. The difference in restricted survival time is the mean survival (up to 7 years) under each strategy minus the mean survival under the immediate initiation strategy.
The estimated 7-year risk of the combined endpoint of AIDS-defining illness or death was 7.1% (6.8, 7.3) for immediate initiation, 7.5% (7.2, 7.8) for initiation at CD4<500 cell/mm3, and 8.5% (8.2, 8.8) for initiation at CD4<350 cell/mm3. Compared with immediate initiation, the risk ratio (95% CI) was 1.06 (1.06, 1.07) under initiation at CD4<500 cell/mm3, and 1.20 (1.17, 1.23) under initiation at CD4<350 cell/mm3. Figure 1 shows the estimated survival curves.
Figure 1.
Survival and AIDS-free survival estimates by cART initiation strategy, HIV-CAUSAL Collaboration 2000-2013
All estimated 7-year risks were lower in analyses restricted to the 17,612 individuals with baseline CD4 cell count>500 cells/mm3 and the 30,558 individuals with baseline CD4 cell count >350 cells/mm3 (Table 3).
Table 3.
Seven-year risks, risk ratios, and risk differences by cART initiation strategy for individuals with CD4 count>500 and >350 cells/mm3 at HIV diagnosis, HIV-CAUSAL Collaboration 2000-2013.*
| CD4 at HIV diagnosis | Clinical Outcome | cART initiation strategy | Risk at 7 years (95% CI) | Risk Ratio (95% CI) | Risk difference (95% CI) | Difference in mean survival time, days (95% CI) |
|---|---|---|---|---|---|---|
| >500 cells/mm3 | All-cause mortality | Immediate universal | 2.7 (2.2,3.5) | Ref. | Ref. | Ref. |
| <500 cells/mm3 | 2.6 (2.2,3.1) | 0.96 (0.87,1.05) | −0.10 (−0.42,0.12) | −1 (−4,3) | ||
| <350 cells/mm3 | 2.7 (2.3,3.1) | 1.00 (0.84,1.14) | −0.01 (−0.49,0.34) | −3 (−7,3) | ||
| AIDS or death | Immediate universal | 4.9 ( 4.4,5.2) | Ref. | Ref. | Ref. | |
| <500 cells/mm3 | 5.7 (5.2,6.0) | 1.21 (1.11,1.33) | 1.00 (0.58,1.35) | −19 (−23,−15) | ||
| <350 cells/mm3 | 7.1 (6.6,7.5) | 1.52 (1.34,1.77) | 2.45 (1.75,3.18) | −38 (−46,−31) | ||
| >350 cells/mm3 | All-cause mortality | Immediate universal | 2.9 (2.7,3.3) | Ref. | Ref. | Ref. |
| <500 cells/mm3 | 2.9 (2.6,3.2) | 0.99 (0.95,1.03) | −0.02 (−0.16,0.08) | −1 (−2,1) | ||
| <350 cells/mm3 | 3.0 (2.7,3.3) | 1.03 (0.92,1.13) | 0.08 (−0.25,0.32) | −3 (−6,1) | ||
| AIDS or death | Immediate universal | 4.9 ( 4.4,5.2) | Ref. | Ref. | Ref. | |
| <500 cells/mm3 | 5.5 (5.1,5.8) | 1.13 (1.09,1.17) | 0.62 (0.47,0.70) | −11 (−13,−9) | ||
| <350 cells/mm3 | 7.0 (6.6,7.5) | 1.43 (1.33,1.53) | 2.11 (1.70,2.56) | −34 (−38,−28) | ||
Estimates based on the parametric g-formula adjusted for measured time-varying confounders (CD4 count, HIV-RNA and AIDS) and baseline characteristics (calendar period and age of HIV diagnosis, risk group, gender, geographical origin, ethnicity and cohort).
The estimated 7-year risk of severe/moderate AIDS or death was 4.7% (4.3, 5.0) under immediate initiation, 4.8% (4.5, 5.1) under initiation at CD4<500 cells/mm3, and 5.2% (4.9, 5.4) under initiation at CD4<350 cells/mm3. Compared with immediate initiation, the risk ratio was 1.03 (1.02, 1.04) under initiation at CD4<500 cell/mm3 and 1.11 (1.07, 1.15) under initiation at CD4<350 cell/mm3. The estimated 7-year risk of tuberculosis was 1.20 (1.09, 1.36) under immediate initiation, and 1.24 (1.15,1.41) and 1.34 (1.26,1.51) under initiation at CD4 initiation <500 and <350 cells/mm3.
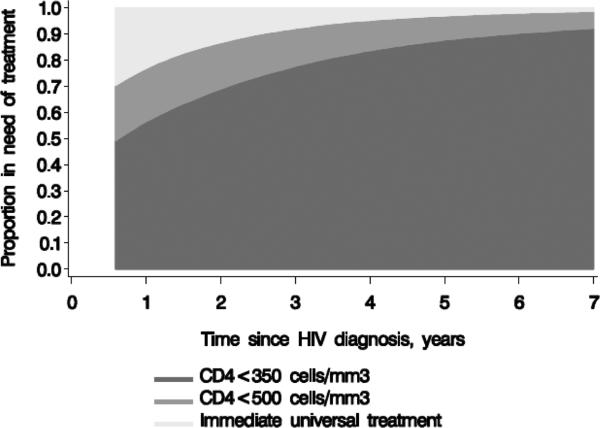
Figure 2 shows the proportion of individuals considered in need of treatment by cART initiation strategy. At 1 year, 100%, 78%, and 58% of individuals were estimated to be on cART under immediate initiation, initiation at CD4<500 cell/mm3, and initiation under CD4<350 cell/mm3, respectively. The corresponding proportions at year 7 are 100%, 99%, and 92% (Appendix Table 3).
Figure 2.
Proportions in need of antiretroviral treatment by cART initiation strategy*, HIV-CAUSAL Collaboration 2000-2013
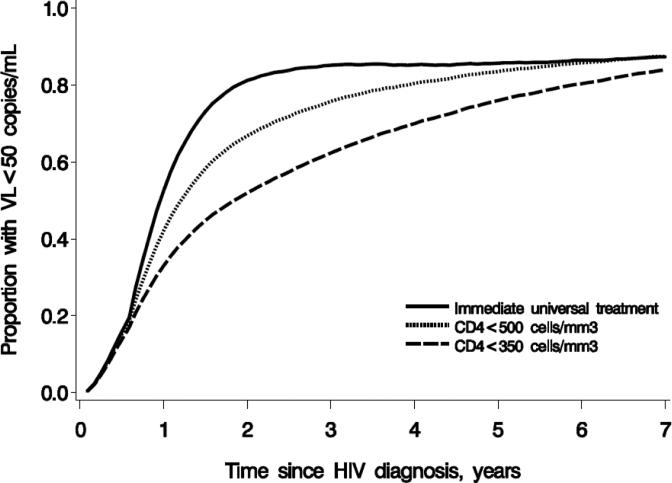
Figure 3 shows the proportion of individuals with virologically suppressed replication by cART initiation strategy. At 1 year, 58%, 46%, and 36% of individuals were estimated to be virologically suppressed under immediate initiation, initiation at CD4<500 cell/mm3, and initiation under CD4<350 cell/mm3, respectively. The corresponding proportions at year 7 are 87%, 87%, and 84% (Appendix Table 4). The estimates were larger when suppressed virological suppression was defined as HIV-RNA<400 copies/mL (Appendix Figure 2).
Figure 3.
Proportion with suppressed virological replication, defined as HIV-RNA<50 copies/mL by cART initiation strategy*. HIV-CAUSAL Collaboration 2000-2013
Discussion
Our estimates from high-income countries in Europe and the USA indicate that, in a population with a fairly low CD4 count at HIV diagnosis, immediate initiation of cART increases survival and AIDS-free survival compared with initiation strategies based on CD4 count. However, over a 7 year period, the average benefit was small both for survival (5 days) and AIDS-free survival (21 days) when comparing immediate initiation with initiation at a CD4 count of less than 350 cells/mm3. For individuals with a CD4 count greater than 500 cells/mm3 at HIV diagnosis, starting cART when the CD4 count fell below 350 cells/mm3 increased the relative risk of death or diagnosis of AIDS by more than 50% when compared with immediate initiation.
Our estimates also indicate that the proportion of individuals with suppressed virological replication was greater for immediate universal cART initiation earlier in the follow-up. By 7 years, all strategies resulted in estimated proportions of virological suppression between 83 and 87%. At that time between 93% and 99% of individuals were estimated to be receiving cART under both CD4-based strategies.
Our findings from high-income countries complement the results from the Temprano12 and the HPTN 05210 trials. The Temprano trial randomised individuals with nadir CD4 cells count > 800 cells/mm3 to either immediate initiation or initiation when CD4 count drops below the threshold recommended by WHO (between 200 and 500 cells/mm3 depending of the year of recruitment). Immediate initiation reduced the risk of serious illness, including tuberculosis and death, by 44%. The HPTN 052 trial randomly assigned individuals in low and mid-income countries with CD4 count between 350 and 550 cells/mm3 to either immediate cART initiation or initiation at the earliest of CD4 dropping below 250 cells/mm3 or a diagnosis of an AIDS-defining illness. A secondary analysis found that immediate initiation reduced the risk of several clinical outcomes. Though these trials cover a shorter period than our study (2.5 versus 7 years) and use outdated CD4 cell count thresholds, a comparison with our estimates suggests that immediate initiation may be more beneficial in low-income countries, where tuberculosis and bacterial infections are more frequent, than in the high-income countries represented in our study.
Our results will also complement the START trial results, when they become available, for the subset of trial participants in high-income countries.34 In preliminary analyses, the data and safety monitoring board of the START trial reported that initiation of treatment at a CD4 count less than 350 cells/mm3 substantially increased the incidence of death or serious disease when compared with immediate initiation in individuals with CD4 counts higher than 500 cells per μL at baseline. We also estimated an increased risk with deferral of cART (52% after 7 years), but of smaller magnitude, perhaps reflecting residual confounding, different populations and periods, or both. Our analysis also provides estimates for all-cause mortality over 7 year follow-up in high income countries. When full results of the START trial are released, we will be able to do a more formal comparison between a randomized and an observational study assessing the benefits of early initiation of cART, which will be useful in informing adjustments that may be required in future analyses of longer term outcomes
Besides the length of follow-up, the main strengths of our study are the large sample size of over 55,0000 individuals and the setting in HIV clinics in Europe and United States that are representative of routine clinical practice. Therefore, our results should be generalisable to a population of HIV-infected, AIDS-free patients in high-income countries. Other observational studies of ART-naïve patients have compared clinical outcomes for cART initiation at different CD4 count thresholds,5-8 and have generally found that earlier initiation was beneficial. Of note, some of these observational studies included HIV cohorts that contributed data to our study, although we are using a more recent data update.
Our study has some limitations. First, we did not have information on non-fatal adverse effects. Because of longer exposure to antiretroviral treatment, patients who initiate cART early might be more susceptive to drug toxicity. Although the safety profiles of antiretroviral drugs has largely improved in the last decade, central nervous system,25,26 renal,27-29 bone30 and cardiovascular31 toxicities have been reported in relation to drugs currently used in high-income countries. Second, like all observational studies the validity of our estimates relies on the assumption of no unmeasured confounding. Although we adjusted our models for time-varying CD4 count, HIV-RNA levels and AIDS diagnosis, the most important factors used by clinicians to decide when to start cART, we cannot exclude the possibility that unmeasured prognostic factors (e.g., hepatitis co-infection) could have influenced the decision to start cART.
The debate on the optimal strategy to initiate cART needs to take into consideration both the public health benefits, i.e., reduction of HIV transmission, and the benefits for HIV-infected individuals, i.e., increase in healthy life years. Our findings help quantify both types of benefits. A public health benefit of earlier initiation is implied by the greater proportion of virologically suppressed individuals, whchi will result in lower transmission to others. However, the success of cART to prevent transmission depends also on HIV testing strategies. Since at least 30% to 80%32,33 of transmissions occur from individuals unaware of their HIV infection, early cART initiation, if implemented, should be put in place together with strategies to encourage HIV testing in early HIV disease.
Our findings also help quantify the individual benefits of earlier initiation in terms of slightly longer survival under the testing strategies present in the study populations. More work is needed to explore whether these small benefits are partly offset by higher risk of toxicity or development of drug resistance in the long term.
In analyses restricted to individuals with CD4 count>350 cells/mm3 at HIV diagnosis, the estimated 7-year risks of mortality and combined outcome mortality or AIDS-defining illness were <3% and <7%, respectively, for all cART initiation strategies. The higher risks (<4.2% and <9%, respectively) in the main analyses, which included approximately 50% individuals with CD4 count<350 cells/mm3 at HIV diagnosis, again suggest the importance of timely cART initiation and better HIV testing strategies.
Finally, our study enables us to quantify the degree to which earlier cART initiation results in increased proportions of individuals considered in need of treatment. The differences between cART initiation strategies decrease over time and become small at 7 years after HIV diagnosis. The early differences might be negligible when considering the total duration of cART, which is expected to be between 40 and 50 years35. More research is needed on the long-term cost-effectiveness of different cART initiation strategies after accounting for the impact on new transmissions.36,37
In conclusion, our estimates indicate that, compared with CD4-based strategies, immediate universal treatment slightly prolongs survival and AIDS-free survival and increases the proportions of individuals with suppressed virological replication and in need of treatment. However, a policy on whether cART is made available at HIV diagnosis irrespective of CD4 count needs to consider many pieces of information—eg, longterm all-cause mortality, AIDS and non-AIDS morbidity, HIV transmission, the health-care setting, and costs that no one study can provide. Results from recently presented randomised trials indicate that there is a health benefit from immediate initiation of cART. Observational studies will continue to have a role in understanding the long-term effects of initiation of cART at high CD4 counts in routine clinical settings. Our results suggest that a focus on better and innovative HIV testing strategies might be as important, if not more, than discussions about early initiation of treatment.
Supplementary Material
Acknowledgments
Funding. NIH grant R01 AI102634.
Footnotes
All authors have contributed to the interpretation of the data and have read and approved the final version of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Principal contributions made by the authors
Data collection: Andrew Phillips, Ashley Olson, Dominique Costagliola, Sophie Abgrall, Ard van Sighem, Peter Reiss, José M. Miró, Elena Ferrer, Amy Justice, Heiner C. Bucher, Hansjakob Furrer, Santiago Moreno, Susana Monge, Giota Touloumi, Nikos Pantazis, Laurence Meyer, Rémonie Seng, Francois Dabis, Marie-Anne Vandenhede, Santiago Pérez-Hoyos, Inma Jarrín, Sophie Jose, Caroline Sabin; Study design: Sara Lodi, Miguel Hernan; Statistical analyses: Sara Lodi, Roger Logan, Jessica Young; Interpretation of results: All authors; Read and approved the manuscript: All authors; Revised the work for important intellectual content: All authors; Drafted the manuscript: Sara Lodi, Miguel Hernan. Sara Lodi, the corresponding author, had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
CS declares research grants from the MRC during this study; personal fees for membership of data safety and advisory boards and for development of educational materials from Gilead Sciences and Janssen-Cilag, outside the submitted work; and personal fees for speaking from Bristol-Myers Squibb and MSD, outside the submitted work. JS declares research grants from the National Institutes of Health during this study. AP declares personal fees for consultancy and speaking from Gilead Sciences, Abbvie, and GlaxoSmithKline, outside the submitted work. DC reports personal fees for travel from Gilead Sciences, outside the submitted work; and research grants and personal fees for travel, consultancy, and speaking from Janssen-Cilag, MSD, and ViiV Healthcare, outside the submitted work. PR reports unrestricted research grants to their institution from Gilead Sciences, ViiV Healthcare, Janssen Pharmaceutica, Bristol-Myers Squibb, and Merck, outside the submitted work; and honoraria paid to their institution from Gilead Sciences and ViiV Healthcare, outside the submitted work. SJ declares research grants paid to their institution from MRC (UK) during this study. LM reports research grants from ANRS Inserm (France) during this study; and research grants from ANRS Inserm (France) and European FP7 through the MRC (UK), outside the submitted work. JMM declares personal fees for consultancy from Abbvie, Bristol-Myers Squibb, Cubist, Gilead Sciences, Merck, Novartis, and ViiV Healthcare, outside the submitted work; and research grants from Bristol-Myers Squibb, Cubist, Gilead Sciences, Merck, Novartis, and ViiV Healthcare, outside the submitted work. All other authors declare no competing interests.
Panel: Research in context
Evidence before the study
Several observational studies and clinical trials have addressed the question of when to start combined antiretroviral therapy (cART). A summary of the evidence was presented in a systematic review and meta-analysis by WHO. Results of the TEMPRANO trial, and interim findings of the START trial, suggest that immediate initiation is the best cART initiation strategy. However, estimates from observational studies remain important to assess long-term outcomes of early cART initiation in populations representative of routine clinical practice.
Added value of this study
We have used data from a large collaboration of cohort studies in Europe and the USA to compare the effectiveness of three cART initiation strategies: immediate initiation; initiation at a CD4 count less than 500 cells/mm3 or a diagnosis of AIDS; and initiation at a CD4 count less than 350 cells/mm3 or a diagnosis of AIDS. The CD4 count at HIV diagnosis was low for many patients. In this population with a fairly low CD4 count at HIV diagnosis, immediate initiation increased survival and AIDS-free survival but, overall, the benefit was small. A strategy of immediate initiation of cART substantially increases the proportion of individuals with suppressed virological replication and the proportion of individuals in need of cART.
Implications of all the available evidence
Recent trials suggest that immediate initiation is the best ART initiation strategy. However, the benefi ts of a strategy of immediate initiation of cART, such as prolonged survival and AIDS-free survival and increased virological suppression, might be small in high-income settings with relatively low CD4 count at HIV diagnosis. More widespread and frequent HIV testing is likely to be at least as important as a policy of early cART initiation.
REFERENCES
- 1.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. Jama. 2014;312(4):410–25. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services (DHHS), editor. HHS panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2014 [Google Scholar]
- 3.World Health Organization (WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2013 [PubMed] [Google Scholar]
- 4.European AIDS clinical society (EACS) European guidelines for treatment of HIV infected adults in Europe. 2014 doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 5.Young JG, Cain LE, Robins JM, O'Reilly EJ, Hernan MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–43. doi: 10.1007/s12561-011-9040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Writing committee for the CASCADE Collaboration Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171(17):1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154(8):509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anglemyer A, Rutherford GW, Easterbrook PJ, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. Aids. 2014;28(Suppl 2):S105–18. doi: 10.1097/QAD.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 12.Danel C GD, Le Carrou J, Anglaret X, Moh R, Eholie S, Ménan H, Badje A, Kouame G, Ntakpe JB. Early ART and IPT in HV-infected African adults with high CD4 count (Temprano trial).. Conference of retrovirus and opportunistic infections (CROI); Seattle. 2015. [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundgren JD, Babiker AG, Gordin FM, Borges AH, Neaton JD. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med. 2013;11:148. doi: 10.1186/1741-7015-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 16.Ancelle-Park R. Expanded European AIDS case definition. Lancet. 1993;341(8842):441. doi: 10.1016/0140-6736(93)93040-8. [DOI] [PubMed] [Google Scholar]
- 17.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocroft A, Sterne JA, Egger M, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis. 2009;48(8):1138–51. doi: 10.1086/597468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM. A new approach to causal inference in mortality studies with a sustained exposure period: application to the healthy worker survivor effect. Mathematical Modelling. 1986;7(9-12):1393–512. [Google Scholar]
- 21.Robins J, Hernan M. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice GD, Verbeke M, G. Molenberghs G, editors. Advances in longitudinal data analysis. Chapman and Hall/CRC Press; Boca Raton: 2009. pp. 553–99. [Google Scholar]
- 22.Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grijsen ML, Steingrover R, Wit FW, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 2012;9(3):e1001196. doi: 10.1371/journal.pmed.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fidler S, Porter K, Ewings F, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207–17. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav. 2011;15(8):1803–18. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- 26.Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–99. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood N, Mandalia S, Bower M, Gazzard B, Nelson M. Ritonavir-boosted atazanavir exposure is associated with an increased rate of renal stones compared with efavirenz, ritonavir-boosted lopinavir and ritonavir-boosted darunavir. Aids. 2011;25(13):1671–3. doi: 10.1097/QAD.0b013e32834a1cd6. [DOI] [PubMed] [Google Scholar]
- 28.Young J, Schafer J, Fux CA, et al. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. Aids. 2012;26(5):567–75. doi: 10.1097/QAD.0b013e32834f337c. [DOI] [PubMed] [Google Scholar]
- 29.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207(9):1359–69. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. Aids. 2012;26(7):825–31. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 31.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 32.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. Aids. 2012;26(7):893–6. doi: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 33.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS One. 2013;8(2):e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babiker AG, Emery S, Fatkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(1 Suppl):S5–S36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May M, Gompels M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ. 2011;343:d6016. doi: 10.1136/bmj.d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2(1):e23–34. doi: 10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 37.Stover J, Gopalappa C, Mahy M, et al. The impact and cost of the 2013 WHO recommendations on eligibility for antiretroviral therapy. Aids. 2014;28(Suppl 2):S225–30. doi: 10.1097/QAD.0000000000000235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.