Abstract
Background
Inflammation biomarkers are associated with the venous thromboembolism (VTE) risk factors obesity and age, however the relationships of inflammation with VTE risk remain controversial.
Objectives
To examine associations of four inflammation biomarkers, C-reactive protein (CRP), serum albumin, white blood cell count (WBC), and platelet count (PLTC), with incident VTE, and determine whether they mediate the association of age or obesity with VTE.
Patients/Methods
Hazards models adjusted for VTE risk factors were used to calculate prospective associations of each biomarker with incident VTE in 30,239 participants of the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Mediation of the associations of obesity and age with VTE were examined by bootstrapping. Over 4.6 years, there were 268 incident VTE events. Adjusting for VTE risk factors, the hazard ratio (HR) (95% confidence interval (CI)) was 1.25 (1.09, 1.43) per standard deviation (SD) higher log-CRP and 1.25 (1.06, 1.48) per SD lower albumin, with no associations for WBC or PLTC. The association of BMI, but not age, with VTE was partially mediated by CRP and albumin. In risk factor-adjusted models, the percent attenuation of the BMI HR for VTE by introducing CRP or albumin to the models was 15.4% (95% CI: 7.7%, 33.3%) and 41.0% (95% CI: 12.8%, 79.5%), respectively.
Conclusion
Higher CRP and lower serum albumin were associated with increased VTE risk, and statistically mediated part of the association of BMI with VTE. These data suggest inflammation may be a potential mechanism underlying the relationship of obesity and VTE risk.
Keywords: Biomarkers, C-reactive protein, Inflammation, Serum albumin, Venous Thromboembolism
Venous thromboembolism (VTE) is thought to result from a combination of risk factors which may exceed an individual’s “thrombosis potential” and lead to thrombosis [1, 2]. Several VTE risk factors, such as age, obesity, genetics, immobilization, and cancer are well recognized, while others, such as increased inflammation, remain a matter of debate [3, 4]. An understanding of the interrelations of VTE risk factors also remains incomplete.
Relationships between inflammation and coagulation are well established [5, 6], and inflammation is associated with atherothrombosis risk [7]. While several biomarkers of inflammation are higher in the presence of some VTE risk factors, such as older age and obesity, the relationships between inflammation and VTE risk remain uncertain. Higher C-reactive protein (CRP), a well-established biomarker of inflammation, was independently associated with VTE in two prospective studies [8, 9], however several other studies have reported positive associations that were attenuated in multivariable analyses [10, 11] or observed no associations [12–14]. Other inflammation biomarkers including cytokines and acute phase reactants have been suggested as VTE risk markers [15, 16], but these findings are also inconsistent [13, 17]. At present, the independent nature of the relationship between inflammation and VTE is not established, and it remains controversial whether inflammation biomarkers play a causal role in VTE, or are elevated as a consequence of disease pathogenesis [3, 4].
We studied the prospective relationships of four inflammation biomarkers, C-reactive protein (CRP), serum albumin, white blood cell count (WBC), and platelet count (PLTC), with incident VTE in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Our goal was to evaluate whether these biomarkers of chronic inflammation were associated with VTE independent of traditional VTE risk factors and comorbidities. We also sought to assess whether these biomarkers potentially mediated the associations of two established VTE risk factors, age and obesity, with VTE.
Methods
Study Participants
REGARDS is a prospective longitudinal cohort study of 30,239 black and white men and women aged ≥45 years; detailed methods have been published previously [18]. Briefly, participants were enrolled between 2003 and 2007 from the contiguous United States, designed for an equal representation of race and gender, with an oversampling of individuals living in the Southeastern U.S. (AL, AR, GA, LA, MS, NC, SC, TN). Individuals were recruited using a commercial list (Genysis Inc, Daly City, CA) by use of mail and telephone contact. Persons with active cancer or undergoing current cancer treatment were excluded from the study.
Following enrollment and verbal consent, demographic information and medical histories were obtained using a computer-assisted telephone interview (CATI). This was followed by an in-home examination including written informed consent, along with anthropomorphic and blood pressure measures, phlebotomy, electrocardiogram, and medication inventory, assessed using standardized protocols (Exam Management Systems Incorporated, Irving, Texas). Questionnaires were left with participants to assess family history of vascular disease, and participants were followed by telephone every 6 months for surveillance of medical events. Deaths were ascertained by proxy report and through periodic searches of the National Death Index [19]. Study methods were reviewed and approved by the institutional review boards at each study institution.
Event Ascertainment and Definitions
VTE events were identified and classified through February 2011 as previously described [20]. Briefly, participants or proxies were asked during each telephone call for an update on their medical status and the reasons for any hospitalizations, with each hospitalization reviewed by a research nurse through February, 2011; additional VTE events were identified by self-report between February, 2010 and February, 2011; in addition, causes of death, and other events (e.g., stroke and coronary heart disease), were reviewed for possible VTE. Validation of VTEs was performed by review of abstracted medical records (79.5% of requested records were obtained) for each potential VTE case by two of three physicians (NAZ, ARF, and MC). In the case of disagreements the third physician provided the final VTE classification, or if VTE status remained uncertain, cases were discussed and resolved.
Provoked VTE was defined as a VTE preceded within 90 days by major trauma, surgery, marked immobility, or associated with active cancer or chemotherapy. All other VTE events were considered unprovoked. Coronary heart disease (CHD) was defined by participant self-report of a prior myocardial infarction (MI) or a coronary artery revascularization procedure, or by electrocardiogram evidence of an MI at baseline. Stroke was ascertained by participant self-report at baseline. Diabetes was defined by self-reported physician diagnosis with use of antidiabetic medications, fasting glucose >126 mg/dL, or nonfasting glucose >200 mg/dL. Hypertension was defined as systolic blood pressure >140 mmHg, diastolic pressure >90 mmHg, or use of antihypertensive medications. Body mass index (BMI) was defined as weight in kilograms divided by height in meters squared (kg/m2), and BMI categories were defined as: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2).
Laboratory Measurements
Blood samples were collected, centrifuged, and serum and plasma was separated and shipped overnight on ice packs to the Laboratory for Clinical Biochemistry Research at the University of Vermont. High sensitivity CRP was analyzed by particle-enhanced immunonephelometry using the BNII nephelometer according to the manufacturer’s instructions (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL); the interassay coefficients of variation (CV) were 2.1%–5.7%. Albumin and glucose were measured in serum by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics; Rochester, NY); the CV was < 5% for albumin and 1% for glucose.
Complete blood counts were performed the day after sample collection using a Beckman Coulter LH 755 Hematology Workcell (Beckman Coulter, Incorporated; Fullerton, California); CVs of 5% and 10% for WBC and PLTC, respectively, were considered acceptable. Albumin, WBC, and PLTC were measured among consecutive individuals as part of an ancillary study that began after enrollment had started, resulting in 19,393 participants with serum albumin measured, 18,334 with WBC information, and 17,850 with PLTC measured.
Statistical Analyses
The present study included 29,556 participants with follow-up in REGARDS. Participants who reported VTE at baseline (n=1,749) were excluded from analyses, leaving 27,807 participants. The distribution of CRP was highly skewed, and a natural logarithm (ln) transformation was used. Analysis of variance or t-tests were used to describe associations of unadjusted inflammation biomarker means with demographic variables and VTE risk factors; geometric (back-transformed) means (95% confidence intervals (CI)) were presented for CRP. All statistical analyses were conducted using the Statistical Analysis System (SAS, version 9.3; SAS Institute, Inc., Cary, NC).
Cox proportional hazards models were used to estimate hazard ratios (HR) of inflammation biomarkers with incident VTE. Biomarkers were modeled per standard deviation (SD) higher for lnCRP, WBC, and PLTC, and per SD lower serum albumin. Demographic-adjusted models included age, sex, race, region, and race*region interaction (due to a known interaction of race and region on VTE incidence in REGARDS [20]). Partially-adjusted models added adjustment for BMI, smoking status, and hypertension. Risk factor-adjusted models included these variables with additional adjustment for diabetes and history of CHD and/or stroke. Interaction terms were assessed for the inflammation biomarkers with race and were considered significant if the interaction p-value was ≤ 0.10.
Non-linear response relationships of CRP and albumin with VTE were evaluated using restricted cubic spline functions [21] with knots at quartiles of CRP (25th %: 0.94 mg/L; 50th %: 2.17 mg/L; and 75th %: 4.96 mg/L) and albumin (25th %: 4.0 g/dL; 50th %: 4.2 g/dL; and 75th % 4.4 g/dL), adjusting for age, sex, race, region, and race*region. To reduce the influence of acute clinical inflammation, participants in the top 5th percentile of CRP were excluded from the analyses.
We tested whether the inflammation biomarkers significantly associated with VTE mediated the associations of BMI and age with VTE using bootstrapping. We generated 1000 replicate samples with replacement and computed the HRs (95% CIs), modeled per 1-SD increment higher, for BMI (6.2 kg/m2) and age (10 years) without and with lnCRP or, in separate models, albumin, included as covariates. The 95% CI of the change in the BMI or age HR for VTE was calculated from the 2.5th and 97.5th percentile of the replicate sample distribution for the age or BMI HR. The percent change in the HR was calculated by the following formula:
Secondary analyses were performed for provoked and unprovoked VTE, and for DVT versus PE ± DVT. A sensitivity analysis was performed by excluding participants taking warfarin at baseline, and by including additional adjustment for acetylsalicylic acid and/or statin use in risk factor-adjusted models in the entire study population, and these medications plus hormone replacement therapy in analyses of women.
Results
In participants with follow-up in REGARDS and free of VTE at baseline, the median (interquartile range) CRP was 2.2 mg/L (0.94, 5.0). The mean (SD) values of WBC, PLTC, and serum albumin were 5.9 x109 cells/L (2.8), 237 x109 cells/L (69), and 4.2 g/dL (0.33), respectively. The characteristics of the study population by VTE status are shown in Table 1 and Table S1 in Supporting Information. Table 2 presents the associations of the inflammation biomarkers with VTE risk factors. Older participants had lower albumin levels and lower PLTC than younger participants. Women, blacks, and those living in the southeast had higher CRP and PLTC. Women and blacks also had lower albumin and lower WBC. Obesity category, hypertension, and smoking status were positively associated with each of the inflammation biomarkers, except for albumin which was not associated smoking status (Table 2).
Table 1.
Biomarkers of inflammation at the baseline exam in those who did or did not develop VTE during follow-up in REGARDS
| Variable | No VTE (n=27,539) | VTE(n=268) |
|---|---|---|
| CRP, mg/L (median, 25th %, 75th %) | 2.2 (0.94, 5.0) | 2.7 (1.3, 6.0) |
| CRP >3.0 mg/L (n, %) | 10,332 (38) | 112 (42) |
| White cell count, ×109 cells/L (mean, SD)* | 5.9 (2.8) | 5.9 (2.1) |
| Platelet count, ×109 cells/L (mean, SD)* | 237 (68.7) | 228 (73.0) |
| Serum Albumin, g/dL (mean, SD)* | 4.2 (0.33) | 4.1 (0.34) |
Albumin, white cell count, and platelet count were measured in a subset of participants as part of an ancillary study as described in Methods.
CRP: C-reactive protein; VTE: venous thromboembolism.
Table 2.
Associations of biomarkers of inflammation with VTE risk factors at baseline
| Means (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | CRP(n=26,057) | P-Value | Albumin (n=19,393) | P-Value | WBC (n=18,334) | P-Value | Platelet Count(n=17,850) | P-Value |
| Age, years | <0.0001 | <0.0001 | 0.55 | <0.0001 | ||||
| <58 | 2.1 (2.0, 2.2) | 4.2 (0.34) | 5.9 (2.1) | 249 (68) | ||||
| 58–63 | 2.3 (2.3, 2.4) | 4.2 (0.32) | 5.9 (1.9) | 241 (69) | ||||
| 64–70 | 2.3 (2.2, 2.3) | 4.2 (0.31) | 6.0 (4.4) | 234 (69) | ||||
| >70 | 2.1 (2.0, 2.2) | 4.1 (0.32) | 5.9 (2.2) | 223 (66) | ||||
| Gender | <0.0001 | <0.0001 | 0.0007 | <0.0001 | ||||
| Men | 1.8 (1.7, 1.8) | 4.2 (0.34) | 6.0 (3.8) | 215 (70) | ||||
| Women | 2.7 (2.6, 2.7) | 4.1 (0.32) | 5.9 (1.9) | 251 (60) | ||||
| Race | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Black | 2.7 (2.6, 2.8) | 4.1 (0.33) | 5.6 (3.6) | 241 (70) | ||||
| White | 1.9 (1.8, 1.9) | 4.2 (0.32) | 6.2 (2.1) | 235 (67) | ||||
| Region | <0.0001 | 0.63 | 0.01 | 0.0003 | ||||
| Southeast | 2.3 (2.2, 2.3) | 4.2 (0.33) | 6.0 (3.2) | 239 (68) | ||||
| Other States | 2.1 (2.0, 2.1) | 4.2 (0.33) | 5.9 (2.1) | 235 (69) | ||||
| BMI Category, kg/m2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Underweight (<18.5) | 1.1 (1.0, 1.3) | 4.2 (0.39) | 5.9 (1.9) | 249 (84) | ||||
| Normal(18.5–24.9) | 1.4 (1.4, 1.5) | 4.2 (0.33) | 5.7 (4.3) | 234 (70) | ||||
| Overweight(25.0–29.9) | 1.9 (1.9, 2.0) | 4.2 (0.32) | 5.8 (2.0) | 233 (65) | ||||
| Obese(30+) | 3.4 (3.3, 3.5) | 4.1 (0.32) | 6.1 (2.2) | 243 (71) | ||||
| Hypertension | <0.0001 | <0.0001 | <0.0001 | 0.004 | ||||
| no | 1.8 (1.7, 1.80) | 4.2 (0.32) | 5.7 (2.0) | 236 (66) | ||||
| yes | 2.6 (2.5, 2.6) | 4.1 (0.33) | 6.1 (3.3) | 239 (70) | ||||
| Smoking Status | <0.0001 | 0.37 | <0.0001 | <0.0001 | ||||
| Never | 2.1 (2.0, 2.1) | 4.2 (0.32) | 5.6 (1.8) | 238 (66) | ||||
| Former | 2.2 (2.1, 2.2) | 4.2 (0.33) | 5.9 (3.8) | 233 (69) | ||||
| Current | 2.9 (2.8, 3.0) | 4.2 (0.34) | 7.0 (2.2) | 246 (76) | ||||
Data are means (SD) for albumin, white blood cell count (WBC), and platelet count. CRP was log-transformed and data are rounded geometric (back-transformed) means (95% CI).
There were 268 incident VTE events over 4.6 years of follow-up. Mean CRP was higher, and albumin lower, in those with incident VTE (p<0.001). There were no significant differences in WBC or PLTC (Table 1). In demographic-adjusted (age, gender, race, region, race*region) Cox proportional hazards models, higher CRP and lower albumin, but not WBC or PLTC, were positively associated with incident VTE. The VTE hazard ratio (HR) (95% CI) was 1.34 (1.18, 1.52) per SD higher lnCRP and 1.29 (1.09, 1.51) per SD lower albumin, respectively. In risk factor-adjusted models that included additional adjustment for BMI, smoking, hypertension, and comorbidities (diabetes, and history of CHD and stroke), lnCRP and albumin remained associated with VTE. The HRs (95% CI) per SD increment higher lnCRP was 1.25 (1.09, 1.43) and per SD lower albumin was 1.25 (1.06, 1.48) (Table 3). Interactions of the inflammation biomarkers with race were not statistically significant (all p-interactions >0.10).
Table 3.
Cox proportional hazards models of biomarkers of inflammation with incident VTE (per SD increment)
| Biomarker | Model 1(HR, 95% CI) | Model 2(HR, 95% CI) | Model 3(HR, 95% CI) |
|---|---|---|---|
| lnCRP (per 1.18) | 1.34 (1.18, 1.52) | 1.26 (1.10, 1.44) | 1.25 (1.09, 1.43) |
| Albumin (per 0.33 g/dL) | 1.29 (1.09, 1.51) | 1.24 (1.05, 1.47) | 1.25 (1.06, 1.48) |
| White Cell Count(per 2.8 ×109 cells/L) | 1.00 (0.87, 1.17) | 0.98 (0.79, 1.23) | 1.00 (0.83, 1.21) |
| Platelet Count(per 69.0 ×109 cells/L) | 1.03 (0.87, 1.22) | 1.07 (0.90, 1.26) | 1.05 (0.88, 1.25) |
Biomarkers were modeled per standard deviation (SD) (shown in parentheses) higher for lnCRP, white cell count, and platelet count, and per SD lower serum albumin.
Model 1: Age, sex, race, region, race*region interaction.
Model 2: Model 1 + body mass index, smoking, hypertension.
Model 3: Model 2 + diabetes, and history of coronary heart disease or stroke.
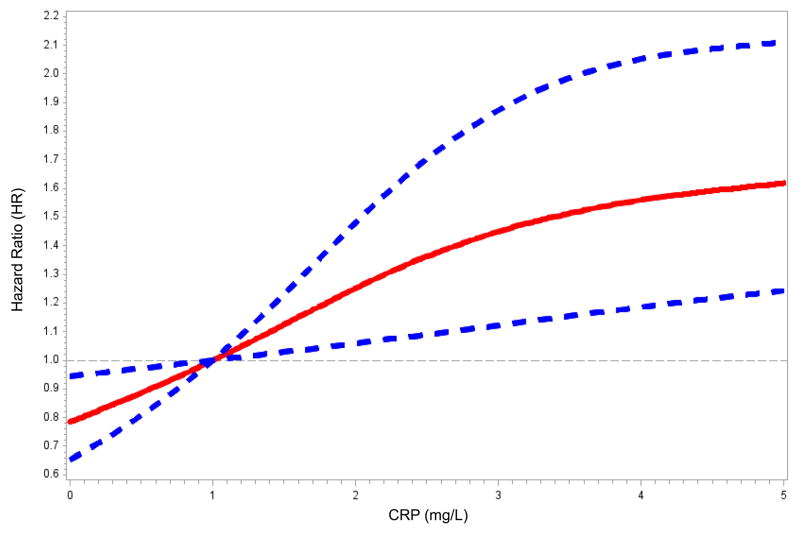
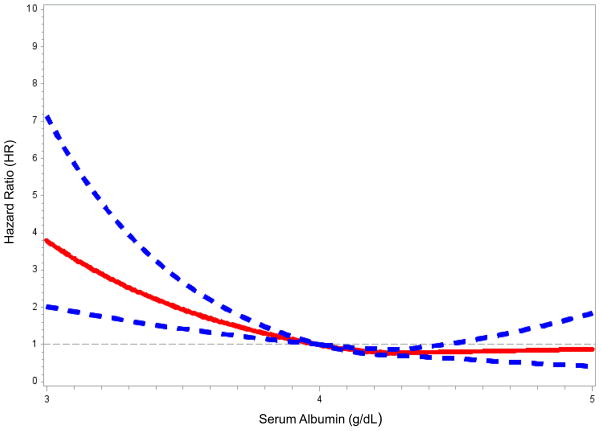
Figure 1 presents the association of CRP and albumin with VTE risk using restricted cubic splines with the reference category set at 1 mg/L for CRP and 4.0 g/dL for albumin. VTE risk increased linearly with a CRP > ~1 mg/L (Fig 1A) and below an albumin concentration of ~4.0 g/dL (Fig 1B).
Figure 1.
Restricted cubic spline modeling of C-reactive protein (CRP) and serum albumin with VTE. Restricted cubic spline functions were analyzed with knots located at quartiles of the population distribution for (A) CRP (25th %: 0.94 mg/L; 50th %: 2.17 mg/L; and 75th %: 4.96 mg/L) with the reference category set at 1 mg/L; and, (B) serum albumin (25th %: 4.0 g/dL; 50th %: 4.2 g/dL; and 75th % 4.4 g/dL) with the reference category set at 4.0 g/dL. Solid lines represent hazard ratios; dashed lines represent 95% confidence intervals. Analyses were adjusted for age, sex, race, region, and race*region interaction.
In demographic-adjusted analyses, lnCRP and, separately, albumin did not mediate the relationship of age with VTE (Table 4). However, higher CRP and, separately, lower albumin partially attenuated the HR of BMI for VTE. With the inclusion of lnCRP in demographic-adjusted models, the association between BMI (modeled per SD increment, 6.2 kg/m2) and VTE was attenuated by 21.2% (95% CI: 2.7%, 36.4%), and in risk-factor adjusted models was attenuated by 15.4% (95% CI: 7.7%, 33.3%). The percent attenuation of the HR of BMI for VTE by including albumin was 42.4% (95% CI: 6.1%, 81.8%) in demographic-adjusted models, and 41.0% (95% CI: 12.8%, 79.5%) in risk factor-adjusted models (Table 4).
Table 4.
Percent attenuation of the associations of age and BMI with VTE by CRP and serum albumin
| Inflammation Biomarker | Age HR (95% CI) | BMI HR (95% CI) |
|---|---|---|
| Model 1 | 1.55 (1.36, 1.76) | 1.33 (1.18, 1.50) |
| Model 1 + lnCRP | 1.54 (1.19, 2.0) | 1.26 (1.23, 2.08) |
| Model 1 + Albumin | 1.57 (1.33, 1.85) | 1.19 (1.01, 1.40) |
| Change in HR (%) | ||
| CRP | 1.8% (−9.1%, 9.1%) | 21.2% (2.7%, 36.4%) |
| Albumin | 3.6% (−21.8%, 32.7%) | 42.4% (6.1%, 81.8%) |
| Model 2 | 1.70 (1.47, 1.96) | 1.39 (1.23, 1.58) |
| Model 2 + lnCRP | 1.68 (1.45, 1.95) | 1.33 (1.16, 1.52) |
| Model 2 + Albumin | 1.66 (1.39, 1.99) | 1.23 (1.04, 1.46) |
| Change in HR (%) | ||
| CRP | 2.9% (−4.3%, 8.6%) | 15.4% (7.7%, 33.3%) |
| Albumin | 5.7% (−20.0%, 30.0%) | 41.0% (12.8%, 79.5%) |
Age and BMI were modeled per standard deviation (SD) higher (Age SD= 10 years; BMI SD= 6.2 kg/m2).
Model 1: Age, sex, race, region, race*region interaction.
Model 2: Model 1 + BMI, diabetes, smoking status, hypertension, and history of coronary heart disease or stroke.
In analyses of obesity categories (underweight, normal weight, overweight, and obese), rather than BMI as a continuous variable, the percent attenuation of the obesity HR (95% CI) for VTE by lnCRP was 14.3% (−23.1%, 38.2%) for the overweight category and 24.1% (3.2%, 44.0%) for the obese category, respectively (using normal weight as the reference). In risk factor-adjusted models the percent attenuation by lnCRP was 12.7% (95% CI: 5.5%, 30.0%) for the overweight category and 20.6% (95% CI: 10.8%, 38.9%) for the obese category, respectively. Albumin did not mediate the relationships of either the overweight or obese BMI categories with VTE.
In secondary analyses evaluating provoked (n= 142) and unprovoked VTE (n= 126), the HR (95% CI) per SD increment higher lnCRP was 1.40 (1.18, 1.67) for provoked VTE and 1.27 (1.06, 1.53) for unprovoked VTE (demographic-adjusted models). For serum albumin, the HRs (95% CI) were 1.14 (0.91, 1.43) for provoked VTE and 1.46 (1.16, 1.84) for unprovoked VTE, per SD increment, respectively. Results were slightly attenuated but remained significant in risk factor-adjusted models. There were no associations of WBC or PLTC for provoked or unprovoked VTE.
In demographic-adjusted secondary analyses of DVT (n= 139) or PE ± DVT (n= 123), the HR (95% CI) per SD increment higher lnCRP was 1.37 (1.15, 1.63) for DVT and 1.33 (1.11, 1.60) for PE ± DVT. For serum albumin, the demographic-adjusted HR (95% CI) was 1.19 (0.94, 1.51) for DVT and 1.37 (1.09, 1.71) for PE ± DVT. Results remained similar in risk factor-adjusted models.
A sensitivity analysis removing 779 participants (774 non-VTE and 5 VTE) on warfarin at baseline did not affect the interpretation of the results. Results remained robust in risk factor-adjusted models with added adjustment for acetylsalicylic acid and statin use in the entire study population, and these medications plus hormone replacement therapy in analyses of women.
Discussion
In this prospective population-based study we demonstrated that higher CRP and lower serum albumin, but not WBC or PLTC, were associated with increased VTE risk. CRP, and separately, albumin, partially attenuated the HR of obesity, but not age, for VTE.
The positive associations of CRP with incident VTE in our study are consistent with two previous prospective population-based studies reporting associations of elevated CRP with increased VTE risk [8, 9]. Our prospective study of a large biracial population from the entire contiguous United States provided support that relationships of CRP with VTE were independent of other VTE risk factors and comorbidities included in our models, and suggested that CRP mediated part of the association of obesity with VTE. However, other cohort and case-control studies reported no associations of CRP with VTE [12–14, 22], and two case-control studies reported positive associations that were attenuated in multivariable analyses [10, 11]. Limited statistical power or differences among study populations, including age, race, and nationality, may partially account for the discrepant results.
Previous prospective population-based studies have reported associations of low serum albumin with risk of incident and recurrent VTE [22–24]. Although patients with nephrotic syndrome are at increased VTE risk [25, 26], our results are unlikely to be confounded by nephrotic syndrome as the prevalence of gross proteinuria in REGARDS is low [27].
We did not observe associations of WBC or PLTC with VTE risk in our study. WBC and PLTC were associated with VTE in hospitalized medical patients [28–32], but not in previous population-based studies [13, 33]. Our current hypothesis is that elevated WBC and PLTC may represent acute pathologies associated with VTE risk, while CRP and albumin may represent chronic risk markers associated with persistent low-level inflammation or hypercoagulability.
Whether inflammation biomarkers are causally related to VTE remains controversial. CRP can influence tissue factor synthesis, hemostatic activation, and fibrinolysis which could provide a possible causal mechanism with VTE [34–36]. However, polymorphisms in the CRP gene associated with higher CRP levels were not associated with VTE risk [9]. Further, inflammatory gene loci were not identified in a genome-wide association study (GWAS) of VTE [37]. These findings suggest that CRP and albumin reflect underlying inflammatory or hypercoagulable states and are not causally related to VTE [38].
While CRP and albumin may be risk markers that are not in the causal pathway, emerging data suggests inflammation may be causally associated with VTE. A follow-up multi-cohort genotyping analysis identified HIVEP1, which codes for a protein involved in the transcriptional regulation of inflammatory genes, as a susceptibility locus for VTE [39]. Further, recent results from experimental animal models have demonstrated the importance of endothelial cell activation, monocyte, platelet and neutrophil recruitment, and the formation of neutrophil extracellular traps (NETs) in the initiation and progression of DVT [40–42].
Results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial demonstrated that rosuvastatin use lowered CRP levels [43] and reduced VTE risk [44]. Rosuvastatin use lowered CRP levels by 37% at the 12-month visit [43], and resulted in a VTE HR (95% CI) of 0.57 (0.37, 0.86) [44]. The association was largest in those with CRP >5. These results taken together with the positive associations between CRP and albumin with VTE observed here, may suggest that some of the reduction in VTE risk by statins occur by reducing inflammation, although it was recently proposed that suppression of coagulation activation is also important [45].
Our results showed that CRP and albumin statistically mediated some of the obesity HR, but not the age HR, for VTE. This finding is consistent with inflammation as one potential mechanism underlying the relationship of obesity and VTE risk. The lack of mediation of albumin when obesity was stratified into clinical categories likely represents reduced power due to albumin not being measured in approximately 1/3 of the cohort. Biologic mediation between obesity, inflammation, and VTE, however, cannot be established by our observational study design, and we cannot rule out the possibility of confounding.
Several limitations of our study are noted. Importantly, the inflammation biomarkers were measured at baseline, which in most cases was years prior to the VTE event. As such, the biomarker levels evaluated in the analyses may not reflect their levels at the time of the VTE. The biomarkers investigated are also limited in scope, and other potential biomarkers of inflammation and adiposity (e.g., myeloperoxidase, IL-6, leptin, adiponectin, NETs) were not measured. We also do not have complete information on some important VTE risk factors, such as incident cancer. Second, as previously discussed, several genetic analyses to date suggest there is not a causal role of inflammation with VTE, and causality cannot be established from our study design. Genotyping (for e.g., CRP, Factor V Leiden, or prothrombin G20210A) information was not available in our study. Third, VTE case ascertainment relied predominately on participant report and record retrieval in REGARDS was ~80% [20]. Finally, we lacked statistical power to examine the associations of extreme cell count values due to the small number of VTE events in these categories. Strengths of the present study include the large population-based sample of black and white participants from the entire contiguous United States with physician validated VTE events.
In summary, higher CRP and lower serum albumin were associated with increased VTE risk, while WBC and PLTC were not. Inflammation, as assessed by CRP or albumin, statistically mediated part of the association of BMI with VTE. These data support the hypothesis that inflammation is associated with VTE risk and may suggest that inflammation partially mediates the association of obesity with VTE.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of REGARDS. REGARDS was funded by cooperative agreement NS 041588 from the National Institute of Neurological Disorders and Stroke with additional funding from the American Recovery and Reinvestment Act grant RC1HL099460 from the National Heart, Lung, and Blood Institute (PI Zakai). Funding for measurements of serum albumin was from Amgen for the REGARDS Renal Ancillary study. Amgen did not have any role in the design and conduct of the study, in the collection and management of the data, or in the review or revision of the manuscript. NCO was supported by the National Heart, Lung, and Blood Institute post-doctoral training award 5T32HL007594.
Footnotes
Conflicts of Interest
The authors state that they have no conflicts of interest.
Addendum
N. C. Olson conceived the research and analytic plan, performed statistical analyses, interpreted data, and wrote the manuscript; M. Cushman conceived the research, interpreted data, and revised the manuscript; P. L. Lutsey, L. A. McClure, and S. Judd contributed to data analysis, interpreted data, and contributed to revisions of the manuscript; R. P. Tracy interpreted data; A. R. Folsom interpreted data, and contributed to revisions of the manuscript; N. A. Zakai conceived the research and analytical plan, obtained funding, interpreted data, and revised the manuscript.
References
- 1.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–73. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 2.Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis - current understanding from an epidemiological point of view. Br J Haematol. 2010;149:824–33. doi: 10.1111/j.1365-2141.2010.08206.x. [DOI] [PubMed] [Google Scholar]
- 3.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–5. doi: 10.1267/thro05020362. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Favaloro EJ, Montagnana M, Franchini M. C-reactive protein and venous thromboembolism: causal or casual association? Clin Chem Lab Med. 2010;48:1693–701. doi: 10.1515/cclm.2010.335. [DOI] [PubMed] [Google Scholar]
- 5.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–30. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 6.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost. 2009;102:615–9. doi: 10.1160/th09-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30:1672–8. doi: 10.1161/atvbaha.109.198473. [DOI] [PubMed] [Google Scholar]
- 10.Kamphuisen PW, Eikenboom JC, Vos HL, Pablo R, Sturk A, Bertina RM, Rosendaal FR. Increased levels of factor VIII and fibrinogen in patients with venous thrombosis are not caused by acute phase reactions. Thromb Haemost. 1999;81:680–3. [PubMed] [Google Scholar]
- 11.Vormittag R, Vukovich T, Schonauer V, Lehr S, Minar E, Bialonczyk C, Hirschl M, Pabinger I. Basal high-sensitivity-C-reactive protein levels in patients with spontaneous venous thromboembolism. Thromb Haemost. 2005;93:488–93. doi: 10.1267/thro05030488. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/nejm199704033361401. [DOI] [PubMed] [Google Scholar]
- 13.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) Am J Med. 2002;113:636–42. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 14.Hald EM, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Brox J, Hansen JB. High-sensitivity C-reactive protein is not a risk factor for venous thromboembolism: the Tromso study. Haematologica. 2011;96:1189–94. doi: 10.3324/haematol.2010.034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Aken BE, den Heijer M, Bos GM, van Deventer SJ, Reitsma PH. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. 2000;83:536–9. [PubMed] [Google Scholar]
- 16.Reitsma PH, Rosendaal FR. Activation of innate immunity in patients with venous thrombosis: the Leiden Thrombophilia Study. J Thromb Haemost. 2004;2:619–22. doi: 10.1111/j.1538-7836.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen SC, Naess IA, Cannegieter SC, Hammerstrom J, Rosendaal FR, Reitsma PH. Inflammatory cytokines as risk factors for a first venous thrombosis: a prospective population-based study. PLoS Med. 2006;3:e334. doi: 10.1371/journal.pmed.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 19.Wojcik NC, Huebner WW, Jorgensen G. Strategies for using the National Death Index and the Social Security Administration for death ascertainment in large occupational cohort mortality studies. American journal of epidemiology. 2010;172:469–77. doi: 10.1093/aje/kwq130. [DOI] [PubMed] [Google Scholar]
- 20.Zakai NA, McClure LA, Judd SE, Safford MM, Folsom AR, Lutsey PL, Cushman M. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129:1502–9. doi: 10.1161/CIRCULATIONAHA.113.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–8. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoodi BK, Gansevoort RT, Veeger NJ, Matthews AG, Navis G, Hillege HL, van der Meer J. Microalbuminuria and risk of venous thromboembolism. JAMA. 2009;301:1790–7. doi: 10.1001/jama.2009.565. [DOI] [PubMed] [Google Scholar]
- 23.van Schouwenburg IM, Mahmoodi BK, Veeger NJ, Kluin-Nelemans HC, Gansevoort RT, Meijer K. Elevated albuminuria associated with increased risk of recurrent venous thromboembolism: results of a population-based cohort study. Br J Haematol. 2012;156:667–71. doi: 10.1111/j.1365-2141.2011.09018.x. [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Lutsey PL, Heckbert SR, Cushman M. Serum albumin and risk of venous thromboembolism. Thromb Haemost. 2010;104:100–4. doi: 10.1160/th09-12-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbano B, Gigante A, Amoroso A, Cianci R. Thrombosis in nephrotic syndrome. Semin Thromb Hemost. 2013;39:469–76. doi: 10.1055/s-0033-1343887. [DOI] [PubMed] [Google Scholar]
- 26.Lionaki S, Derebail VK, Hogan SL, Barbour S, Lee T, Hladunewich M, Greenwald A, Hu Y, Jennette CE, Jennette JC, Falk RJ, Cattran DC, Nachman PH, Reich HN. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. doi: 10.2215/cjn.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warnock DG, Muntner P, McCullough PA, Zhang X, McClure LA, Zakai N, Cushman M, Newsome BB, Kewalramani R, Steffes MW, Howard G, McClellan WM. Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2010;56:861–71. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakai NA, Callas PW, Repp AB, Cushman M. Venous thrombosis risk assessment in medical inpatients: the medical inpatients and thrombosis (MITH) study. J Thromb Haemost. 2013;11:634–41. doi: 10.1111/jth.12147. [DOI] [PubMed] [Google Scholar]
- 29.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost. 2004;2:2156–61. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 30.Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126:113–8. doi: 10.1016/j.thromres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, Steger G, Jaeger U, Zielinski C, Pabinger I. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) J Thromb Haemost. 2010;8:114–20. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 32.Trujillo-Santos J, Di Micco P, Iannuzzo M, Lecumberri R, Guijarro R, Madridano O, Monreal M. Elevated white blood cell count and outcome in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:905–11. [PubMed] [Google Scholar]
- 33.van der Bom JG, Heckbert SR, Lumley T, Holmes CE, Cushman M, Folsom AR, Rosendaal FR, Psaty BM. Platelet count and the risk for thrombosis and death in the elderly. J Thromb Haemost. 2009;7:399–405. doi: 10.1111/j.1538-7836.2008.03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–20. [PubMed] [Google Scholar]
- 35.Bisoendial RJ, Kastelein JJ, Levels JH, Zwaginga JJ, van den Bogaard B, Reitsma PH, Meijers JC, Hartman D, Levi M, Stroes ES. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res. 2005;96:714–6. doi: 10.1161/01.res.0000163015.67711.ab. [DOI] [PubMed] [Google Scholar]
- 36.Speidl WS, Zeiner A, Nikfardjam M, Geppert A, Jordanova N, Niessner A, Zorn G, Maurer G, Schreiber W, Wojta J, Huber K. An increase of C-reactive protein is associated with enhanced activation of endogenous fibrinolysis at baseline but an impaired endothelial fibrinolytic response after venous occlusion. J Am Coll Cardiol. 2005;45:30–4. doi: 10.1016/j.jacc.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 37.Tregouet DA, Heath S, Saut N, Biron-Andreani C, Schved JF, Pernod G, Galan P, Drouet L, Zelenika D, Juhan-Vague I, Alessi MC, Tiret L, Lathrop M, Emmerich J, Morange PE. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113:5298–303. doi: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- 38.Vormittag R, Funk M, Mannhalter C, Schonauer V, Vukovich T, Minar E, Bialonczyk C, Hirschl M, Pabinger I. C-reactive protein 3′ UTR +1444C>T polymorphism in patients with spontaneous venous thromboembolism. Atherosclerosis. 2006;188:406–11. doi: 10.1016/j.atherosclerosis.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Morange PE, Bezemer I, Saut N, Bare L, Burgos G, Brocheton J, Durand H, Biron-Andreani C, Schved JF, Pernod G, Galan P, Drouet L, Zelenika D, Germain M, Nicaud V, Heath S, Ninio E, Delluc A, Munzel T, Zeller T, Brand-Herrmann SM, Alessi MC, Tiret L, Lathrop M, Cambien F, Blankenberg S, Emmerich J, Tregouet DA, Rosendaal FR. A follow-up study of a genome-wide association scan identifies a susceptibility locus for venous thrombosis on chromosome 6p24.1. Am J Hum Genet. 2010;86:592–5. doi: 10.1016/j.ajhg.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–44. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. The Journal of experimental medicine. 2012;209:819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 44.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams NB, Lutsey PL, Folsom AR, Herrington DH, Sibley CT, Zakai NA, Ades S, Burke GL, Cushman M. Statin therapy and levels of hemostatic factors in a healthy population: the Multi-Ethnic Study of Atherosclerosis. J Thromb Haemost. 2013;11:1078–84. doi: 10.1111/jth.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.