Abstract
Myasthenia gravis (MG) is an autoimmune disease caused by complement-fixing antibodies against acetylcholine receptors (AChR); antigen-specific CD4+ T cells, regulatory T cells (Tregs) and T helper (Th) 17+ cells are essential in antibody production. Target-specific therapeutic interventions should therefore be directed against antibodies, B cells, complement and molecules associated with T cell signaling. Even though the progress in the immunopathogenesis of the disease probably exceeds any other autoimmune disorder, MG is still treated with traditional drugs or procedures that exert a non-antigen specific immunosuppression or immunomodulation. Novel biological agents currently on the market, directed against the following molecular pathways, are relevant and specific therapeutic targets that can be tested in MG: (a) T cell intracellular signaling molecules, such as anti-CD52, anti-interleukin (IL) 2 receptors, anti- costimulatory molecules, and anti-Janus tyrosine kinases (JAK1, JAK3) that block the intracellular cascade associated with T-cell activation; (b) B cells and their trophic factors, directed against key B-cell molecules; (c) complement C3 or C5, intercepting the destructive effect of complement-fixing antibodies; (d) cytokines and cytokine receptors, such as those targeting IL-6 which promotes antibody production and IL-17, or the p40 subunit of IL-12/1L-23 that affect regulatory T cells; and (e) T and B cell transmigration molecules associated with lymphocyte egress from the lymphoid organs. All drugs against these molecular pathways require testing in controlled trials, although some have already been tried in small case series. Construction of recombinant AChR antibodies that block binding of the pathogenic antibodies, thereby eliminating complement and antibody-depended-cell-mediated cytotoxicity, are additional novel molecular tools that require exploration in experimental MG.
Keywords: myasthenia gravis, immunotherapies, target-specific immunomodulation
Introduction
Myasthenia gravis (MG) fulfils all the prerequisites of a classic antibody-mediated autoimmune disease, as supported by the following [Vincent and Rothwell, 2004; Engel, 2006; Drachman, 2008]: (a) the antigen, acetylcholine receptor (AChR), is known and well-characterized; (b) antibodies against the AChRs are detected and measured in more than 85% of the patients’ sera; (c) the immunoglobulin (Ig) G from MG sera binds in situ to the AChRs at the postsynaptic endplate causing degradation of the AChRs by fixing complement or crosslinking of adjacent receptors; (d) the AChR antibodies are pathogenic because they transmit the disease to experimental animals and cause destruction of the AChRs in cultured myotubes;(e) immunization of healthy animals with AChRs leads to clinical signs of myasthenia which can be subsequently passed to other animals with purified IgG; and (f) removal of the pathogenic autoantibodies results in clinical improvement [Vincent and Rothwell, 2004; Engel, 2006; Drachman, 2008]. This antibody response is T-cell dependent because regulatory T cells (Tregs) and CD4+ T cells recognize AChR epitopes in the context of major histocompatibility complex (MHC) class II molecules and exert a helper function on B cells to produce antibodies [Vincent and Rothwell, 2004; Engel, 2006; Drachman, 2008].
Accordingly, MG is the most suitable disorder to apply antigen-specific immunotherapies, either by targeting the sensitized T or B cell subpopulations to inhibit the AChR production or by modifying the pathogenic antibodies not to cause lysis of the AChRs. This process is, however, technically difficult because the autoimmune T cell and antibody responses are highly heterogeneous [Sabatos-Peyton et al. 2010; Meriggioli et al. 2008]. Furthermore, high doses of immunodominant (and potentially pathogenic) epitopes are needed to generate Tregs that recognize only the disease-inducing epitopes and induce tolerance, a process likely to lead to uncontrolled T-cell activation [Sabatos-Peyton et al. 2010]. Because of these limitations, and in spite of the tremendous progress in the immunobiology of the disease, MG is still treated with traditional drugs or procedures that exert a non-antigen specific immunosuppression or immunomodulation [Sanders and Evoli, 2010; Dalakas, 2012, 2013, 2015]. These therapies, especially the application of plasmapheresis and intravenous immunoglobulin (IVIg), have been arguably quite successful; they have increased survival and improved the quality of life for the majority of MG patients to the point that we do not consider MG anymore as ‘gravis’.
A number of patients, however, do not respond sufficiently well to the available therapies or suffer severe side effects from the long-term use of corticosteroids or immunosuppressants, necessitating the need for newer more effective and longer-lasting therapies with less severe side effects [Dalakas, 2012, 2013, 2015]. Such therapies are now accomplished by the use of biological agents of the kind that have led to breakthrough therapies in other chronic autoimmune diseases such as rheumatoid arthritis and multiple sclerosis. In MG, the application of these agents is long overdue because the immunobiology of the disease is much better understood compared with other diseases, while the industry is providing us with drugs specific for the cellular pathways involved in antibody production and antibody-mediated tissue damage.
This paper identifies the targets of immunotherapies in MG and discusses the currently available biological agents that have the potential to offer target-specific therapies, as successfully applied in the other autoimmune diseases [Dalakas, 2012, 2013, 2015].
Synopsis of current immunotherapies in MG
The present immunotherapies in MG include the following two categories [Dalakas, 2012, 2013, 2015].
Conventional and nonspecific
This category consists of corticosteroids and immunosuppressants. These drugs have been serving the patients for many years and continue to be the cornerstone of current immunotherapies. They have been arguably responsible for reducing mortality and increasing quality of life, but at a significant cost regarding long-term side effects. High doses of corticosteroids, even up to 100 mg daily, are still needed to induce remission and lower doses are essential to maintain a response (Dalakas 2012,2013). The common immunosuppressive drugs, such as azathioprine, mycophenolate mofetil, cyclosporine, methotrexate or tacrolimus, are routinely used in an effort to reduce the high daily dose of corticosteroids to the lowest possible doses that prevent relapses and diminish the long-term steroid side effects. The effectiveness of these drugs is, however, variable while tolerability and patient compliance are overall suboptimal. Most importantly, their reported efficacy has been either based on small-scale and underpowered randomized trials, or on empirical basis with at times unconvincing evidence. As a result, many patients with generalized myasthenia gravis, experience unacceptable side effects or suboptimal quality of life after many years, necessitating the need for alternative, safer and more effective therapies.
Immunomodulating, non-antigen specific, for short-term benefit
This category includes IVIg and plasmapheresis. Both therapies are used when there is a need for immediate help, until the aforementioned agents take effect, or during an acute worsening and periods of crises [Drachman, 2008; Sanders and Evoli, 2010; Dalakas, 2010a, 2012, 2013, 2014; Gajdos et al. 2008]. Plasmapheresis and IVIg provide lifesaving benefit to a large number of patients and probably account for the reduced mortality we have observed the past 20 years. However, they are costly and impractical for long-term therapies because both of them exert a transient effect which is immunomodulating and not immunosuppressive, as needed to bring finality or long-term remission to the ongoing immune process. In reference to IVIg, there is mounting criticism [Dalakas, 2014] that its efficacy has not been tested in the chronic management of MG or as a steroid-sparing agent. A testament to these uncertainties is the two new control trials that have just began designed to specifically address both of these questions [ClinicalTrials.gov identifier: NCT 02473952, NCT 02473965].
Collectively, in MG more specific therapies with long-term efficacy are needed, hence the consideration of the new biologic agents [Dalakas, 2012, 2013], as discussed below.
Target-specific immunotherapies in MG
A number of biological agents currently on the market or in clinical trials for various autoimmune disorders offer target-specific, ‘missile-like’ therapy, quite relevant to the pathogenesis of MG [Dalakas, 2012, 2013]. These agents come as: (a) monoclonal antibodies, characterized either as chimeric when only the fragment, crystallisable (Fc) portion of the IgG is human, or humanized when the whole IgG molecule is human except for the hypervariable region that remains from the mouse [Dalakas, 2012, 2013]; or (b) as therapeutic fusion proteins (-cepts), engineered when the Fc region of IgG1 is fused to the extracellular domain of key immune molecules.
An exciting new technology aimed at re-engineering of the pathogenic antibodies not to be pathogenic may be a promising futuristic therapeutic tool very appropriate in MG because the AChR antibodies can be modified to act as molecular decoys effectively blocking the binding of the pathogenic antibodies [Steinman and Zamvil, 2012].
Therapeutic targets based on immunopathogenesis of MG
To understand the rationale for applying the new biological agents in MG, the main network involved in the immunopathogenesis of the disease is briefly discussed to highlight the key molecules need to be targeted in order to induce tolerance or restore immune balance.
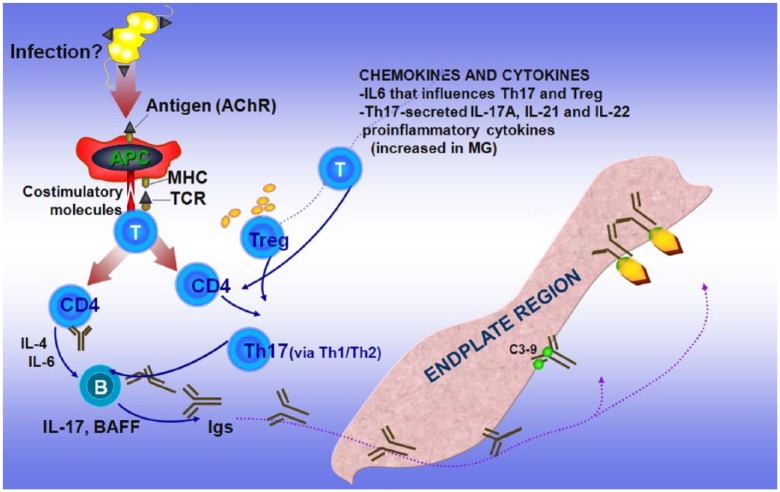
As previously discussed [Dalakas, 2012, 2013], it is unclear what triggers MG but, like all the other autoimmune disorders, the process begins when tolerance is broken probably by infections or via molecular mimicry when the AChR protein shares sequence homologies with microbial antigens, resulting in crossreactivity and autoimmunity [Dalakas, 2013]. When this happens, the AChR is presented by antigen-presenting cells (APCs) (probably dendritic cells in the thymus or B cells in the periphery) to CD4+ T cells leading to upregulation of key cytokines, such as IL4 and IL6, which stimulate B cells to produce anti-AChR antibodies. These antibodies fix complement at the endplate region, leading to destruction of the AChRs and simplification of the endplate region (Figure 1a).
Figure 1a.
Main players crucial in the immunopathogenetic network involved in myasthenia gravis (MG) as related to therapeutic targets.
Acetylcholine receptors (AChRs), presented via antigen-presenting cells (APCs) to CD4+ T cells via costimulatory molecules, lead to upregulation of cytokines that stimulate B cells to produce anti-AChR antibodies which, by fixing complement at the endplate region, cause destruction of the AChRs. Regulatory T cells (Tregs) and T helper (Th) 17+ cells, cytokines such as interleukin (IL) 6 that affect the induction of Tregs, and proinflammatory cytokines such as IL-17A, IL-21 and IL-22, which are increased in MG patients, enhance and sustain the immune imbalance (modified from Dalakas 2012).
BAFF, B-cell activating factor; Igs, immunoglobulins; MHC, major histocompatibility complex; TCR, T-cell receptor.
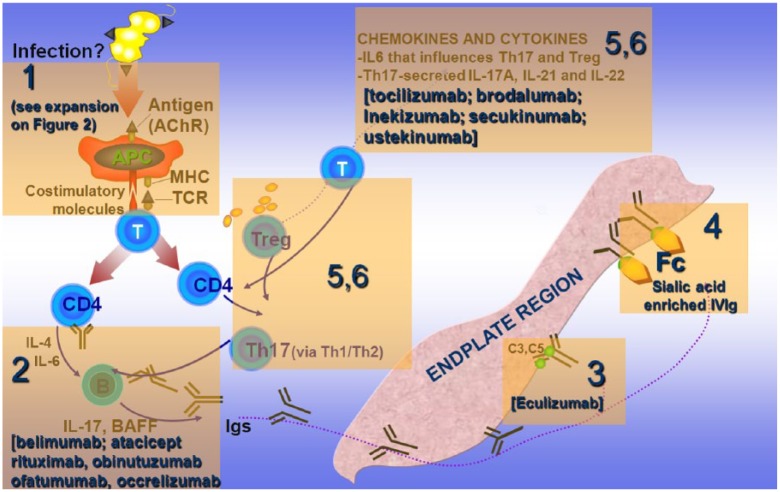
The involvement of Treg and Th17+ cells is fundamental because they affect antibody production via a Th1/Th2 cytokine balance [Aricha et al. 2011; Masuda et al. 2010]. Cytokines, such as IL-6, affect the induction of Tregs to pathogenic Th1 cells, while proinflammatory cytokines, such as IL-17A, IL-21 and IL-22, which are increased in MG, are fundamental in maintaining the immune imbalance [Meriggioli et al. 2008; Aricha et al. 2011; Masuda et al. 2010]. Accordingly, as numerically depicted in Figure 1b, the targets of action of specific immunotherapeutic drugs applicable for targeted therapy in MG are directed against the following: (a) molecules involved in T-cell activation; (b) antibodies, B cells and B-cell trophic factors; (c) complement; (d) modulation of the Fc receptor (FcR) of the IgG antibodies; (e) cytokines involved in antibody production or immunoregulation; and (f) Treg and Th17+ cells that affect the production of antibodies via Th1/Th2 cytokine balance [Dalakas, 2012, 2013]. Agents against these targets (mentioned in Figure 1b), already available for the treatment of other systemic autoimmune or neurological disorders [Dalakas, 2010b, 2011a, 2011b; Gold et al. 2003; Hohlfeld and Dalakas, 2003], need to be considered as future therapeutic options in MG, as discussed below.
Figure 1b.
Crucial targets of action of specific immunotherapeutic drugs as related to the pathogenesis of myasthenia gravis (MG).
In MG, new therapies might be directed against the following sequential targets (in boxes): (1) molecules involved in T-cell activation and costimulation (expanded in Figure 2); (2) antibodies, B cells and B-cell trophic factors, with most representative the anti-CD20 molecules (rituximab, occrelizumab, ofatumumab) and the anti B-cell activating factor (BAFF) and B lymphocyte stimulator (Blys) (belimumab and atacicept); (3) complement C5 (targeted by the drug eculizumab); (4) fragment, crystallizable receptor (FcR) of immunoglobulin G (IgG) by enriching the sialic acid content of IgG; (5) cytokines such as interleukin (IL) 6 that facilitate antibody production by B cells (targeted by tocilizumab); and (6) regulator T cells (Tregs) and helper T (Th) 17+ cells that affect production of antibodies via Th1/Th2 cytokine balance (targeted by brodalumab, inekizumab, sekinumab and ustekinumab). Three other biological agents not depicted in the figure, but discussed in the text, are those affecting T-cell transmigration or trapping activated T cells in the lymphoid organs (targeted by natalizumab, vedolizumab and fingolimod) (extensively modified from Dalakas 2013).
AChR, acetylcholine receptor; IVIg, intravenous immunoglobulin; MHC, major histocompatibility complex; TCR, T-cell receptor.
Biological agents as future therapies in MG
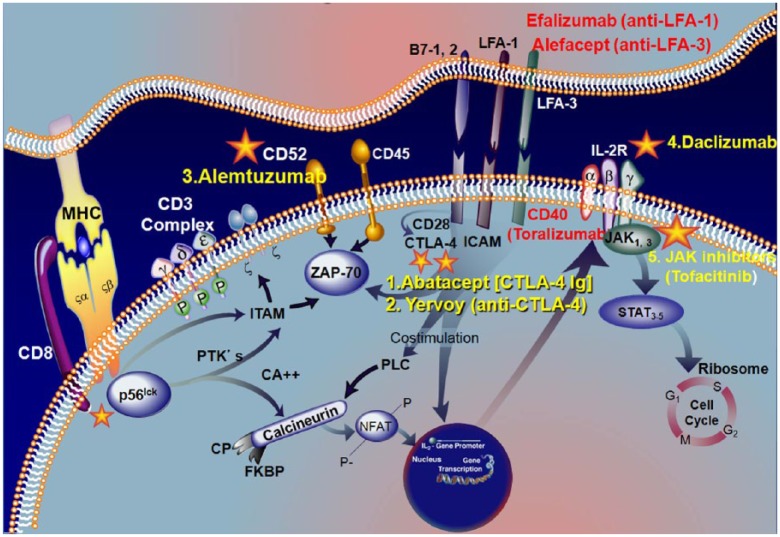
T-cell intracellular signaling pathways and molecules associated with antigen presentation (#1 in Figure 1b and Figure 2)
Figure 2.
Signaling pathways activated by major histocompatibility complex (MHC)/T-cell receptor (TCR) engagement and successful drugs (1–4) that inhibit specific signaling molecules (expansion of box 1 in Figure 1b).
The interaction of TCR with antigen/MHC complex activates intracellular phosphotyrosine kinases (ZAP-70) that mediate signaling via phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) and transduction molecules such as CD52. T-cell activation is meditated via costimulatory factors delivered by LFA-I/ICAM and LFA-3/CD2, CD40 and CD28/CTLA-4 interactions. Monoclonal antibodies against LFA1 [efalizumab (Raptiva)] and anti-CD40 (toralizumab) or fusion proteins against LFA3 [alefacept] that block the T-cell activation process have either failed or withdrawn from the market (see text). However, two drugs in this group targeting CTLA-4, abatacept and yervoy [Vincent and Rothwell, 2004; Engel, 2006] have been effective and are approved. TCR engagement and costimulation activates sequentially downstream events that, via phospholipase (PLC) and activation of calcineurin, activate the nuclear factor of activated T cells, which is translocated into the nucleus where it binds to interleukin (IL) 2 promoter to induce cell proliferation and differentiation. Antibodies against CD52 alemtuzumab (3*) can stop the T0cell activation. Activated T cells synthesize the T-cell growth factor, IL-2 and its receptor, which binds to IL-2 with moderate affinity. This receptor can be blocked by the monoclonal antibody to CD25 [daclizumab (4*)]. Binding of IL-2 to the receptor in turn activates an intracellular signaling cascade via Janus kinases (JAK1, JAK3), with subsequent phosphorylation of signal transducer and activator of transcription (STAT) proteins. The compound against JAK kinases (tofacitinib [5*]) inhibits IL-2 dependent differentiation of helper T cells and suppresses B and T cell functions [adapted from Aricha et al. 2011; Masuda et al. 2010; Dalakas, 2010b, 2011a, 2011b]. The asterisks denote the approved drugs on the market (extensively modified from Dalakas 2013).
CTLA-4, cytotoxic T lymphocyte antigen; ICAM, intercellular adhesion molecule; LFA-1, lymphocyte function antigen 1; LFA-3, lymphocyte function antigen 3; NFAT, nuclear factor of activated T cells; PLC, phospholipase C; PTK, protein tyrosine kinase; ZAP-70, zeta-chain associated protein 70.
As expanded in Figure 2, the antigen presentation by the MHC complex to the T-cell receptor (TCR) activates intracellular phosphotyrosine kinases (p56, ZAP-70) that mediate signaling via phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) and transduction molecules such as CD52 [Gold et al. 2003; Hohlfeld and Dalakas, 2003; Dalakas, 2010b, 2011b; Peterson and Koretzky, 1999; Halloran, 2000; Tak and Kalden, 2011]. T-cell activation is predominantly mediated via costimulatory factors delivered by the following interactions: B7/CD28, cytotoxic T lymphocyte antigen 4 (CTLA-4); lymphocyte function antigen 1 (LFA-1)/intercellular adhesion molecule (ICAM); lymphocyte function antigen 3 (LFA-3)/CD2,CD40; CTLA-4/CD40; and ICOS/ICOSL (Figure 2). These costimulatory molecules, which are fundamental for T-cell activation, have been targeted by monoclonal antibodies during the past decade with mixed results. Three drugs (depicted in red in Figure 2) have failed or have been withdrawn. Alefacept (directed against LFA-3), which exerts a downregulatory effect on memory T cells and cytokine production, and efalizumab (against LFA-1), which blocks reactivation of memory T cells [Gold et al. 2003; Hohlfeld and Dalakas, 2003; Dalakas, 2010b, 2011b; Peterson and Koretzky, 1999; Halloran, 2000; Tak and Kalden, 2011] were FDA-approved for the treatment of psoriasis in 2002, but were withdrawn from the market 5 years later because of association with progressive multifocal leukoencephalopathy (PML). A third drug toralizumab (directed against CD40/CD154) was ineffective in rheumatic diseases, despite promising results in autoimmune animal models [Tak and Kalden, 2011].
Two other drugs in this category targeting CTLA-4 (#1,2 in Figure 2) have, however, been effective and they are already on the market. One, abatacept (Orencia), a fusion protein with CTLA-4-Ig, which inhibits binding of CD28 on T cells, has been approved for rheumatoid arthritis and is undergoing a phase II trial in inflammatory myopathies. Because CTLA-4 is altered in MG and aberrant cellular mechanisms involving CTLA-4 may predispose to developing MG based on a recent genome-wide association study [Renton et al. 2015], abatacept is of direct relevance in MG and a good candidate drug especially in some genetically predefined patient subsets. A second drug, yervoy (against CTLA-4), a humanized monoclonal antibody that blocks the activity of CTLA-4, has been approved for the immunotherapy of melanoma. Yervoy is powerful and works like taking the breaks off the immune system, allowing T cells to activate and proliferate in order to attack melanoma cells. Consequently, an important side effect is autoimmune complications, such as developing other autoimmune diseases including neuropathies. For these reasons, such therapy is aimed only for life-threatening metastatic melanoma.
In contrast to the mixed results in efficacy and safety observed with anti-costimulatory molecules, agents against other factors associated with TCR engagement, such as transduction molecules and cytokine receptors, have been successful immunosuppressants and they are already on the market. They target downstream substrates of T-cell activation molecules, including activation of calcineurin via phospholipase (PLC) and nuclear factor of activated T cells (NFAT) which translocate to the nucleus, bind to IL-2 promoter, and induce cell proliferation and differentiation [Gold et al. 2003; Hohlfeld and Dalakas, 2003; Peterson and Koretzky, 1999; Halloran, 2000]. Agents currently on the market that target such molecules, also relevant to the immunobiology of MG, are: (a) alemtuzumab, (b) daclizumab; and (c) tofacitinib.
Alemtuzumab (CAMPATH), a monoclonal antibody against CD52, results in long-lasting lymphocyte depletion via apoptosis [CAMMS223 Trial Investigators, 2008] (#3 in Figure 2). Alemtuzumab has been approved for multiple sclerosis, resulting in almost 70% reduction of relapses and disability prevention [CAMMS223 Trial Investigators, 2008]. The drug has been also promising in chronic inflammatory demyelinating polyneuropathy (CIDP) where a controlled trial is planned [Marsh et al. 2010]. (b) daclizumab, a monoclonal antibody that binds to CD25 (IL-2 receptor antagonist) and inhibits T-cell proliferation (#4 in Figure 2).
Daclizumab is well tolerated, has been approved for one form of leukemia, and has been very promising in patients with multiple sclerosis in two phase III clinical trials [Wynn et al. 2011; Gold et al. 2013]. It is an excellent candidate agent to consider for trials in MG.
Tofacitinib is an oral Janus kinase inhibitor (#5 in Figure 2). When IL-2 binds to its IL-2 receptor, it activates an intracellular signaling cascade via Janus kinases (JAK1, JAK3) with subsequent phosphorylation of signal transducer and activator of transcription (STAT) proteins. The JAK1 and JAK3 tyrosine kinases mediate signal transduction activity involving the surface receptors of multiple cytokines including IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, all integral to lymphocyte activation, function and proliferation, relevant to the immunopathology of MG. In vitro, tofacitinib inhibits IL-2 dependent differentiation of Th2 and Th17, and attenuates signaling by proinflammatory cytokines, such as IL-6 and interferon-γ [van Hollenhoven et al. 2012; Fleischmann et al. 2012; Sandborn et al. 2012]. Blockade of the Janus kinases results in suppression of both T and B cells while maintaining Treg function. Tofacitinib has been effective in ulcerative colitis and rheumatoid arthritis [van Hollenhoven et al. 2012; Fleischmann et al. 2012; Sandborn et al. 2012; Lee et al. 2014] and is an excellent candidate agent for future trials in MG.
B cells, B-cell trophic factors and autoantibodies (#2 in Figure 1b)
B cells are involved not only in complement activation and antibody production but also in antigen presentation and cytokine production, such as IL-1, IL-6, IL-10 and tumor necrosis factor (TNF) [Dalakas, 2008a, 2008b; Tsokos, 2011]. Accordingly, targeting B cells may restore immune balance as these cells are involved in several areas of the immune activation processes. Apart for targeting circulating B cells, two trophic B-cell factors, BAFF (B-cell activating factor) and APRIL (a proliferating inducing ligand), both TNF-α ligands, are also relevant and potential therapeutic targets in MG because BAFF serum levels is increased in patients with active disease [Ragheb, 2008].
At least 10 drugs in the form of monoclonal antibodies or fusion proteins against B cells or B-cell growth factors have been successfully tested or undergone clinical trials in autoimmune diseases [Dalakas, 2008a, 2008b; Tsokos, 2011]. Among those targeting trophic factors, belimumab, directed against the human soluble BAFF has been approved as the first drug for the treatment of lupus [Tsokos, 2011]. Because the soluble BAFF level is increased in MG and belimumab, by targeting BAFF affects the differentiation of B cells into antibody-producing plasma cells, the drug is relevant as a potential treatment option in MG. A phase II study of belimumab in both AChR and muscle-specific tyrosine kinase (MuSK) positive MG is now in progress [ClinicalTrials.gov identifier: NCT01480596]. Atacicept, an anti-transmembrane activator and calcium modulating ligand (CAML) interactor (TACI) IgG fusion protein, which prevents B lymphocyte stimulator (Blys) and APRIL from binding to its TACI receptor, was tried in multiple sclerosis but it was unsuccessful probably because: (a) Blys may have a protective role stimulating IL-10 producing regulatory B cells (Bregs) that help plasma cell survival; (b) Blys and APRIL are expressed late in B-cell maturation; and (c) atacicept causes incomplete B-cell depletion [Kappos et al. 2014].
Among the drugs against B-cell antigens three that target the CD20 molecule on B cells [rituximab, ofatumumab (Arzera) and obinutuzumab (Gazyva] are currently on the market for US Food and Drug Administration (FDA) approved indications (#2 in Figure 1b). Rituximab, a chimeric monoclonal antibody is directed against CD20, a 297 amino acid, not secreted, membrane-associated phosphoprotein of 33–37 kD present on all B cells, except stem cells, pro-B cells and plasma cells [Dalakas, 2008a, 2008b]. Occrelizumab, the humanized version of rituximab, is very promising in multiple sclerosis, and is now undergoing a second phase III clinical trial. Ofatumumab (Arzerra) targets different CD20 epitopes because it binds not only to the large loop of the CD20 molecule but also the small loop closer to B-cell membrane and results in more effective B-cell lysis. Ofatumumab has shown great promise in multiple sclerosis in phase II/III trials [Sorensen et al. 2014]. Obinutuzumab (Gazyva), the latest humanized anti-CD20 monoclonal antibody approved for chronic lymphocytic leukemia, is much more effective than the others in causing a more profound B-cell lysis [Goede et al. 2015]. The first three, referred to as type I anti-CD20 monoclonals, cause B cell depletion by antibody-dependent cellular cytotoxicity (ADCC), by complement-depended cytotoxicity (CDC) or apoptosis, and affect mainly the circulating B cells but not the B-cell population in the bone marrow or lymph nodes and do not lyse the antibody-producing plasma cells. In contrast, obinutuzumab belongs to type II anti-CD20 because it is glycoengineered by defucosylation of IgG oligosaccharides in the Fc region to enhance its binding affinity to the FcγRIII receptor on immune effector cells. As a result, the ADCC activity of obinutuzumab is: (a) 35–100 times higher than rituximab and ofatumumab; (b) recruits more monocytes, neutrophils and dendritic cells via Fc-FcγR interactions, thereby increasing the phagocytotic and cytotoxic activity effected by monocytes and macrophages; and (c) has a minimal effect on B-cell killing via CDC. Obinutuzumab, by its different binding topology on the CD20, achieves superior rituximab B-cell depletion not only in the periphery but also in lymphoid tissue, including lymph nodes and spleen [Goede et al. 2015].
Based on a number of reports (but not controlled studies), rituximab at 375 mg/m2 once a week for 4 weeks, or 2 g (divided in two, 1 g each, biweekly infusions) has been effective in patients with MG and seems especially promising in MuSK-positive MG [Díaz-Manera et al. 2012]. In one study, improvement was noted in up to 96% of MuSK-positive MG patients and 81% of AChR-positive patients. In MuSK-positive MG where the antibodies are of IgG1 and IgG4 subclass, the response to rituximab was more robust with long-lasting remissions [Diaz-Manera et al. 2012]. A controlled multi-center study has now begun [ClinicalTrials.gov identifier: NCT02110706].
In general, the duration of the response varies, but most of the time, follow-up infusions are required after 6–12 months. We have seen, however, long-lasting remissions even up to 2–3 years in patients requiring for years an immunosuppressant and not being able to lower the prednisone beyond 35 mg every other day. The timing of the second infusion may be dictated by the reappearance of CD20+CD27+ memory B cells, which are directly involved in antibody production and usually re-emerge after 6–8 months [Dalakas, 2008b; Maurer et al. 2012]. Experience with the use of rituximab in anti-myelin-associated glycoprotein (MAG) neuropathies has shown that the expansion of CDR3 sequences of memory B cells 8 months after the infusion may be a predictor of response to therapy; in this study, the non-responders had a higher load of IgM memory B cell expansions that persisted after therapy [Maurer et al. 2012]. This observation may suggest that a low efficiency to reduce B-cell expansions may predict poor clinical response; whether such patients may potentially benefit from repeated therapy to further reduce the clonally related autoreactive B cells or need higher doses or a more potent anti-CD20 agent like ofatumumab or obinutuzumab remains unclear.
Another drug in this family with a novel action on plasma cells is bortezomib, which inhibits proteasome activity in plasma cells leading to plasma cell depletion [Verbrugge et al. 2015]. Bortezomib has been studied in animal models of autoimmune diseases including experimental MG, where it caused reduction of AChR antibody titers, inhibited damage to the postsynaptic endplate region and resulted in clinical improvement [Verbrugge et al. 2015; Gomez et al. 2011]. The drug has been approved for multiple myeloma and mantle cell lymphoma and, although it is an excellent candidate agent in MG, enthusiasm is diminished because it causes peripheral neuropathy, a considerable concern in MG patients.
Complement (#3 in Figure 1b)
The most effective agent in inhibiting complement activation is IVIg. Based on a series of studies in vitro, in animal models and in patients, IVIg inhibits complement uptake and intercepts, at the C3 level, the formation and deposition of membranolytic attack complex (MAC) on the targeted tissues [Dalakas, 2012, 2013; Basta et al. 1994]. IVIg has multiple actions [Dalakas, 2010a], but some of the most likely mechanisms of effectiveness in MG are probably via complement inhibition and supply of idiotypic antibodies.
The second, very specific and direct anti-complement agent is a monoclonal antibody against C5 (eculizumab), which inhibits C5 and intercepts the formation of MAC and the subsequent generation of proinflammatory molecules [Dalakas, 2012, 2013; Tüzün et al. 2011]. Eculizumab, approved for paroxysmal hemoglobinuria, is a suitable drug to test in difficult AChR-positive MG cases because the pathogenic AChR antibodies fix complement at the end plate region [Vincent and Rothwell, 2004; Engel, 2006]. A small, randomized, double blind, placebo-controlled, crossover, multicenter phase II study in patients with refractory generalized MG has shown that eculizumab is effective [Howard et al. 2013], prompting an ongoing phase III study [ClinicalTrials.gov identifier: NCT01997229]. Eculizumab is appropriate for AChR-positive MG and not for MuSK-positive because MuSK antibodies do not fix complement. Eculizumab has been also very promising in neuromyelitis optica (NMO), another complement-fixing antibody-mediated neurological disease [Pittock et al. 2013].
Modulation of the Fc receptors (#4 in Figure 1b)
Fc receptors are important because they determine antibody-mediated effector functions and antibody-dependent cell-mediated cytotoxicity [Quast and Lunemann, 2014]. In antibody-mediated animal models of autoimmune diseases, the Fc-linked sugar moieties serve as a molecular switch shifting IgG activity to anti-inflammatory pathways. An agent known to have an effect on Fc receptors is IVIg. Sialic acid-rich IVIg suppresses inflammation by upregulating the inhibitory FcγRIIB receptors [Anthony et al. 2008]. Normally, 1–2% of the IgG within the IVIg preparations possess the fully (tetrasialylated) sialic acid containing anti-inflammatory glycoform, while mono- and bi-sialylated glycoforms are much higher; enriching them can therefore enhance the anti-inflammatory effect of IVIg by 20% [Anthony et al. 2008]. Whether new IVIg products engineered to have increased sialic acid within the Fc portion may be more effective in the management of MG, as shown in an animal model of autoimmune arthritis [Othy et al. 2014], remains to be determined.
Cytokines, cytokine receptors and Tregs (#5, 6 in Figure 1b)
The most widely available anti-cytokine agents for clinical use are those directed against TNF-α, as approved for rheumatoid arthritis, based on controlled trials. They include etanercept (Embrel), infliximab (Remicade) and adalimumab (Humira). These agents have, however, a paradoxical effect in several autoimmune neurological diseases causing exacerbation of multiple sclerosis, autoimmune myopathies and neuropathies. This has been also the case for MG. Following therapies with these agents, it has been observed that some patients develop AChR antibodies or a full-blown clinical MG [Fee and Kasarskis, 2009], with resolution of symptoms and reduction of antibodies after therapy discontinuation. These agents are not therefore recommended in MG. In contrast, other anti-cytokine agents more relevant to the pathogenesis of the disease (Figure 1a), such as those targeting IL-6 and IL-17 [Mu et al. 2009; Roche et al. 2011], are serious contenders as future treatment options. These agents include the following (#5,6 in Figure 1b). Tocilizumab, an IL6 receptor antagonist approved for rheumatoid arthritis [Yamamoto et al. 2015], is quite relevant in MG because IL-6 affects the induction of Tregs to pathogenic Th1 cells. The drug has shown effectiveness in neuromyelitis optica, an autoimmune neurological disease mediated by IgG antibodies against aquaporin-4 (AQP-4) [Ayzenberg et al. 2013]. Brodalumab, inekizumab and secukinumab, all monoclonal antibodies against IL-17 or IL-17A, are effective in psoriasis in phase III clinical trials [Papp et al. 2012; Leonardi et al. 2012; Langley et al. 2014]. Ustekinumab, a human monoclonal antibody against the p40 subunit of IL-12/1L-23, has shown effectiveness in psoriatic arthritis and has been approved for plaque psoriasis [Gottlieb et al. 2008].
Agents involved in cell adhesion and T-cell migration
The prototypic drug in this category is natalizumab, approved for multiple sclerosis and Crohn’s disease, which prevents adhesion and transmigration of T cells by binding to integrins α4β1 (VLA4) and a4b7 on leucocytes [Castro-Borrero et al. 2012]. Because it affects both T and B cells, it might be a reasonable drug for a new trial in difficult MG cases, provided the PML safety concerns are resolved. A more appropriate anti-integrin monoclonal antibody for MG, however, may be vedolizumab, because it targets only the a4b7 integrin and modulates only the gut but not the brain T, B lymphocytes [Feagan et al. 2013] which are not relevant in MG; furthermore, vedolizumab by not affecting the α4β1-mediated lymphocyte trafficking to the brain, does not seem to be associated with PML. The drug has been effective in Crohn’s disease and ulcerative colitis [Feagan et al. 2013].
A different family of drugs targeting T-cell migration is fingolimod, which binds to sphingosin receptors and traps the egress of lymphocytes from the lymphoid organs. The drug, which is approved for multiple sclerosis [Kappos et al. 2010], is a good candidate agent for future MG trials because it also affects B cells and exerts a trophic action that might be relevant in enhancing AChR recovery and endplate regeneration. This concept is based on the idea that local factors at the endplate region, such as the chronic release of toxic cytokines, TNF-α or matrix metalloproteinases (MMPs), may affect AChR recovery and re-synthesis owing to the ongoing antibody-mediated immune attack. The neuroprotective effect of fingolimod might therefore be relevant in MG by providing an additional effect on preventing axonal degeneration of distal nerve terminals or endplate fibrosis.
Re-engineering of pathogenic antibodies (molecular decoys)
Generation of recombinant antibodies that block binding of circulating antibodies and eliminating complement and cell-mediated cytotoxicity is a new concept pioneered recently for another antibody-mediated disease, NMO, caused by pathogenic antibodies to AQP-4 [Steinman et al. 2012; Tradtrantip et al. 2012]. Recombinant monoclonal antibodies were produced from clonally expanded plasma blasts derived from the cerebrospinal fluid (CSF) of NMO patients by introducing amino acid mutations into the IgG1Fc sequences to generate constructs deficient in CDC and ADCC. These antibodies exert their action by blocking the binding of circulating antibodies, thereby eliminating complement-dependent cytotoxicity and ADCC due to steric competition owing to their large physical size compared with the native AQP-4. Because these antibodies do not block all B cells or inhibit the universal complement pathway, they offer an ideal therapeutic tool for an antibody-mediated disorder like MG where the pathogenic antibodies fix complement [Steinman et al. 2012; Tradtrantip et al. 2012]. The same group has also shown that enzymatic deglycosylation of IgG can convert the pathogenic anti-AQP-4 antibody into a therapeutic one offering a clever tool by removing only the sugar moieties from the IgG [Tradtrantip et al. 2013]. These techniques of manipulating the structure of pathogenic antibodies should be tried in experimental autoimmune MG and, if successful, might offer an exciting new futuristic tool for the human disease.
Concerns over new immunobiologic agents: safety and cost
The new biological agents in the form of monoclonal antibodies or fusion proteins as discussed above offer guarded optimism as future new therapeutic options in MG. Many of these drugs, however, pose two major concerns: excessive cost and long-term safety. Although the cost is sometimes prohibitive, the idea that they can improve quality of life and diminish the disfiguring side effects of long-term steroid use or the bone-marrow toxicity of the currently used immunosuppressive drugs, may overcome the concerns of the third party carriers, provided their effectiveness is proven with controlled trials. Perhaps the most alarming concern is their long-term safety. Bacterial, fungal or opportunistic infections have been rarely reported with several of these drugs; reactivation of latent viral infections, such as herpes or John Cunningham (JC) virus, as well as latent tuberculosis have been additional concerns necessitating clinical and laboratory vigilance especially in patients with MG who have been already exposed to prior immunosuppressive therapy. Overall, the balance of risk–safety ratio should be viewed in the context of the other available options and the need to increase long-term quality of life [Dalakas, 2013].
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Anthony R., Nimmerjahn F., Ashline D., Reinhold V., Paulson J., Ravetch J. (2003) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320: 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricha R., Mizrachi K., Fuchs S., Souroujon M. (2011) Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. J Autoimmun 36: 135–141. [DOI] [PubMed] [Google Scholar]
- Ayzenberg I., Kleiter I., Schröder A., et al. (2013) Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol 70: 394–397. [DOI] [PubMed] [Google Scholar]
- Basta M., Dalakas M. (1994) High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest 94: 1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMMS223 Trial Investigators (2008) Alemtuzumab versus. interferon beta-1a in early multiple sclerosis. N Engl J Med 359: 1786–1801. [DOI] [PubMed] [Google Scholar]
- Castro-Borrero W., Graves D., Frohman T., Flores A., Hardeman P., Logan D., et al. (2012) Current and emerging therapies in multiple sclerosis: a systematic review. Ther Adv Neurol Disord 5: 205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. (2008a) Inhibition of B cell functions: implications for neurology. Neurology 70: 2252–2260. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2008b) B cells as therapeutic targets in autoimmune neurological disorders. Nat Clin Pract Neurol 4: 557–567. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2010a) Evidence-based efficacy of intravenous immunoglogulin in human myasthenia gravis and mechanisms of action. In: Christadoss P. (ed.), Myasthenia Gravis: Mechanisms of Disease and Immune intervention, 2nd edn. New York: Linus Publications, pp. 89–102. [Google Scholar]
- Dalakas M. (2010b) Immunotherapy of myositis: issues, concerns future prospects. Nat Rev Rheumatol 6: 129–137. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2011a) Immunotherapy of inflammatory myopathies: practical approach and future prospects. Curr Treat Options Neurol 13: 311–323. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2011b) Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 7: 507–517. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2012) Biologics and other novel approaches and new therapeutic options in myasthenia gravis: a view to the future. Ann NY Acad Sci 1274: 1–8. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2013) Novel future therapeutic options in myasthenia gravis. Autoimmun Rev 12: 936–941. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2014) IVIg in the chronic management of Myasthenia Gravis: Is it enough for your money? J Neurol Sci 338: 1–2. [DOI] [PubMed] [Google Scholar]
- Dalakas M. (2015) Inflammatory muscle diseases N Engl J Med 372: 1734–1747. [DOI] [PubMed] [Google Scholar]
- Díaz-Manera J., Martínez-Hernández E., Querol L., Klooster R., Rojas-García R., Suárez-Calvet X., et al. (2012) Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology 78: 189–193. [DOI] [PubMed] [Google Scholar]
- Drachman D. (2008) Therapy of myasthenia gravis. Handb Clin Neurol 91: 253–272. [DOI] [PubMed] [Google Scholar]
- Engel A. (2006) Acquired autoimmune myasthenia gravis. In: Engel A., Franzini-Armstrong C. (eds), Myology. New York: McGraw-Hill, pp. 1769–1792. [Google Scholar]
- Feagan B., Rutgeerts P., Sands B., Hanauer S., Colombel J., Sandborn W., et al. (2013) Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369: 699–710. [DOI] [PubMed] [Google Scholar]
- Fee D., Kasarskis E. (2009) Myasthenia gravis associated with etanercept therapy. Muscle Nerve 39: 866–870. [DOI] [PubMed] [Google Scholar]
- Fleischmann R., Kremer J., Cush J., Schulze-Koops H., Connell C., Bradley J., et al. (2012) Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367: 495–507. [DOI] [PubMed] [Google Scholar]
- Gajdos P., Chevret S., Toyka K. (2008) Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev: CD002277. [DOI] [PubMed] [Google Scholar]
- Goede V., Klein C., Stingenbauer S. (2015) Obinutuzumab (GA101) for the treatment of chronic lymphocytic leukemia and other B-cell non-Hodgkin’s lymphomas: a glycoengineered type II CD20 antibody. Oncol Res Treat 38: 185–192. [DOI] [PubMed] [Google Scholar]
- Gold R., Dalakas M., Toyka K. (2003) Immunotherapy in autoimmune neuromuscular disorders. Lancet Neurol 2: 22–32. [DOI] [PubMed] [Google Scholar]
- Gold R., Giovannoni G., Selmaj K., Havrdova E., Montalban X., Radue E., et al. (2013) Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 381: 2167–2175. [DOI] [PubMed] [Google Scholar]
- Gomez A., Vrolix K., Martinez-Martinez P., et al. (2011) Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol 186: 2503–2513. [DOI] [PubMed] [Google Scholar]
- Gottlieb A., Korman N., Gordon K., Feldman S., Lebwohl M., Koo J., et al. (2008) Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 58: 851–864. [DOI] [PubMed] [Google Scholar]
- Halloran P. (2000) Sirolimus and cyclosporin for renal transplantation. Lancet 356: 179–180. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Dalakas M. (2003) Basic principles of immunotherapy in neurological diseases. Sem Neurol 23: 121–132. [DOI] [PubMed] [Google Scholar]
- Howard J., Jr., Barohn R., Cutter G., Freimer M., Juel V., Mozaffar T., et al. (2013) A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve 48: 76–84. [DOI] [PubMed] [Google Scholar]
- Kappos L., Hartung H., Freedman M., Boyko A., Radü E., Mikol D., et al. (2014) Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol 13: 353–363. [DOI] [PubMed] [Google Scholar]
- Kappos L., Radue E., O’Connor P., Polman C., Hohlfeld R., Calabresi P., et al. (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362: 387–401. [DOI] [PubMed] [Google Scholar]
- Langley R., Elewski B., Lebwohl M., Reich K., Griffiths C., Papp K., et al. (2014) Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med 371: 326–338. [DOI] [PubMed] [Google Scholar]
- Lee E., Fleischmann R., Hall S., Hanauer S., Colombel J., Sandborn W., et al. (2014) Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 370: 2377–2386. [DOI] [PubMed] [Google Scholar]
- Leonardi C., Matheson R., Zachariae C., Cameron G., Li L., Edson-Heredia E., et al. (2012) Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366: 1190–1199. [DOI] [PubMed] [Google Scholar]
- Marsh E., Hirst C., Llewelyn J., Cossburn M., Reilly M., Krishnan A., et al. (2010) Alemtuzumab in the treatment of IVIG-dependent chronic inflammatory demyelinating polyneuropathy. J Neurol 257: 913–919. [DOI] [PubMed] [Google Scholar]
- Masuda M., Matsumoto M., Tanaka S., Nakajima K., Yamada N., Ido N., et al. (2010) Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimmunol 225: 123–131. [DOI] [PubMed] [Google Scholar]
- Maurer M., Rakocevic G., Leung C., Quast I., Lukačišin M., Goebels N., et al. (2012) Rituximab induces sustained reduction of pathogenic B cells in patients with peripheral nervous system autoimmunity. J Clin Invest 122: 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriggioli M., Sheng J., Li L., Prabhakar B. (2008) Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis. Ann N Y Acad Sci 1132: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L., Sun B., Kong Q., Wang J., Wang G., Zhang S., et al. (2009) Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 128(Suppl. 1): e826–e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othy S., Topçu S., Saha C., et al. (2014) Sialylation May be dispensable for reciprocal modulation of helper T cells by intravenous immunoglobulin.Eur J Immunol 44: 2059–2063. [DOI] [PubMed] [Google Scholar]
- Papp K., Leonardi C., Menter A., Ortonne J., Krueger J., Kricorian G., et al. (2012) Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366: 1181–1189. [DOI] [PubMed] [Google Scholar]
- Peterson E., Koretzky G. (1999) Signal transduction in T lymphocytes. Clin Exp Rheumatol 17: 107–114. [PubMed] [Google Scholar]
- Pittock S., Lennon V., McKeon A., Mandrekar J., Weinshenker B., Lucchinetti C., et al. (2013) Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol 12: 554–562. [DOI] [PubMed] [Google Scholar]
- Quast I., Lunemann J. (2014) Fc glycan-modulated immunoglobulin G effector functions. J Clin Immunol 34(Suppl. 1): S51–S55. [DOI] [PubMed] [Google Scholar]
- Ragheb S., Lisak R., Lewis R., Van Stavern G., Gonzales F., Simon K. (2008) A potential role for B-cell activating factor in the pathogenesis of autoimmune myasthenia gravis. Arch Neurol 65: 1358–1362. [DOI] [PubMed] [Google Scholar]
- Renton A., Pliner H., Provenzano C., Evoli A., Ricciardi R., Nalls M., et al. (2015) A genome-wide association study of myasthenia gravis. JAMA Neurol 72: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J., Capablo J., Larrad L., Gervas-Arruga J., Ara J., Sánchez A., et al. (2011) Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve 44: 278–280. [DOI] [PubMed] [Google Scholar]
- Sabatos-Peyton C., Verhagen J., Wraith D. (2012) Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol 22: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W., Ghosh S., Panes J., Vranic I., Su C., Rousell S., et al. (2012) Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 367: 616–624. [DOI] [PubMed] [Google Scholar]
- Sanders D., Evoli A. (2010) Immunosuppressive therapies in myasthenia gravis. Autoimmunity 43: 428–435. [DOI] [PubMed] [Google Scholar]
- Sorensen P., Lisby S., Grove R., Derosier F., Shackelford S., Havrdova E., et al. (2014) Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 82: 573–581. [DOI] [PubMed] [Google Scholar]
- Steinman L., Zamvil S. (2012) Re-engineering of pathogenic aquaporin 4-specific antibodies as molecular decoys to treat neuromyelitis optica. Ann Neurol 71: 287–288. [DOI] [PubMed] [Google Scholar]
- Tak P., Kalden J. (2011) Advances in rheumatology: new targeted therapeutics. Arthritis Res Ther 13(Suppl. 1): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tradtrantip L., Ratelade J., Zhang H., Verkman A. (2013) Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann Neurol 73: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tradtrantip L., Zhang H., Saadoun S., Phuan P., Lam C., Papadopoulos M., et al. (2012) Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol 71: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokos G. (2011) Systemic lupus erythematosus. N Engl J Med 365: 2110–2121. [DOI] [PubMed] [Google Scholar]
- Tüzün E., Huda R., Christadoss P. (2011) Complement and cytokine based therapeutic strategies in myasthenia gravis. J Autoimmun 37: 136–143. [DOI] [PubMed] [Google Scholar]
- Van Vollenhoven R., Fleischmann R., Cohen S., Lee E., García Meijide J., Wagner S., et al. (2012) Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367: 508–519. [DOI] [PubMed] [Google Scholar]
- Verbrugge S., Scheper R., Lems W., de Gruijl T., Jansen G. (2015) Proteasome inhibitors as experimental therapeutics of autoimmune diseases. Arthritis Res Ther 17: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Rothwell P. (2004) Myasthenia gravis. Autoimmunity 37: 317–319. [DOI] [PubMed] [Google Scholar]
- Wynn D., Kaufman M., Montalban X., Vollmer T., Simon J., Elkins J., et al. (2011) Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol 9: 381–390. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Goto H., Hirao K., et al. (2015) Long-term safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan.J Rheumatol 42: 1368–1375. [DOI] [PubMed] [Google Scholar]