Abstract
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide. Death rates have been declining, largely as a result of early detection through mammography and improved treatment, but mammographic screening is controversial because of over-diagnosis of breast disease that might not require treatment, and under-diagnosis of cancer in women with dense breasts. Breast cancer screening could be improved by pairing mammography with a tumor circulating marker, of which there are currently none. Given genomic similarities between the basal breast cancer subtype and serous ovarian cancer, and given our success in identifying circulating markers for ovarian cancer, we investigated the performance in hormone receptor-negative breast cancer detection of both previously identified ovarian serum markers and circulating markers associated with transcripts that were differentially expressed in breast cancer tissue compared to healthy breast tissue from reduction mammaplasties.
Methods
We evaluated a total of 15 analytes (13 proteins, 1 miRNA, 1 autoantibody) in sera drawn at or before breast cancer surgery from 43 breast cancer cases (28 triple-negative—TN—and 15 hormone receptor-negative—HRN—/ HER2-positive) and 87 matched controls.
Results
In the analysis of our whole cohort of breast cancer cases, autoantibodies to TP53 performed significantly better than the other selected 14 analytes showing 25.6% and 34.9% sensitivity at 95% and 90% specificity respectively with AUC: 0.7 (p<0.001). The subset of 28 TN cancers showed very similar results. We observed no correlation between anti-TP53 and the 14 other markers; however, anti-TP53 expression correlated with Body-Mass-Index. It did not correlate with tumor size, positive lymph nodes, tumor stage, the presence of metastases or recurrence.
Conclusion
None of the 13 serum proteins nor miRNA 135b identified women with HRN or TN breast cancer. TP53 autoantibodies identified women with HRN breast cancer and may have potential for early detection, confirming earlier reports. TP53 autoantibodies are long lasting in serum but may be affected by storage duration. Autoantibodies to TP53 might correlate with Body-Mass-Index.
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide. In 2012 female breast cancer accounted for 25% (1.67 million) of the total new female cancer cases and 15% (521,900) of the total female cancer deaths [1]. Breast cancer death rates have been decreasing in North America and several European countries over the past 25 years, largely as a result of early detection through mammography and improved treatment [2].
Mammography is the standard breast cancer screening tool, which has led to the discovery of cancers in their early stages, pushing 5-year survival to 90% as opposed to 20% when the disease has already spread to distant organs [3]. However, its utility as a screening method is controversial [4, 5], due in part to an increased diagnosis of in situ breast disease [6], a condition that may or may not progress to invasive cancer. Furthermore, mammographic screening is less sensitive in women with dense breasts, a feature commonly found in younger women [7, 8]. Screening output could potentially be improved by pairing mammography with a tumor circulating marker. Currently, no markers for breast cancer screening or detection are in clinical use, but a few markers, e.g. carcinoembryonic antigen (CEA), or carbohydrate antigens 15–3 and 27.29, are being used and seem helpful for making decisions in the metastatic [9] or surveillance [10] setting. Many studies have focused on analyzing transcript markers in breast cancer, starting with Perou’s molecular portraits [11] and followed by numerous others. This has culminated in clinical tests for cancer outcome, such as Oncotype DX [12] and Mammaprint [13]. Few studies have catalogued protein expression in breast cancer [14, 15]. These did not provide information in healthy breast tissue, which prevent the markers’ use as diagnostic biomarkers. In 2011, autoantibodies to several glycopeptides of MUC1, the protein recognized by the cancer antigens CA15-3 and CA27.29, were found to discriminate sera drawn from breast cancer patients at diagnosis from benign and healthy controls [16]. However, when the same group tested autoantibodies to MUC1 in a nested case-control study involving over 1000 serum samples from women who later developed breast cancer and over 1300 matched controls, no differences were observed [17]. Another promising breast marker is autoantibodies to TP53 [18–20], found to be present in sera that were collected on average more than 150 days before a breast cancer diagnosis, although with a relatively small sample size [21].
Finding and validating new biomarkers is a difficult task [22]. We previously discovered Human epididymis protein 4 (HE4, WFDC2) as a marker for ovarian cancer, first as a transcript in tissue and, four years later, as protein in serum [23, 24], and identified several other genes and serum markers that similarly discriminated ovarian cancer from controls [25]. Given the genomic similarities between the aggressive basal breast cancer subtype and serous ovarian cancer [26, 27] and the hypothesis that biomarkers may overlap between these two subtypes [27], we investigated whether any of the serum markers that signal the presence of ovarian cancer could also signal presence of HRN or TN breast cancer.
Additionally, applying the successful ovarian marker discovery strategy, transcripts that we found to be differentially expressed in breast cancer tissue compared to healthy breast tissue from reduction mammaplasties [28] were also tested for their discriminatory potential as proteins in serum.
Next to proteins and autoantibodies, miRNAs are being studied as circulating markers. Micro-RNA-135b has been shown to be over-expressed in TNBC [29–31] and was chosen as a further candidate.
We tested women diagnosed with basal-like breast cancers because they exhibit genomic similarity to serous ovarian cancer [26] and have poor prognosis, potentially greatly benefitting from a serum marker. For reasons of availability of clinical data, we chose patients whose cancers were negative for estrogen, progesterone and HER2 receptor (triple-negative or TN), being aware that not all TN breast cancers have basal-like histology [32]. Independently, survival data for TN cancers are among the worst [33, 34]. Our population was augmented with another poor-prognosis sub-group, hormone receptor-negative (HRN) breast cancers positive for HER2 [35, 36].
We tested a total of 15 analytes in sera drawn at or before breast cancer diagnosis from 43 HRN cases (including a subset of 28 TN cases) and 87 matched controls. As reported below, we found that autoantibodies to TP53 discriminated HRN breast cancer cases from screening controls.
Methods
Study Population
Cases included 43 women with HRN breast cancer, enrolled prior to therapeutic surgery (lumpectomy or mastectomy) for invasive cancer at Swedish Medical Center (SMC) in Seattle, WA, USA. All cancers had ductal histology, 28 were TN and 15 were HRN and HER2-positive. Four patients received neo-adjuvant chemotherapy. Blood was drawn prior to surgery in all cases, either on the day of surgery (n = 12) or up to one week (n = 16), two to six weeks (n = 10) or seven to 30 weeks (n = 5) before surgery. For 21 of the 43 cases, a post-treatment blood draw was also obtained. To the 43 cases, 87 healthy controls were matched by age, time period of blood draw and ethnicity (Table 1 and S1 Table). Controls were participating in mammography screening at SMC and had no history of cancer per SMC or the local Cancer Surveillance System [37], and did not develop cancer within four years. Blood draws for cases and controls followed the same protocol implemented by the same staff at Fred Hutchinson Cancer Research Center (FHCRC). For cases and controls, questionnaire data were available. Patient characteristics are summarized in S1 Table.
Table 1. Matching Cases to Controls.
| Variable | Value | Invasive Cases | Healthy Controls | p-Value (Invasive vs. Healthy) |
|---|---|---|---|---|
| N | Sample Size | 43 (33.1%) | 87 (66.9%) | |
| Ethnicity | Asian-Filipino | 1 (2.3%) | 0 (0.0%) | 0.42 |
| Black | 0 (0.0%) | 2 (2.3%) | ||
| Caucasian | 31 (72.1%) | 71 (81.6%) | ||
| Hispanic | 1 (2.3%) | 3 (3.4%) | ||
| Mixed | 3 (7.0%) | 5 (5.7%) | ||
| Unknown | 7 (16.3%) | 6 (6.9%) | ||
| Collection Date | mean (sd) | 2006-01-30 (317 days) | 2005-11-29 (406 days) | 0.34 |
| Date Range | Oldest Collection | 2004-04-23 | 2004-05-12 | |
| Newest Collection | 2008-06-13 | 2008-06-06 | ||
| Age at Blood Draw | mean (sd) | 56 (12) | 54 (10) | 0.55 |
Four criteria for selection of 15 analytes (Table 2)
Table 2. Selection of 15 analytes and their sensitivity/specificity as serum markers.
| Selection Criterion | Sensitivity at 100% specificity | Sensitivity at 95% specificity | Sensitivity at 90% specificity | |
|---|---|---|---|---|
| CA125 | 1 | 2.3% | 11.6% | 14.0% |
| GDF15 | 1 | 4.7% | 11.6% | 14.0% |
| PKM | 1 | 0.0% | 0.0% | 17.5% |
| SPARC | 1 | 2.3% | 7.0% | 14.0% |
| WFDC2 | 1 | 4.7% | 9.3% | 11.6% |
| COL1A1 | 2 | 0.0% | 2.3% | 2.3% |
| CTGF | 2 | 7.0% | 7.0% | 9.3% |
| FN1 | 2 | 0.0% | 11.6% | 18.6% |
| S100A7 | 2 | 0.0% | 2.3% | 16.3% |
| SPP1 | 2 | 10.0% | 10.0% | 15.0% |
| CCL5 | 3 | 2.4% | 12.2% | 24.4% |
| miR-135b | 3 | 0.0% | 0.0% | 4.7% |
| Anti-TP53 | 3 | 4.7% | 25.6% | 34.9% |
| HOXA5 | 4 | 2.3% | 11.6% | 11.6% |
| SFRP1 | 4 | 0.0% | 7.0% | 11.6% |
Data from 43 cases and 87 controls. Marker selection criteria: (1) serum marker potential in high-grade serous ovarian cancer and involvement in breast cancer; (2) transcript involved in breast cancer; (3) association with poor breast cancer outcome; (4) protein decreased in breast cancer tissue compared to normal breast.
The goal of our research is the identification of markers or analytes for the detection of breast cancer. Expression of an analyte in cancer must therefore be known in relation to it its expression in a non-cancerous state (e.g. healthy blood or healthy breast tissue). This is reflected in the following four selection criteria. Criterion 1 (ovarian cancer markers): Five protein analytes were chosen based on their performance as circulating markers in identifying high-grade serous ovarian cancer [25] and evidence for their role in breast or other cancers: GDF15 (MIC-1) protein elevation in human serum is associated with oral squamous cell carcinoma [38], pancreatic cancer [39] and colon cancer [40]; PKM (pyruvate kinase muscle) protein was suggested a marker of clinical breast cancer disease [41] and is expressed in advanced breast cancers [42]; SPARC (osteonectin) protein is expressed in breast cancer tissue with potential prognostic value [43]; CA125 (MUC16 protein) is associated with poor breast cancer prognosis [44] and is able to discriminate ER-negative breast cancer patients from controls with unknown ER status [45]; and WFDC2 (HE4) protein, which we and others [46] have found to have low expression in benign breast disease and its expression in breast cancer tissue may be associated with worse survival [47]. Criterion 2 (breast tissue transcript markers): Five protein analytes were selected based on their performance in our transcript-based breast cancer work comparing breast cancer tissue to that of normal tissue from breast reduction surgeries [28]: COL1A1 (collagen, type 1, alpha 1) and FN1 (fibronectin 1) showed higher mRNA expression in breast cancer tissues than in controls, CTGF (connective tissue growth factor) was less highly expressed in aggressive cancers vs. healthy controls, and S100A7 (psoriasin) and SPP1 (osteopontin) expression was associated with poor-outcome cancer [28]. All five transcripts were previously associated with breast cancer [11, 48–59] and the corresponding proteins are all expressed in breast cancer tissue [60]. Furthermore, CTGF protein expression in cancer tissue is associated with poor prognosis in some cancer types [61], S100A7 protein is expressed in breast cancer [62] and SPP1 protein is implicated in breast tumor progression [63] and significantly elevated in patients with TN breast cancer [64]. CTGF protein is present in blood of liver cancer patients [65] and S100A7 protein is found reduced in serum of psoriasin patients [66], FN1 is expressed in plasma of breast cancer patients [67] and higher in breast cancer serum than controls [68], SPP1 protein is present in serum [69] and elevated in that of breast cancer patients [70], SPP1 however failed to discriminate breast cancer cases from healthy controls in pre-diagnostic serum samples [71]. Criterion 3 (poor outcome breast cancer): Three analytes, CCL5 (RANTES) protein, hsa-miR-135b (microRNA 135b) and Anti-TP53 (autoantibodies to p53) were chosen because of their association with poor breast cancer outcome: CCL5 was reported to be over-expressed in HRN breast cancer and to increase with cancer stage [72, 73], miR-135b is over-expressed in basal-like breast cancers and associated with estrogen receptor negativity and with poor survival and early metastasis [29–31]. Anti-TP53 was shown to be expressed in TN cancers [74]. CCL5 and autoantibodies to TP53 were also reported in breast cancer serum [21, 73]. Criterion 4 (low expression in breast cancer): Lastly, two protein analytes were included which showed decreased protein expression in breast cancer tissue compared to healthy control tissue, HOXA5 (homeobox A5) [75] and SFRP1 (secreted frizzled-related protein 1) [76].
Assays
Enzyme-linked immunosorbent assays (ELISA) were run in two formats, a conventional 96-well plate format (P) or on beads (B) on the Bio-Plex immunoassay platform (LifeSciences, Hercules, CA, USA). Assays were purchased when commercially available or developed from antibodies that were obtained from commercial sources or from collaborators. ELISA values were normalized by the mean of four samples of normal human serum (a pool of serum from 73 healthy female donors) present on each 96-well plate. Expression of miR-135b and a spiked-in control miRNA (cel-miR-39) was analyzed using individual Taqman RT-qPCR assays (Life Technologies, Foster City, CA), qPCR reactions were run on Viia7 Real-Time PCR System (Life Technologies, Foster City, CA) in duplicate using no-template controls. Cycle threshold (Ct) values were normalized as previously described [77]. Following is a list of the assays, if commercial, or the antibodies, if in-house (first: capture, second: detection). CCL5 (B): Bio-Plex Pro Hu Cytokine Croup I RANTES (LifeSciences, Cat No 171G5022M). COL1A1 (P): Rabbit pAb to Collagen I (Abcam, Cat No ab34710-100, 1 μg/ml), Col1A1 goat polyclonal IgG (Santa Cruz, Cat No SC8783, 1 μg/ml). CTGF (P): Anti-human CTGF Ab (Antigenix America, Cat No RHF461CK, 5 μg/ml), Anti-human CTGF Biotin Tracer (Antigenix America, Cat No FHF461CK, 0.5 μg/ml). FN1 (P): Human Fibronectin Platinum ELISA (eBioscience, Cat No BMS2028). GDF15 (P): A10.3 (diaDexus, 20 μg/ml), Biotinylated B2.2 (diaDexus, 1 μg/ml). HOXA5 (P): 171–270 mAb (Abnova, Cat No H00003202-M06, 0.1 μg/ml), H-125 rabbit polyclonal IgG (Santa Cruz, Cat No SC28599, 2 μg/ml). PKM (P): Tumor M2-PK EDTA Plasma (ScheBo BioTech Inc, Cat No 8). S100A7 (P): Ab817 (Kornelia Polyak, 1 μg/ml), Biotinylated Ab1068 (Kornelia Polyak, 1 μg/ml). SFRP1 (P): H-90 (Santa Cruz, Cat No SC13939, 1 μg/ml), Human sFRP-1 Affinity Purified Polyclonal Ab, Goat IgG (R&D Systems, Cat No AF1384, 0.75 μg/ml). SPARC (P): Human SPARC Physcoerythrin mAb Clone 122511 (R&D Systems, Cat No IC941P, 1 μg/ml), SPARC [N50] Antibody (GeneTex Inc, Cat No GTX19528, 0.5 μg/ml). SPP1 (B): Human Osteopontin Quantikine ELISA kit (R&D Systems, Cat No DOST00). WFDC2 (B): mAb#172 (Brad Nelson, 1 μg/ml), Biotinylated mAb#144 (Brad Nelson, 1 μg/ml). CA125 (B): RDI-TRK4C29-X306, RDI-TRK4C29-X52 (Research Diagnostics)[78]. Anti-TP53 (B): p53-GST Recombinant Protein matching wild type and several isoforms, expressed in E. coli (Millipore, Cat No 14–865, 20 μg/ml) was coupled to magnetic beads (Life Sciences, Cat No MC10026-01) as described earlier [78] and serum was tested as described in the Supplement. miR-135b (rt-qPCR): For combined analysis with the ELISA values, Ct values were linearized using the formula e (40-Ct) where "e" is the base of the natural logarithm. Raw data (normalized) are summarized in S2 Table.
Statistical analysis
Fisher's exact test was used to test for differences in distribution of ethnicity between cases and controls, and Student's t-test was used to test for differences in collection date and age at blood draw. Sensitivity and specificity of each analyte were evaluated using the receiver operating characteristic (ROC) curve analysis and the area under the ROC curve (AUC) was tested (H0: AUC = 0.5: no difference between cases and controls) using the Mann-Whitney test. Kendall's tau statistic was used to test for correlations between covariates and individual markers. Combination markers were calculated by fitting a logistic regression model with case status as the dependent variable and individual analyte as the independent variables, and were defined as the linear combination of the analyte values using the coefficients estimated by the regression model. A bootstrap analysis with 10,000 iterations was conducted to determine if the combination marker demonstrated a better than random improvement over the best marker alone. This analysis was performed using data from 40 cases and 86 controls because the analyte SPP1 had missing values for three cases and one control. Backward stepwise model selection was done using the Akaike's Information Criterion (AIC) via the 'stepAIC' procedure [79]. All statistical analyses were conducted in the R software package [80].
Results
Analyte Expression
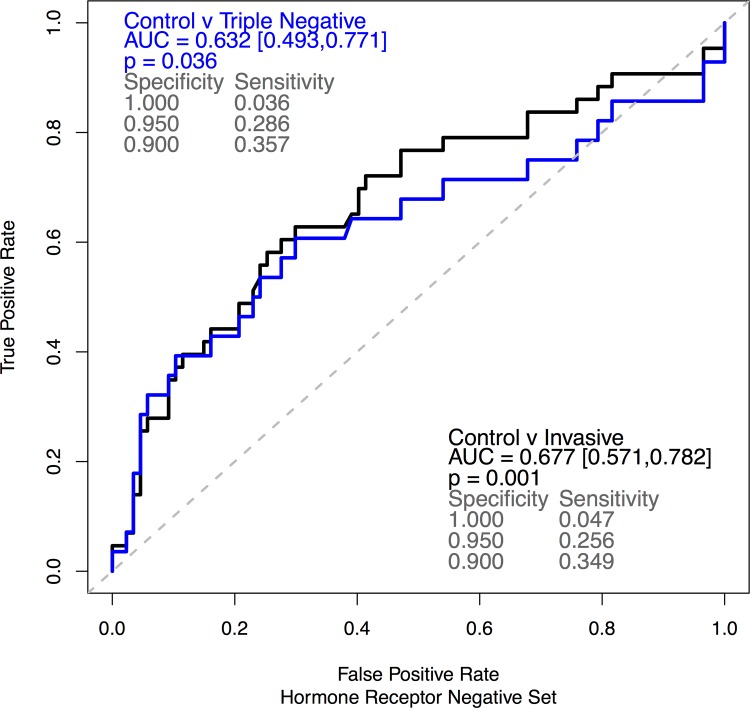
All 15 assays were run on pre-surgical sera from 43 HRN breast cancer patients and 87 controls (Table 1). Analysis was performed on the full set of 43 HRN cases and on the subset of 28 TN cases. Comparing analyte sensitivities at 100%, 95% and 90% specificity ranked anti-TP53 ahead of all other 14 analytes (AUC: 0.677, p = 0.001, Fig 1 and Table 2). ROC curves for the full HRN and the TN set are shown in S1 Fig and the corresponding dot plots are shown in S2 Fig. Autoantibodies to TP53 also outperformed the other markers in the TN subset, showing sensitivity values very similar to the ones obtained in the full set of cases (Fig 1). Conversely, some of the other 14 analytes performed slightly better in the TN subset than in the full set of breast cancer cases (S2 Table).
Fig 1. Autoantibodies to TP53 discriminate breast cancer sera from control sera.
ROC curves showing the 43 HRN cases (black) and the 28 TN subset (blue) vs. 87 matched healthy controls.
To test if any of the other 14 analytes may contribute to the sensitivity of anti-TP53, we selected two complementary analyte sets: the first set was chosen by hand using the sensitivities of individual analytes. It combines anti-TP53 with SPP1, FN1, CCL5 and GDF15. The second set was chosen through backward stepwise model selection using AIC, which resulted in a combination of anti-TP53 with FN1 and CTGF. Neither panel was found to have a better than random increase in either area under the curve (AUC) or the sensitivity of the panel at 90% or 95% specificity (p>0.094 for all comparisons, unadjusted for multiple comparisons, see S4 Table). The TN subset resulted in similar numbers (S5 Table).
Thus, of the 15-analyte panel only anti-TP53 warranted further analysis. Its relatively good performance was not influenced by the single outlier measurement in patient 5, which had a relative Mean Fluorescence Intensity (MFI) value of 22.45 compared to a mean of 0.78 (sd = 0.59) in the remaining 42 patients (S2 Fig). Removal of this serum sample marginally reduced anti-TP53 sensitivity at 95% and 90% specificity from 25.6% to 23.8% and from 34.9% to 34.5% respectively.
Anti-TP53 levels throughout treatment
To test whether TP53 autoantibody levels remain steady throughout treatment, we compared anti-TP53 values of the pre-surgical samples to those in post-therapeutic samples which were available from 21 of the 43 cases. Post-therapy values dropped to an average of 81% of the diagnostic values. However, the same reduction in relative MFI values was observed in paired samples from 10 control individuals whose draws dated from the same time period and thus had similar blood draw intervals as the 21 cases (S6 Table). After adjustment for the date of serum collection, diagnostic and post-therapy values (as well as the values of the two control draws) were significantly correlated (S3 Fig).
Anti-TP53 and confounding factors
To test whether anti-TP53 levels correlated with patient- and disease-related characteristics, we used Kendall's Correlation Test (S7 Table). Of the 22 covariates, body-mass-index (BMI) was the only with a significant correlation to anti-TP53 levels (p = 0.05, unadjusted for multiple comparisons), though the observed correlation was weak (0.317, S4 Fig) and was not significant when adjusted for multiple comparisons (p = 1, Bonferroni correction). Unfortunately, BMI data were only available in the cancer patients, and hence, statistical tests adjusted for BMI, which could eliminate the possibility that the correlation between anti-TP53 and case status were attributable to systematic differences in BMI, were not possible.
Tumor size, which is often correlated with analytes secreted by tumors, did not correlate with anti-TP53 and neither did the severity of the disease (positive lymph nodes, tumor stage, the presence of metastases and recurrence).
Discussion
To investigate their potential as early detection markers, we measured the expression of 15 analytes in serum that was drawn prior to surgery in 43 cases with HRN breast cancer (and a 28-case TN subset thereof) and 87 matched healthy controls. Five of the evaluated analytes were proteins with marker potential in high-grade serous ovarian cancer (HGSOC), but this potential could not be replicated in this subset of breast cancer cases that is thought to be molecularly related to ovarian cancer [26].
Likewise, none of the analytes previously showing transcript-level over-expression in breast cancer cases gave a sufficient signal in serum, indicating that transcript expression in breast tissue is a poor indicator for a successful protein marker expressed in blood of HRN or TN breast cancer patients.
Given that years of research have not resulted in a protein marker for detection of breast cancer, and given that none of the protein analytes tested in this study and a recent study with over 800 cases and controls [45] display a significant signal at diagnosis, it may very well be that circulating proteins make poor breast markers.
Similarly, our selected microRNA, miR-135b, lacked adequate signal in serum for the diagnosis of HRN breast cancer.
Our best performing marker is indeed the body’s own antibodies to TP53 (anti-TP53), the expression of which has been associated with breast cancer before. Incidentally, anti-TP53 has been shown to have detection marker potential in HGSOC [81] and preliminary results by our group in a set of 728 sera (123 ovarian cancer cases, 369 healthy controls, 144 benign ovarian disease, 92 surgical controls) showed performance similar to that reported here in breast cancer (unpublished).
In 1982 Crawford et al. reported the presence of autoantibodies to TP53 in sera of women with breast cancer [82], and since then, multiple surveys have found anti-TP53 in 9–34% of women with breast cancer [18–20]. Comparing cases to controls, in 2012 Lu et al. reported that anti-TP53 had 35% sensitivity at 90% specificity in detecting breast cancer. Most importantly though, they also found 6% of pre-diagnostic breast cancer samples to be positive for this analyte [21], hinting at its potential for early diagnosis. Our data confirm these results and further suggest that anti-TP53 is potentially useful for the detection of poor-outcome breast cancers.
Our data further show that TP53 autoantibody levels are independent of tumor size, cancer stage, the presence of metastases or the presence of positive lymph nodes, again suggesting a potential to detect breast cancer in its early stages. Furthermore, diagnostic and post-therapeutic samples from the same patient show similar anti-TP53 expression after adjustment for time in the freezer. Due to the relatively small sample size and the wide confidence interval for the trend line for time in the freezer, the latter statement must be interpreted carefully, in particular because some autoantibody levels decrease with increased storage time [83] rather than increase as we observed for anti-TP53.
Given the relative stability of TP53 autoantibodies in blood, an antibody reaction caused by a small lesion may be detectable over an extended period of time, suggesting, and confirming results by Lu et al. [21], that autoantibodies to TP53 have potential as a screening marker. While anti-TP53 may be the best-performing serum marker for breast cancer detection, its sensitivity and specificity (namely, low false-negative and false-positive rates), are limited, suggesting the need to combine it with one or several other well-performing complementary markers. More research is needed to identify such markers.
Lastly, the presence of antibodies to TP53 is weakly correlated with increased body mass. This is an unexpected finding because obesity is associated with an attenuated immune response [84] and one would therefore have expected decreased anti-TP53 with increasing BMI. Replication is needed to confirm this result; measurement of BMI is advised in studies using autoantibodies to TP53.
Conclusions
Serum proteins examined and miR-135b failed to identify women with HRN or TN breast cancer.
TP53 autoantibodies identified women with HRN or TN breast cancer and may have potential for early detection, confirming earlier findings.
TP53 autoantibodies are long lasting in serum but may be affected by storage duration.
Supporting Information
HRN breast cancer cases (43, blue) or TN breast cancer cases (28, black) vs. 87 matched controls.
(PDF)
Figure showing the values of the 15 evaluated analytes measured in 43 HRN breast cancer cases and 87 matched controls.
(PDF)
When adjusted for the time each serum was stored in the freezer, anti-TP53 values of the first draw (diagnostic for patients) and the second draw (post-therapy for patients) from each individual correlated (p<0.005 for cases and p = 0.029 for controls).
(PDF)
As depicted in the graph, there is a weak correlation (Kendal correlation statistic = 0.317; p = 0.005) between anti-TP53 expression and BMI.
(PDF)
Listing the following characteristics: Surgery Year, Time from mammogram (Mx) to Draw (weeks:days), Time Draw to Surgery (weeks:days), BI-RADS at Diagnosis (Dx), Density at Dx, Ethnicity, Death as of Dec 2014, Recurrence as of June 2014, No Ovaries at Dx, ER/PR status, HER2 status, Ducal histology, Stage, Lymph Nodes (positive / total), Metastasis, Neo-adjuvant treatment (Tx), Age at Dx, Age at Menarche, Age at Menopause, BMI, Age at first contraceptive, Current Hormone Therapy, Years of Hormone Therapy, Currently pregnant, Parity, Children, Smoking (Pack years), Drinking (g Alc / yr), Other Cancer(s).
(CSV)
(CSV)
Numbers are given for the full 43 HRN set and the TN subset.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Kornelia Polyak for antibodies to S100A7, diaDexus for antibodies to GDF15 (MIC-1), Brad Nelson for antibodies to HE4, Kathy O'Briant for specimen retrieval and processing, Hannah Purkey for running the immunoassays, Carole Shaw for clinical data retrieval, Shirley Gough for clinical chart abstractions, Jamie Guenthoer for her assistance in coordination of the miR-135b studies and David Beatty for discussions. The acquisition of some control samples was facilitated through the Marsha Rivkin Center in Seattle.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the US Department of Defense (http://cdmrp.army.mi/funding/): DAMD17-02-1-0691; National Institutes of Health (http://grants.nih.gov/): P50 CA083636, U01 CA152637 and R01 DK085714; Río Hortega (http://www.eng.isciii.es/ISCIII/es/contenidos/fdinvestigacion/fd-financiacion/fd-convocatorias-ayudas-accion-estrategica-salud/fdseguimiento-cientifico-economico/seguimiento-ayudas-para-contratos-post-formacionrio-hortega.shtml): CM10/00084; Fundación Martín Escudero (www.fundame.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society. Global Cancer Facts and Figures 2012. 2012.
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. Epub 2011/02/08 06:00. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3. SEER Cancer Statistics Review, 1975–2008 Bethesda, MD: National Cancer Institute; [updated 2011]. Available: http://seer.cancer.gov/csr/1975_2008. [Google Scholar]
- 4. Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–19. Epub 2002/03/29 10:00. . [DOI] [PubMed] [Google Scholar]
- 5. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. Epub 2012/11/23 06:00. 10.1056/NEJMoa1206809 [DOI] [PubMed] [Google Scholar]
- 6. Wickerham DL, Julian TB. Ductal carcinoma in situ: a rose by any other name. J Natl Cancer Inst. 2013;105(20):1521–2. Epub 2013/09/27 06:00. 10.1093/jnci/djt268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinclair N, Littenberg B, Geller B, Muss H. Accuracy of screening mammography in older women. AJR Am J Roentgenol. 2011;197(5):1268–73. Epub 2011/10/25 06:00. 10.2214/AJR.10.5442 [DOI] [PubMed] [Google Scholar]
- 8. Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. 2012;198(3):W292–5. Epub 2012/02/24 06:00. 10.2214/AJR.10.6049 [DOI] [PubMed] [Google Scholar]
- 9. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312. Epub 2007/10/24 09:00. . [DOI] [PubMed] [Google Scholar]
- 10. Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, et al. Tumor markers in breast cancer- European Group on Tumor Markers recommendations. Tumour Biol. 2005;26(6):281–93. Epub 2005/10/29 09:00. . [DOI] [PubMed] [Google Scholar]
- 11. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. [DOI] [PubMed] [Google Scholar]
- 12. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. . [DOI] [PubMed] [Google Scholar]
- 13. van 't Veer L, Dai H, van de Vijver M, He Y, Hart A, Bernards R, et al. Expression profiling predicts outcome in breast cancer. Breast Cancer Res. 2003;5(1):57–8. 10.1186/bcr562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geiger T, Madden SF, Gallagher WM, Cox J, Mann M. Proteomic portrait of human breast cancer progression identifies novel prognostic markers. Cancer Res. 2012;72(9):2428–39. Epub 2012/03/15. 10.1158/0008-5472.can-11-3711 . [DOI] [PubMed] [Google Scholar]
- 15. Kennedy JJ, Abbatiello SE, Kim K, Yan P, Whiteaker JR, Lin C, et al. Demonstrating the feasibility of large-scale development of standardized assays to quantify human proteins. Nat Methods. 2014;11(2):149–55. Epub 2013/12/10. 10.1038/nmeth.2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blixt O, Bueti D, Burford B, Allen D, Julien S, Hollingsworth M, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13(2):R25 Epub 2011/03/10 06:00. 10.1186/bcr2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burford B, Gentry-Maharaj A, Graham R, Allen D, Pedersen JW, Nudelman AS, et al. Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer. Br J Cancer. 2013;108(10):2045–55. Epub 2013/05/09 06:00. 10.1038/bjc.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernández Madrid F. Autoantibodies in breast cancer sera: candidate biomarkers and reporters of tumorigenesis. Cancer Lett. 2005;230(2):187–98. Epub 2005/11/22 09:00. . [DOI] [PubMed] [Google Scholar]
- 19. Casiano CA, Mediavilla-Varela M, Tan EM. Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol Cell Proteomics. 2006;5(10):1745–59. Epub 2006/05/31 09:00. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller M, Meyer M, Schilling T, Ulsperger E, Lehnert T, Zentgraf H, et al. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol. 2006;29(4):973–80. Epub 2006/09/12 09:00. . [PubMed] [Google Scholar]
- 21. Lu H, Ladd J, Feng Z, Wu M, Goodell V, Pitteri SJ, et al. Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer Prev Res (Phila). 2012;5(8):1036–43. Epub 2012/06/21 06:00. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzoli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013;66(4):273–81. Epub 2013/02/22. 10.1136/jclinpath-2012-201031 . [DOI] [PubMed] [Google Scholar]
- 23. Schummer M, Ng W, Bumgarner R, Nelson P, Schummer B, Bednarski D, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999;238(2):375–85. . [DOI] [PubMed] [Google Scholar]
- 24. Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter J, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–700. . [PubMed] [Google Scholar]
- 25. Cramer DW, Bast RC Jr., Berg CD, Diamandis EP, Godwin AK, Hartge P, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res (Phila). 2011;4(3):365–74. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012. Epub 2012/09/25. 10.1038/nature11412 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jönsson JM, Johansson I, Dominguez-Valentin M, Kimbung S, Jönsson M, Bonde JH, et al. Molecular subtyping of serous ovarian tumors reveals multiple connections to intrinsic breast cancer subtypes. PLoS One. 2014;9(9):e107643 Epub 2014/09/17 06:00. 10.1371/journal.pone.0107643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schummer M, Green A, Beatty JD, Karlan BY, S. K, Gross J, et al. Comparison of Breast Cancer to Healthy Control Tissue Discovers Novel Markers with Potential for Prognosis and Early Detection. PLoS ONE. 2010;5(2):e9122 10.1371/journal.pone.000912 PubMed Central PMCID: PMCPMC2817747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8(10):R214 Epub 2007/10/10 09:00. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lowery AJ, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C, et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11(3):R27 Epub 2009/05/13 09:00. 10.1186/bcr2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Enerly E, Steinfeld I, Kleivi K, Leivonen SK, Aure MR, Russnes HG, et al. miRNA-mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS One. 2011;6(2):e16915 Epub 2011/03/03 06:00. 10.1371/journal.pone.0016915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18(2):123–33. Epub 2013/02/14 06:00. 10.1634/theoncologist.2012-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. Epub 2007/08/03 09:00. . [DOI] [PubMed] [Google Scholar]
- 34. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. Epub 2010/11/12 06:00. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 35. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. Epub 1987/01/09. . [DOI] [PubMed] [Google Scholar]
- 36. Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–90. Epub 2007/04/04 09:00. . [DOI] [PubMed] [Google Scholar]
- 37.Schwartz SM, Li CI, Potts M, Hafterson J. Cancer Surveillance System 2014 [cited 2014 2014-11-20]. Available: http://www.fredhutch.org/en/labs/phs/projects/cancer-surveillance-system.html.
- 38. Yang CZ, Ma J, Luo QQ, Neskey DM, Zhu DW, Liu Y, et al. Elevated level of serum growth differentiation factor 15 is associated with oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2013;26(10):12091. Epub 2013/05/29 06:00. [DOI] [PubMed] [Google Scholar]
- 39. Özkan H, Demirbaş S, İbiş M, Akbal E, Köklü S. Diagnostic validity of serum macrophage inhibitor cytokine and tissue polypeptide-specific antigen in pancreatobiliary diseases. Pancreatology. 2011;11(3):295–300. Epub 2011/07/16 06:00. 10.1159/000328963 [DOI] [PubMed] [Google Scholar]
- 40. Brown DA, Hance KW, Rogers CJ, Sansbury LB, Albert PS, Murphy G, et al. Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): a potential screening tool for the prevention of colon cancer? Cancer Epidemiol Biomarkers Prev. 2012;21(2):337–46. Epub 2011/12/07 06:00. 10.1158/1055-9965.EPI-11-0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoopmann M, Warm M, Mallmann P, Thomas A, Gohring UJ, Schondorf T. Tumor M2 pyruvate kinase—determination in breast cancer patients receiving trastuzumab therapy. Cancer Lett. 2002;187(1–2):223–8. Epub 2002/10/03 04:00. . [DOI] [PubMed] [Google Scholar]
- 42. Lüftner D, Mesterharm J, Akrivakis C, Geppert R, Petrides PE, Wernecke KD, et al. Tumor type M2 pyruvate kinase expression in advanced breast cancer. Anticancer Res. 2000;20(6D):5077–82. Epub 2001/05/01 10:00. . [PubMed] [Google Scholar]
- 43. Nagai MA, Gerhard R, Fregnani JH, Nonogaki S, Rierger RB, Netto MM, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat. 2011;126(1):1–14. Epub 2010/04/07 06:00. 10.1007/s10549-010-0867-2 [DOI] [PubMed] [Google Scholar]
- 44. Norum LF, Erikstein B, Nustad K. Elevated CA125 in breast cancer—A sign of advanced disease. Tumour Biol. 2001;22(4):223–8. Epub 2001/06/16 10:00. . [DOI] [PubMed] [Google Scholar]
- 45. Marks JR, Anderson KS, Engstrom P, Godwin AK, Esserman LJ, Longton G, et al. Construction and Analysis of the NCI-EDRN Breast Cancer Reference Set for Circulating Markers of Disease. Cancer Epidemiol Biomarkers Prev. 2014;3. Epub 2014/12/05 06:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hertlein L, Stieber P, Kirschenhofer A, Fürst S, Mayr D, Hofmann K, et al. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin Chem Lab Med. 2012;50(12):2181–8. Epub 2012/10/25 06:00. . [DOI] [PubMed] [Google Scholar]
- 47. Kamei M, Yamashita S, Tokuishi K, Hashioto T, Moroga T, Suehiro S, et al. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. 2010;30(11):4779–83. Epub 2010/12/01 06:00. . [PubMed] [Google Scholar]
- 48. Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abba MC, Drake JA, Hawkins KA, Hu Y, Sun H, Notcovich C, et al. Transcriptomic changes in human breast cancer progression as determined by serial analysis of gene expression. Breast Cancer Res. 2004;6(5):R499–513. Epub 2004/08/21. 10.1186/bcr899 bcr899 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amatschek S, Koenig U, Auer H, Steinlein P, Pacher M, Gruenfelder A, et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res. 2004;64(3):844–56. Epub 2004/02/12. . [DOI] [PubMed] [Google Scholar]
- 51. Zhao H, Langerød A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15(6):2523–36. Epub 2004/03/23. 10.1091/mbc.E03-11-0786 E03-11-0786 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 1471-2407-7-55 [pii] 10.1186/1471-2407-7-55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Srour N, Reymond MA, Steinert R. Lost in translation? A systematic database of gene expression in breast cancer. Pathobiology. 2008;75(2):112–8. 10.1159/000123849 [DOI] [PubMed] [Google Scholar]
- 54. Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, et al. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res. 2008;14(17):5555–64. 10.1158/1078-0432.CCR-08-0555 [DOI] [PubMed] [Google Scholar]
- 55. Westbury CB, Reis-Filho JS, Dexter T, Mahler-Araujo B, Fenwick K, Iravani M, et al. Genome-wide transcriptomic profiling of microdissected human breast tissue reveals differential expression of KIT (c-Kit, CD117) and oestrogen receptor-alpha (ERalpha) in response to therapeutic radiation. J Pathol. 2009;219(1):131–40. 10.1002/path.2581 [DOI] [PubMed] [Google Scholar]
- 56. Grigoriadis A, Mackay A, Reis-Filho JS, Steele D, Iseli C, Stevenson BJ, et al. Establishment of the epithelial-specific transcriptome of normal and malignant human breast cells based on MPSS and array expression data. Breast Cancer Res. 2006;8(5):R56. Epub 2006/10/04. doi: bcr1604 [pii] 10.1186/bcr1604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paruchuri V, Prasad A, McHugh K, Bhat HK, Polyak K, Ganju RK. S100A7-downregulation inhibits epidermal growth factor-induced signaling in breast cancer cells and blocks osteoclast formation. PLoS ONE. 2008;3(3):e1741 Epub 2008/03/06. 10.1371/journal.pone.0001741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reinholz MM, Iturria SJ, Ingle JN, Roche PC. Differential gene expression of TGF-beta family members and osteopontin in breast tumor tissue: analysis by real-time quantitative PCR. Breast Cancer Res Treat. 2002;74(3):255–69. Epub 2002/09/11. . [DOI] [PubMed] [Google Scholar]
- 59. Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, et al. Osteopontin-c is a selective marker of breast cancer. Int J Cancer. 2008;122(4):889–97. . [DOI] [PubMed] [Google Scholar]
- 60. Uhlén M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28(12):1248–50. Epub 2010/12/09 06:00. 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- 61. Jacobson A, Cunningham JL. Connective tissue growth factor in tumor pathogenesis. Fibrogenesis Tissue Repair. 2012;5 Suppl 1(5):S8 Epub 2012/12/25 06:00. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pucci-Minafra I, Cancemi P, Albanese NN, Di Cara G, Marabeti MR, Marrazzo A, et al. New protein clustering of breast cancer tissue proteomics using actin content as a cellularity indicator. J Proteome Res. 2008;7(4):1412–8. Epub 2008/02/21 09:00. 10.1021/pr700748m [DOI] [PubMed] [Google Scholar]
- 63. Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10(1 Pt 1):184–90. Epub 2004/01/22 05:00. . [DOI] [PubMed] [Google Scholar]
- 64. Wang X, Chao L, Ma G, Chen L, Tian B, Zang Y, et al. Increased expression of osteopontin in patients with triple-negative breast cancer. Eur J Clin Invest. 2008;38(6):438–46. Epub 2008/05/03 09:00. 10.1111/j.1365-2362.2008.01956.x [DOI] [PubMed] [Google Scholar]
- 65. Gressner OA, Fang M, Li H, Lu LG, Gressner AM, Gao CF. Connective tissue growth factor (CTGF/CCN2) in serum is an indicator of fibrogenic progression and malignant transformation in patients with chronic hepatitis B infection. Clin Chim Acta. 2013;421:126–31. Epub 2013/03/19 06:00. 10.1016/j.cca.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 66. Anderson KS, Wong J, Polyak K, Aronzon D, Enerbäck C. Detection of psoriasin/S100A7 in the sera of patients with psoriasis. Br J Dermatol. 2009;160(2):325–32. Epub 2008/11/20 09:00. 10.1111/j.1365-2133.2008.08904.x [DOI] [PubMed] [Google Scholar]
- 67. Cohen A, Wang E, Chisholm KA, Kostyleva R, O'Connor-McCourt M, Pinto DM. A mass spectrometry-based plasma protein panel targeting the tumor microenvironment in patients with breast cancer. J Proteomics. 2013;81:135–47. Epub 2012/11/24 06:00. 10.1016/j.jprot.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 68. Ruiz-Garcia E, Scott V, Machavoine C, Bidart JM, Lacroix L, Delaloge S, et al. Gene expression profiling identifies Fibronectin 1 and CXCL9 as candidate biomarkers for breast cancer screening. Br J Cancer. 2010;102(3):462–8. Epub 2010/01/14 06:00. 10.1038/sj.bjc.6605511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pietrowska M, Marczak L, Polanska J, Behrendt K, Nowicka E, Walaszczyk A, et al. Mass spectrometry-based serum proteome pattern analysis in molecular diagnostics of early stage breast cancer. Journal of Translational Medicine. 2009;7(60):60 Epub 2009/07/15 09:00. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bramwell VH, Doig GS, Tuck AB, Wilson SM, Tonkin KS, Tomiak A, et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006;12(11 Pt 1):3337–43. Epub 2006/06/03 09:00. . [DOI] [PubMed] [Google Scholar]
- 71. Opstal-van Winden AW, Rodenburg W, Pennings JL, van Oostrom CT, Beijnen JH, Peeters PH, et al. A bead-based multiplexed immunoassay to evaluate breast cancer biomarkers for early detection in pre-diagnostic serum. Int J Mol Sci. 2012;13(10):13587–604. Epub 2012/12/04 06:00. 10.3390/ijms131013587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res. 2001;7(2):285–9. Epub 2001/03/10 10:00. . [PubMed] [Google Scholar]
- 73. Gonzalez RM, Daly DS, Tan R, Marks JR, Zangar RC. Plasma biomarker profiles differ depending on breast cancer subtype but RANTES is consistently increased. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1543–51. Epub 2011/05/19 06:00. 10.1158/1055-9965.EPI-10-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nozoe T, Mori E, Kono M, Iguchi T, Maeda T, Matsukuma A, et al. Serum appearance of anti-p53 antibody in triple negative breast cancer. Breast Cancer. 2012;19(1):11–5. Epub 2010/12/09 06:00. 10.1007/s12282-010-0244-3 [DOI] [PubMed] [Google Scholar]
- 75. Henderson GS, van Diest PJ, Burger H, Russo J, Raman V. Expression pattern of a homeotic gene, HOXA5, in normal breast and in breast tumors. Cell Oncol. 2006;28(5–6):305–13. Epub 2006/12/15 09:00. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vargas AC, McCart Reed AE, Waddell N, Lane A, Reid LE, Smart CE, et al. Gene expression profiling of tumour epithelial and stromal compartments during breast cancer progression. Breast Cancer Res Treat. 2012;135(1):153–65. Epub 2012/06/22 06:00. 10.1007/s10549-012-2123-4 [DOI] [PubMed] [Google Scholar]
- 77. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010;50(4):298–301. Epub 2010/02/12. doi: S1046-2023(10)00047-2 [pii] 10.1016/j.ymeth.2010.01.032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scholler N, Crawford M, Sato A, Drescher C, O'Briant K, Kiviat N, et al. Bead-Based ELISA for Validation of Ovarian Cancer Early Detection Markers. Clin Cancer Res. 2006;12(7):2117–24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth ed. New York: Springer; 2002. [Google Scholar]
- 80. R-Development-Core-Team. R: A Language and Environment for Statistical Computing. Wien, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 81. Lu D, Kuhn E, Bristow RE, Giuntoli RL 2nd, Krüger Kjær S, I. S, et al. Comparison of candidate serologic markers for type I and type II ovarian cancer. Gynecol Oncol. 2011;122(3):560–6. Epub 2011/06/28 06:00. 10.1016/j.ygyno.2011.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30(4):403–8. Epub 1982/10/15. . [DOI] [PubMed] [Google Scholar]
- 83. Männistö T, Surcel HM, Bloigu A, Ruokonen A, Hartikainen AL, Järvelin MR, et al. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53(11):1986–7. Epub 2007/10/24 09:00. . [DOI] [PubMed] [Google Scholar]
- 84. Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298–306. Epub 2012/03/15 06:00. 10.1017/S0029665112000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HRN breast cancer cases (43, blue) or TN breast cancer cases (28, black) vs. 87 matched controls.
(PDF)
Figure showing the values of the 15 evaluated analytes measured in 43 HRN breast cancer cases and 87 matched controls.
(PDF)
When adjusted for the time each serum was stored in the freezer, anti-TP53 values of the first draw (diagnostic for patients) and the second draw (post-therapy for patients) from each individual correlated (p<0.005 for cases and p = 0.029 for controls).
(PDF)
As depicted in the graph, there is a weak correlation (Kendal correlation statistic = 0.317; p = 0.005) between anti-TP53 expression and BMI.
(PDF)
Listing the following characteristics: Surgery Year, Time from mammogram (Mx) to Draw (weeks:days), Time Draw to Surgery (weeks:days), BI-RADS at Diagnosis (Dx), Density at Dx, Ethnicity, Death as of Dec 2014, Recurrence as of June 2014, No Ovaries at Dx, ER/PR status, HER2 status, Ducal histology, Stage, Lymph Nodes (positive / total), Metastasis, Neo-adjuvant treatment (Tx), Age at Dx, Age at Menarche, Age at Menopause, BMI, Age at first contraceptive, Current Hormone Therapy, Years of Hormone Therapy, Currently pregnant, Parity, Children, Smoking (Pack years), Drinking (g Alc / yr), Other Cancer(s).
(CSV)
(CSV)
Numbers are given for the full 43 HRN set and the TN subset.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.