Abstract
BST-2 (tetherin, CD317, HM1.24) restricts virus growth by tethering enveloped viruses to the cell surface. The role of BST-2 during influenza A virus infection (IAV) is controversial. Here, we assessed the capacity of endogenous BST-2 to restrict IAV in primary murine cells. IAV infection increased BST-2 surface expression by primary macrophages, but not alveolar epithelial cells (AEC). BST-2-deficient AEC and macrophages displayed no difference in susceptibility to IAV infection relative to wild type cells. Furthermore, BST-2 played little role in infectious IAV release from either AEC or macrophages. To examine BST-2 during IAV infection in vivo, we infected BST-2-deficient mice. No difference in weight loss or in viral loads in the lungs and/or nasal tissues were detected between BST-2-deficient and wild type animals. This study rules out a major role for endogenous BST-2 in modulating IAV in the mouse model of infection.
Introduction
BST-2 (tetherin; CD317, HM1.24) is a host cell protein of importance to viral immunity. BST-2 prevents newly generated viral particles from being released from the infected cell by forming a "tether" that retains virions at the cell surface. Viral tethering by BST-2 was first identified for human immunodeficiency virus [1, 2] with subsequent studies showing activity against many enveloped viruses [3–6]. In addition to viral tethering, BST-2 can exert other immunomodulatory functions during viral infection [7, 8]. Specifically, BST-2 has been shown to modulate type 1 interferon (IFN) production [9, 10] and trigger NFκB activation [11–13]. Consequently, BST-2 is a molecule of significant interest in host viral defense.
Influenza viruses belong to the Orthomyxoviridae family of enveloped viruses and are an important cause of respiratory disease worldwide. Type A influenza virus (IAV) is the major etiological agent capable of causing epidemics and pandemics in humans. Host anti-viral restriction factors have been described that can limit intracellular replication of IAV. Examples include interferon (IFN)-inducible transmembrane-3 [14, 15], viperin [16] and myxovirus resistance gene A [17, 18]. Currently, whether BST-2 acts to restrict the release of infectious IAV is controversial. Initial reports indicated that BST-2 limited release of IAV virus-like particles (VLP) [19, 20], as well as release of infectious IAV [21–23]. In contrast, several studies reported that BST-2 did not restrict IAV release from infected cells [19, 24, 25]. Overall, studies investigating the role of BST-2 during IAV infection have utilized cell lines engineered to overexpress or suppress BST-2. Differences in BST-2 expression levels, the contribution of transformed cell lines and/or expression of BST-2 in heterologous cell lines may be important factors contributing to the conflicting data published to date. Hence, further studies are required to clarify the impact of BST-2 during IAV infection, using primary cells susceptible to IAV infection where physiologically relevant levels of endogenous BST-2 are expressed. To date, one study has examined the role of BST-2 during influenza virus infection in vivo. Surprisingly, BST-2-deficient mice infected with influenza B virus (IBV) displayed a modest, but significant, reduction in lung viral titers at day 3 post-infection, although no significant differences were detected at day 6 [7]. This finding is paradoxical to the proposed role of BST-2 in limiting viral release, and so far remains largely unexplained.
In the airways, primary alveolar epithelial cells (AEC) and macrophages represent two of the major cell types susceptible to IAV infection [26]. Herein, we have compared AEC and macrophages isolated from wild-type and BST-2-deficient mice [7], for their susceptibility to IAV infection and their ability to support productive IAV replication in vitro. In addition, we have compared weight loss and viral replication following intranasal infection of wild type or BST-2-deficient mice with IAV. Together, our data indicate that endogenous BST-2 does not play a major role in host restriction of IAV in the mouse model of infection.
Materials and Methods
Mice
C57BL/6 and BST-2-deficient mice (kindly provided by M. Colonna, Washington University [7]) were bred and housed in specific pathogen-free conditions at the Bio21 Animal House Facility, The University of Melbourne. Mice (female and male) 6–10 weeks of age were used in experiments conducted in accordance with guidelines provided by National Health and Medical Research Council of Australia. Experimental procedures were approved by the Animal Ethics Committees at the University of Melbourne (Application 1112261). Studies comply with Animal Research: Reporting In Vivo Experiment guidelines (S1 Checklist).
Cell lines, primary macrophages and primary alveolar epithelial cells
Cell lines used in this study included Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection, ATCC), LA-4 mouse lung epithelial cells and the RAW264.7 macrophage cell line (ATCC). Resident peritoneal exudate macrophages were obtained from mice as previously described [27]. Macrophages were seeded into 8-well glass chamber-slides (Lab-Tek), incubated for 2 hours at 37°C and cell monolayers were washed to remove non-adherent cells. The next day, any remaining non-adherent cells were removed and the adherent macrophages used in virus infection assays. Mouse primary lung epithelial cells were prepared as previously described [28]. Briefly, lungs from mice were digested in 1.5 mg/ml Pronase (Roche, USA), 0.1 mg/ml DNase I (Sigma-Aldrich, USA) for 1 hour at 37°C in 5% CO2. Single cell suspensions were incubated with purified rat anti-mouse CD45 antibody (BD Biosciences, USA) and epithelial cells negatively enriched with BioMag goat anti-rat immunoglobulin-coupled magnetic beads (Qiagen, USA). Cells were cultured on collagen-coated (MP Biomedicals, USA) plates. To confirm alveolar epithelial cell purity, monolayers were detached with 3 mM EDTA and cells stained with mouse anti-EpCAM, anti-podoplanin (AEC type I) and anti-CD74 (AEC type II) antibodies and examined by flow cytometry.
Viruses
The representative IAV laboratory strain used in this study was HKx31, a reassortant of A/PR8/34 (PR8, H1N1) with A/Aichi/2/68 (H3N2) bearing the H3N2 surface glycoproteins. In some experiments A/Brazil/11/78 (Brazil/78; H1N1) and A/Solomon Islands/3/2006 (Sol Is/06; H1N1) were used as representative seasonal strains. Sol Is/06 was obtained from the World Heath Organization Collaborating Centre for Reference and Research on Influenza, Melbourne, Australia. Virus was amplified in the allantoic cavity of 10-day-old embryonated hen’s eggs and titrated on MDCK cells by standard plaque assay [29].
Virus infection of mice
Groups of 5 mice were lightly anaesthetized (methoxyflurane) and infected with either 102 or 104 PFU of HKx31 via the intranasal route. Mice were weighed daily and assessed for signs of clinical disease. Animals that had lost ≥ 20% of their original body weight were euthanized by CO2 inhalation followed by cervical dislocation. No adverse events occurred during these experiments. Lungs and nasal tissues were removed, homogenized and clarified by centrifugation. Titers of infectious virus in tissue homogenates were determined by standard plaque assays [29].
Virus infection assays
Mouse peritoneal macrophages and primary lung epithelial cells were infected with IAV and the percentage of IAV-infected cells determined as described previously for epithelial cells [30] and macrophages [31]. Briefly, cells were incubated with IAV in serum-free media for 1 hour at 37°C. Virus inoculum was removed and cells incubated at 37°C in serum-free media. IAV-infected cells were fixed with 80% vol/vol acetone at the indicated time points post-infection and stained using monoclonal antibody (mAb) MP3.10g2.1C7 (WHO Collaborating Centre for Reference and Research on Influenza, Melbourne, Australia) specific for IAV nucleoprotein. Virus-infected cells were co-stained with 4',6-diamidino-2-phenylindole (DAPI) or propidium iodide (PI). A minimum of 200 cells were scored for each sample.
Virus growth assays
Cells were infected with IAV as described above. As trypsin counteracts BST-2 anti-viral activity [32], this was omitted in the infection media, thereby limiting IAV replication to a single round. Cell supernatants were collected at the indicated time-points post-infection, and incubated with 4 μg/ml L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK)-treated trypsin (Sigma Aldrich) for 30 minutes at 37°C to facilitate cleavage of the viral hemagglutinin [33] before the titers of infectious virus were determined by standard plaque assay on MDCK cells [29].
BST-2 expression assays
BST-2 expression was determined by staining with anti-BST-2 (clone 120G8) mAb in conjunction with flow cytometric analysis. Briefly, cells were pre-incubated with 2.4G2 mAb to block non-specific staining via Fc receptors, then stained with rat anti-BST-2 mAb conjugated to FITC (generated in house) or rat IgG1k-FITC isotype control antibody (Biolegend). In some experiments, BST-2 expression was monitored post-infection with IAV (as described above) at the indicated virus doses and time-points. Cells were treated with 1000 international units (IU)/ml recombinant murine IFNα (R&D Systems) as a control to upregulate BST-2 surface expression.
Results
BST-2 expression is upregulated on murine macrophages but not alveolar epithelial cells in response to IAV infection
First, flow cytometry was used to examine BST-2 expression by murine AEC and macrophages that were uninfected, or had been infected 4 and 24 hours previously with IAV strain HKx31 (H3N2). BST-2 expression was analysed using the LA-4 AEC line and primary AEC, as well as using the RAW264.7 macrophage cell line and primary macrophages. Peritoneal exudate macrophages were used given the difficulty in obtaining sufficient numbers of alveolar macrophages via bronchoalveolar lavage (BAL). Note that peritoneal and alveolar macrophages exhibit similar susceptibility and ability to support IAV infection [34, 35]. For epithelial cells, uninfected LA-4 cells and primary AEC expressed cell-surface BST-2, however levels did not increase further following IAV infection. Of interest, culture of AEC in the presence of IFNα did result in upregulation of cell-surface BST-2 (Fig 1A). In contrast to epithelial cells, macrophages upregulated cell-surface BST-2 in response to IAV and levels were similar (RAW264.7), or higher (primary cells), than those elicited in response to IFNα (Fig 1B).
Fig 1. BST-2 expression is upregulated on murine macrophages but not alveolar epithelial cells in response to influenza A virus.
Monolayers of (A) the LA-4 AEC line and primary AEC, or (B) RAW264.7 macrophages and primary macrophages were incubated (i) with a MOI of 5 (HKx31) for 1 hour at 37°C and washed to remove excess virus (IAV infection, solid black line), (ii) in 1000 IU/ml recombinant mouse IFNα (dashed line) or (iii) in media alone (no infection, grey histogram). Cells were then incubated at 37°C for a total of 4 or 24 hours and levels of cell-surface BST-2 determined by flow cytometry. For each cell type, the isotype control (solid black histograms) is shown for ‘no infection’ cells only but is representative of profiles obtained using IAV-infected and IFNα-treated cells. Data are representative of 3 independent experiments.
BST-2 does not modulate susceptibility to IAV infection or release of newly synthesized virions from murine AEC and macrophages
Next, we examined the impact of BST-2 on the susceptibility of primary AEC and macrophages to IAV infection using cells from wild type (WT) and BST-2-deficient animals. We confirmed expression patterns of BST-2 by flow cytometry for primary macrophages isolated from BST-2+/+, BST-2+/- and BST-2-/- mice (S1 Fig). Next, cells isolated from WT or BST-2-deficient mice were inoculated with IAV at different multiplicities of infection (MOI) and the percentage of IAV-infected cells was determined 6–8 hours later by detection of newly synthesized viral nucleoprotein (NP) [35]. Viral NP was not detected at 2 hours post infection, indicating its presence at later time points was due to newly synthesized viral protein and not input virus (S2 Fig). Overall, the percentage of IAV-infected AEC did not differ in the presence or absence of BST-2 at any MOI tested for the IAV laboratory strain HKx31, or the representative seasonal strains Brazil/78 and Sol Is/06 (Fig 2A). Similar to AEC, macrophages exhibited equivalent susceptibility to IAV infection regardless of the presence or absence of BST-2 for both the HKx31 and Brazil/78 strains (Fig 2B). The Sol Is/06 IAV strain did not efficiently infect primary murine macrophages, and therefore was not assessed (data not shown).
Fig 2. BST-2 expression does not modulate IAV susceptibility of murine epithelial cells and macrophages to IAV infection.
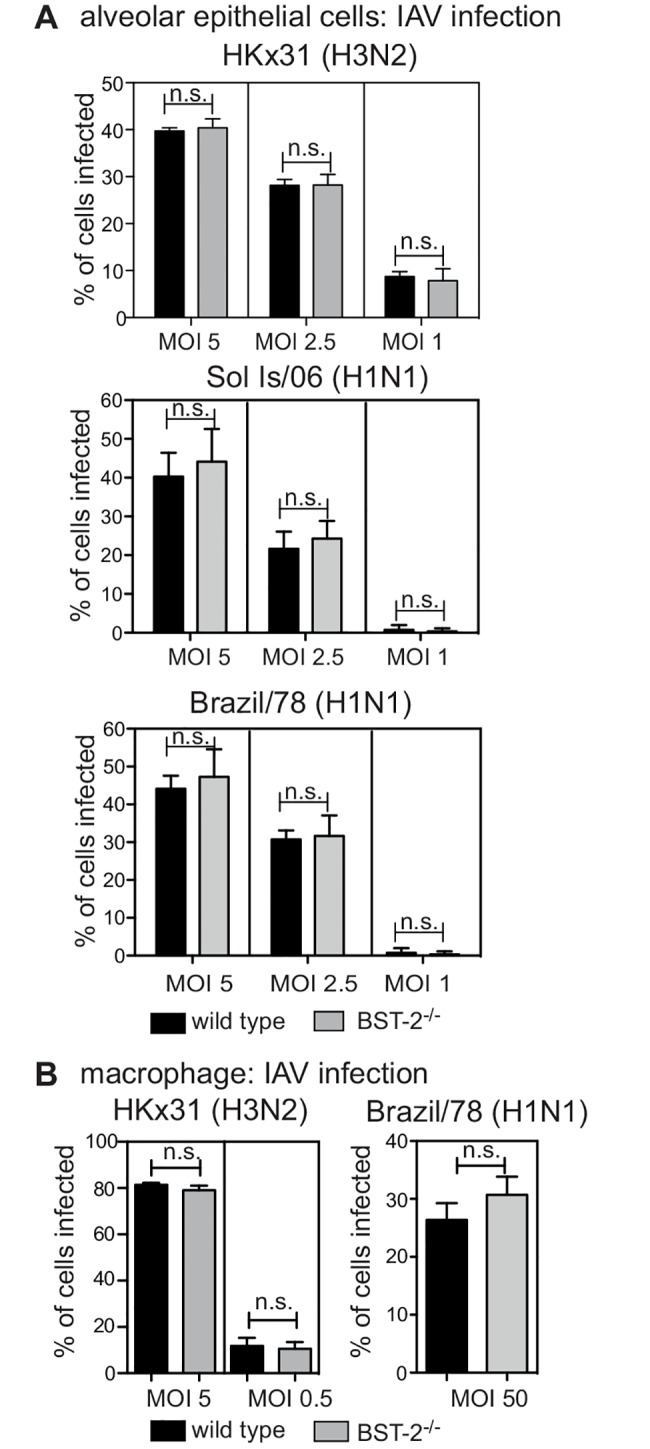
(A) Primary AEC or (B) macrophages isolated from BST-2 wild type (WT) and BST-2-deficient (BST-2-/-) mice were incubated with the indicated MOI and strain of IAV for 1 hour at 37°C, washed to remove excess virus and cultured as indicated. Monolayers were fixed at 8 hours post-infection before staining by immunofluorescence to detect newly synthesized viral NP. Data show the mean (± 1 SD) pooled from 3 independent experiments. n.s. = no significant difference, p = > 0.05, two-way ANOVA followed by Bonferroni analysis.
It is well established that IAV infection of AEC results in productive viral replication, however infection of macrophages with seasonal IAV is abortive, such that viral replication is blocked and infectious viral particles are not released [27, 35]. However, the mechanisms that restrict productive IAV replication macrophages are currently unknown. Whether BST-2 can restrict release of IAV from AEC is contentious, and the role of BST-2 in modulating IAV release from macrophages has not been reported. Therefore, primary AEC and macrophages were inoculated with IAV, washed and cultured for a total of 2 hours (representative of residual inoculum) or 8, 24 or 48 hours, before titres of infectious virus were determined in cell-free supernatants by standard plaque assays. For HKx31, at 24 and 48 hours post-infection, titres of infectious virus released from BST-2-deficient AEC were significantly reduced compared to WT AEC, although this was not the case for the Brazil/78 or Sol Is/06 IAV strains, where viral release of these strains from AEC occurred independently of BST-2 (Fig 3A). For macrophages, IAV titres did not increase between 2 and 24 hours following infection with either HKx31 or Brazil/78, and this was unaffected by the presence or absence of BST-2 (Fig 3B). In these experiments, MDCK cells were included as a control to confirm that the virus inoculum could give rise to productive IAV replication and release from cells (data not shown).
Fig 3. BST-2 expression does not modulate IAV release from murine epithelial cells and macrophages following IAV infection.
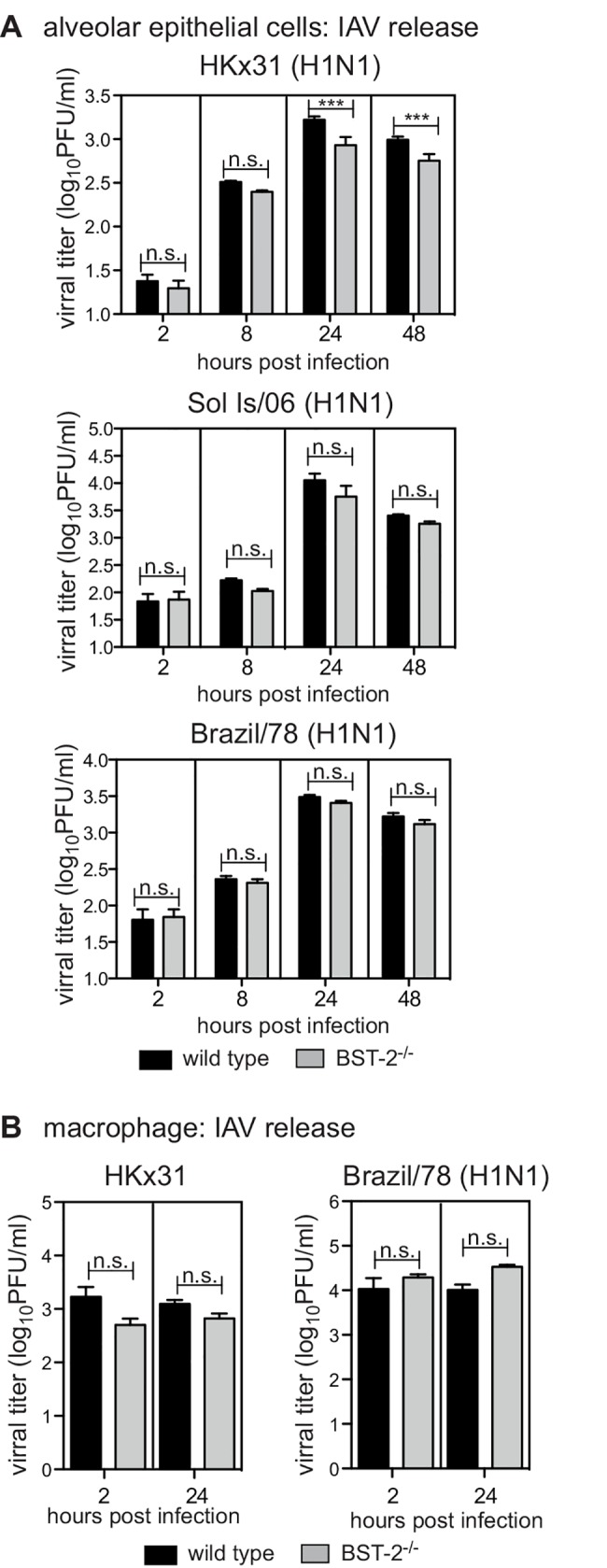
(A) Primary AEC or (B) macrophages isolated from BST-2 wild type (WT) and BST-2-deficient (BST-2-/-) mice were incubated with the indicated strain of IAV for 1 hour at 37°C, washed to remove excess virus and cultured. AEC were infected at a MOI of 1 of HKx31, Brazil/78 and Sol Is/06 and macrophages infected at a MOI 5 for HKx31 and a MOI of 50 for Brazil/78. Culture supernatants were removed at 2 hours, or at 8, 24 or 48 hours as indicated, clarified by centrifugation and titres of infectious virus were determined by plaque assay on MDCK cells. Data is displayed as viral titer (log10PFU/ml) and represent the mean (± 1 SD) from triplicate samples. Data is representative of 2 independent experiments. n.s. = no significant difference, *** p < 0.001, two-way ANOVA followed by Bonferroni analysis.
Lack of BST-2 does not alter the susceptibility of mice to IAV infection or the ability of IAV to replicate in the airways
To assess the impact of BST-2 in vivo we compared the susceptibility of WT BST-2 (bst-2 +/+ or bst-2 +/-) or BST-2-deficient (bst-2 -/-) mice to IAV infection in vivo. First, we assessed the status of BST-2-deficient mice under resting conditions. Proportions of lymphocytes, monocytes, neutrophils and eosinophils in the peripheral blood did not differ significantly between BST-2-deficient and wild type mice (Fig 4A) indicating that the absence of BST-2 did not elicit overt alterations in immune homeostasis. BST-2-deficient mice were inoculated with IAV strain HKx31 via the intranasal route. Infection of mice with this strain elicits a mild respiratory illness and weight loss is a reliable indicator of disease severity [36, 37]. Infection with HKx31 provokes a significant increase in IFNα in the respiratory tract [38] (S3 Fig) and consequently elicits conditions under which BST-2 expression is likely to be elevated. Elevated BST-2 expression has also been detected on immune cells isolated from the respiratory tract on IAV-infected mice [39]. Mice infected with 102 PFU of HKx31 lost little weight by day 3 post-infection whereas infection with 104 PFU resulted in progressive weight loss, however no significant differences were recorded between WT or BST-2-deficient mice (Fig 4B). IAV replication in the airways was assessed in the lung and nasal tissues at day 3 and day 7 post-infection and no significant differences were recorded between WT and BST-2-deficient mice at either time point, irrespective of inoculum dose (Fig 4C). Therefore, endogenous BST-2 does not play a major role in restricting IAV infection in vivo.
Fig 4. Lack of BST-2 does not alter the susceptibility of mice to IAV infection or the ability of IAV to replicate in the airways.

(A) Peripheral blood from wild type and BST-2-/- mice was screened by the Advia2120 automated hematology analyzer. Each symbol represents an individual mouse and the bar indicates the mean. n.s. = no significant difference, p = > 0.05, two way ANOVA followed by Bonferroni analysis. (B) Wild type and BST-2-/- mice were infected with either 102 PFU or 104 PFU of HKx31 via the intranasal route. Mice were weighed daily and the results expressed as the mean percentage weight change per group (± 1 SEM) relative to original body weight. (C) Virus titres were determined in clarified homogenates prepared from lungs and nasal tissues using a standard plaque assay on MDCK cells. Symbols show titres from individual animals and horizontal bars represent the mean virus titre. n.s. = no significant difference, p = > 0.05, Student’s t-test; two-tailed.
Discussion
Here we have undertaken a comprehensive analysis of the role of endogenous BST-2 in restricting IAV infection by performing, to our knowledge, the first analysis of infection of BST-2-deficient primary murine cells with IAV and importantly, IAV infection of BST-2-deficient mice. Our analyses rule out a major role for BST-2 as a host molecule that restricts IAV in the mouse model of infection.
The ability of BST-2 to restrict viral infection relies on its expression by cell types permissive to infection. Analysis of surface BST-2 expression demonstrated its expression at low levels by uninfected macrophages and AEC, two cell types that are susceptible to IAV infection. In addition to inflammatory cytokines, IAV infection results in secretion of type I IFN [40], with BST-2 a type I IFN responsive gene [9]. In accordance with this, macrophages and AEC expressed increased levels of surface BST-2 in the presence of IFNα. In contrast, incubation (and infection) with IAV, increased surface BST-2 expression by macrophages, but not AEC. It is well established that IAV can induce distinct profiles of inflammatory mediators from macrophages and AEC and that macrophages induce more potent type I IFN responses than epithelial cells following IAV exposure [40]. AEC constitutively express BST-2 at high levels, but do not upregulate expression in response to IAV. We observed no major impact of BST-2 deletion on the release of IAV from AEC in single cycle replication assays. IAV may antagonize BST-2 expression in AEC [21], however the ability of IAV to antagonize BST-2 has been disputed [25] and we detect no evidence for impaired BST-2 expression following IAV infection. In summary, we observe that BST-2 is expressed by cell types of importance to IAV infection and consequently has the potential to modulate IAV infection.
Analysis of IAV infection of primary cells in vitro failed to support a major role for BST-2 in restricting productive virus replication and release. In AEC, the role for BST-2 varied depending on the IAV strain tested. For HKx31, BST-2 promotes, rather than inhibits, the release of newly synthesized virions from IAV-infected cells. This was not the case following infection with IAV Brazil/78 or Sol Is/06, and therefore the significance of a role for BST-2 in promoting IAV release is unclear, Regardless, we detect no evidence for a major role for BST-2 in restricting IAV release in AEC under the experimental conditions tested. It is well established that macrophages do not support productive replication of seasonal IAV [27, 35] and herein we demonstrate that the absence of BST-2 did not reverse this phenotype, despite increased expression of BST-2 following IAV infection of macrophages from WT mice. In summary, BST-2 is not acting as a dominant restriction factor for IAV in either murine AEC or macrophages.
Numerous studies have addressed the ability of BST-2 to restrict release of different viruses in vitro, however less is known regarding its ability to modulate viral infections in vivo. The availability of BST-2-deficient mice [7, 41] has allowed the impact of viral infection to be assessed in the absence of endogenous BST-2. Infection of BST-2-deficient mice with Chikungunya virus (CHIKV) results in increased viremia, in accordance with its capacity to tether CHIKV in vitro [42]. Similarly, infection of BST-2 deficient mice with Moloney murine leukemia virus elicits enhanced viral titers [41]. In contrast, BST-2 does not act according to its predicted role as a viral tetherin in several infection models. Infection of BST-2-deficient mice with vesicular stomatitis virus, a target of BST-2 tethering in vitro [6], results in reduced, rather than increased, viral titers [7]. This was also the case following infection with IBV, where BST-2-deficient mice display reduced viral titers in the lung [7]. Herein, we also report reduced levels of infectious IAV in cell supernatants from BST-2-deficient AEC, although this was only under specific experimental conditions and in vivo viral titers in the respiratory tract were not significantly different to those in WT animals. Increased viral titers in the presence of BST-2 is perplexing as it excludes a major role for BST-2 in restricting viral release and instead, implicates a pro-viral role for BST-2. One explanation for this is the potential role for BST-2 in enhancing, rather than inhibiting, viral entry. This is reported for cytomegalovirus, where BST-2 exerts a reverse-tethering mechanism to promote entry [43]. Note, however, that we find no evidence of BST-2 promoting infectious entry of IAV, given that BST-2-deficient AEC and/or macrophages are equally susceptible to infection compared to WT cells. How BST-2 acts to promote, rather than inhibit, IAV release remains to be determined. Regardless, our infection studies revealed similar kinetics of weight loss and viral replication in BST-2-deficient and WT mice. Therefore, in the mouse, BST-does not have a major impact on IAV infection outcomes in vivo.
Together, our data do not support a role for endogenous BST-2 as a major factor restricting infectious entry of primary murine IAV into target cells or in limiting the release of newly synthesized virions from infected cells. In conclusion, based on our studies in the moue model of infection, it is likely that murine IAV infection and replication can be inhibited by the (combined) action of a number of host molecules, of which BST-2 may contribute only a minor role.
Supporting Information
(PDF)
Primary macrophages isolated from bst-2 +/+ (solid black line), bst-2 +/- (dashed line) and bst-2 -/- (black histogram) were cultured for 24 hours with 1000 IU/ml of IFNα before analysis of cell-surface BST-2 expression by flow cytometry. Isotype control shown is for bst-2 +/+ cells and was indistinguishable from that observed for bst-2 -/- and bst-2 +/- cells.
(EPS)
Primary AEC or macrophages were incubated with HKx31 at an MOI of 5 for 1 hour at 37°C, washed to remove excess virus and cultured. Monolayers were fixed with 80% vol/vol acetone at 2 or 8 hours post-infection before staining by immunofluorescence to detect newly synthesized viral NP (green) and with DAPI to stain the nucleus (blue). Similar results were also obtained with Brazil/78 for AEC and macrophages and Sol Is/06 for AEC (data not shown). Images were acquired with a Zeiss LSM700 or Olympus IX70 confocal microscope in conjunction with Zen2012 software.
(PDF)
Mice were infected with 104 PFU HKx31 via the intranasal route. Lungs were removed 3 days following infection. IFNα in total lung homogenates was measured by enzyme-linked immunosorbent assay. Antibodies to IFNα used for capture (22100–1) and detection (32100–1) were from PBL Assay Science (NJ, USA). Data is pooled from two independent experiments. Each symbol represents an individual mouse and the bar indicates the mean. * p < 0.05, Student's unpaired t-test.
(EPS)
Acknowledgments
We acknowledge technical support from J. Corbin (Walter and Eliza Hall Institute).
Abbreviations
- AEC
alveolar epithelial cell
- CHIKV
Chikungunya virus
- IAV
influenza A virus
- IBV
influenza B virus
- MOI
multiplicity of infection
- WT
wild type
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by funding from National Health and Medical Research Council of Australia Project Grant 1083307 (PR), National Health and Medical Research Council (NHMRC) of Australia Early Career Fellowship 1035733 (MT) and the Victorian State Government Operational Infrastructure Scheme (MT). The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
References
- 1. Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–30. Epub 2008/01/18. 10.1038/nature06553 . [DOI] [PubMed] [Google Scholar]
- 2. Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3(4):245–52. 10.1016/j.chom.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83(4):1837–44. Epub 2008/11/28. 10.1128/JVI.02211-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106(8):2886–91. 10.1073/pnas.0811014106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83(5):2382–5. 10.1128/JVI.01607-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84(24):12646–57. 10.1128/JVI.01328-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swiecki M, Wang Y, Gilfillan S, Lenschow DJ, Colonna M. Cutting edge: paradoxical roles of BST2/tetherin in promoting type I IFN response and viral infection. J Immunol. 2012;188(6):2488–92. 10.4049/jimmunol.1103145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li SX, Barrett BS, Heilman KJ, Messer RJ, Liberatore RA, Bieniasz PD, et al. Tetherin promotes the innate and adaptive cell-mediated immune response against retrovirus infection in vivo. J Immunol. 2014;193(1):306–16. 10.4049/jimmunol.1400490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177(5):3260–5. . [DOI] [PubMed] [Google Scholar]
- 10. Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206(7):1603–14. 10.1084/jem.20090547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe. 2012;12(5):633–44. 10.1016/j.chom.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J Virol. 2013;87(4):2046–57. 10.1128/JVI.02272-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8(9):e1002931 10.1371/journal.ppat.1002931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siegrist F, Ebeling M, Certa U. The small interferon-induced transmembrane genes and proteins. J Interferon Cytokine Res. 2011;31(1):183–97. 10.1089/jir.2010.0112 . [DOI] [PubMed] [Google Scholar]
- 15. Feeley EM, Sims JS, John SP, Chin CR, Pertel T, Chen LM, et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7(10):e1002337 10.1371/journal.ppat.1002337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Hinson ER, Cresswell P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe. 2007;2(2):96–105. 10.1016/j.chom.2007.06.009 . [DOI] [PubMed] [Google Scholar]
- 17. Xiao H, Killip MJ, Staeheli P, Randall RE, Jackson D. The human interferon-induced MxA protein inhibits early stages of influenza A virus infection by retaining the incoming viral genome in the cytoplasm. J Virol. 2013;87(23):13053–8. 10.1128/JVI.02220-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pavlovic J, Zurcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64(7):3370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watanabe R, Leser GP, Lamb RA. Influenza virus is not restricted by tetherin whereas influenza VLP production is restricted by tetherin. Virology. 2011;417(1):50–6. 10.1016/j.virol.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yondola MA, Fernandes F, Belicha-Villanueva A, Uccelini M, Gao Q, Carter C, et al. Budding capability of the influenza virus neuraminidase can be modulated by tetherin. J Virol. 2011;85(6):2480–91. 10.1128/JVI.02188-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mangeat B, Cavagliotti L, Lehmann M, Gers-Huber G, Kaur I, Thomas Y, et al. Influenza virus partially counteracts restriction imposed by tetherin/BST-2. J Biol Chem. 2012;287(26):22015–29. 10.1074/jbc.M111.319996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leyva-Grado VH, Hai R, Fernandes F, Belicha-Villanueva A, Carter C, Yondola MA. Modulation of an ectodomain motif in the influenza A virus neuraminidase alters tetherin sensitivity and results in virus attenuation in vivo. J Mol Biol. 2014;426(6):1308–21. 10.1016/j.jmb.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dittmann M, Hoffmann HH, Scull MA, Gilmore RH, Bell KL, Ciancanelli M, et al. A serpin shapes the extracellular environment to prevent influenza A virus maturation. Cell. 2015;160(4):631–43. 10.1016/j.cell.2015.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bruce EA, Abbink TE, Wise HM, Rollason R, Galao RP, Banting G, et al. Release of filamentous and spherical influenza A virus is not restricted by tetherin. J Gen Virol. 2012;93(Pt 5):963–9. 10.1099/vir.0.038778-0 . [DOI] [PubMed] [Google Scholar]
- 25. Winkler M, Bertram S, Gnirss K, Nehlmeier I, Gawanbacht A, Kirchhoff F, et al. Influenza A virus does not encode a tetherin antagonist with Vpu-like activity and induces IFN-dependent tetherin expression in infected cells. PLoS ONE. 2012;7(8):e43337 10.1371/journal.pone.0043337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, Peiris JS, et al. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J Virol. 2011;85(14):6844–55. 10.1128/JVI.02200-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reading PC, Miller JL, Anders EM. Involvement of the mannose receptor in infection of macrophages by influenza virus. J Virol. 2000;74(11):5190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas BJ, Porritt RA, Hertzog PJ, Bardin PG, Tate MD. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. 2014;4:7176 10.1038/srep07176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anders EM, Hartley CA, Reading PC, Ezekowitz RA. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75 (Pt 3):615–22. . [DOI] [PubMed] [Google Scholar]
- 30. Londrigan SL, Turville SG, Tate MD, Deng YM, Brooks AG, Reading PC. N-linked glycosylation facilitates sialic acid-independent attachment and entry of influenza A viruses into cells expressing DC-SIGN or L-SIGN. J Virol. 2011;85(6):2990–3000. 10.1128/JVI.01705-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng WC, Liong S, Tate MD, Irimura T, Denda-Nagai K, Brooks AG, et al. The macrophage galactose-type lectin can function as an attachment and entry receptor for influenza virus. J Virol. 2014;88(3):1659–72. 10.1128/JVI.02014-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hammonds J, Wang JJ, Yi H, Spearman P. Immunoelectron microscopic evidence for Tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 2010;6(2):e1000749 10.1371/journal.ppat.1000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–69. 10.1146/annurev.biochem.69.1.531 . [DOI] [PubMed] [Google Scholar]
- 34. Tate MD, Brooks AG, Reading PC. Correlation between sialic acid expression and infection of murine macrophages by different strains of influenza virus. Microbes Infect. 2011;13(2):202–7. 10.1016/j.micinf.2010.10.004 . [DOI] [PubMed] [Google Scholar]
- 35. Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84(15):7569–80. 10.1128/JVI.00291-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24(2):77–88. 10.1089/vim.2010.0118 . [DOI] [PubMed] [Google Scholar]
- 37. Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS ONE. 2011;6(3):e17618 10.1371/journal.pone.0017618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol. 2013;14(3):238–45. Epub 2013/01/29. 10.1038/ni.2525 . [DOI] [PubMed] [Google Scholar]
- 39. Moffat JM, Segura E, Khoury G, Caminschi I, Cameron PU, Lewin SR, et al. Targeting antigen to bone marrow stromal cell-2 expressed by conventional and plasmacytoid dendritic cells elicits efficient antigen presentation. Eur J Immunol. 2013;43(3):595–605. Epub 2013/01/11. 10.1002/eji.201242799 . [DOI] [PubMed] [Google Scholar]
- 40. Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12(2–3):171–80. . [DOI] [PubMed] [Google Scholar]
- 41. Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci U S A. 2011;108(44):18097–101. 10.1073/pnas.1113694108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahauad-Fernandez WD, Jones PH, Okeoma CM. Critical role for bone marrow stromal antigen 2 in acute Chikungunya virus infection. J Gen Virol. 2014;95(Pt 11):2450–61. 10.1099/vir.0.068643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viswanathan K, Smith MS, Malouli D, Mansouri M, Nelson JA, Fruh K. BST2/Tetherin enhances entry of human cytomegalovirus. PLoS Pathog. 2011;7(11):e1002332 10.1371/journal.ppat.1002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Primary macrophages isolated from bst-2 +/+ (solid black line), bst-2 +/- (dashed line) and bst-2 -/- (black histogram) were cultured for 24 hours with 1000 IU/ml of IFNα before analysis of cell-surface BST-2 expression by flow cytometry. Isotype control shown is for bst-2 +/+ cells and was indistinguishable from that observed for bst-2 -/- and bst-2 +/- cells.
(EPS)
Primary AEC or macrophages were incubated with HKx31 at an MOI of 5 for 1 hour at 37°C, washed to remove excess virus and cultured. Monolayers were fixed with 80% vol/vol acetone at 2 or 8 hours post-infection before staining by immunofluorescence to detect newly synthesized viral NP (green) and with DAPI to stain the nucleus (blue). Similar results were also obtained with Brazil/78 for AEC and macrophages and Sol Is/06 for AEC (data not shown). Images were acquired with a Zeiss LSM700 or Olympus IX70 confocal microscope in conjunction with Zen2012 software.
(PDF)
Mice were infected with 104 PFU HKx31 via the intranasal route. Lungs were removed 3 days following infection. IFNα in total lung homogenates was measured by enzyme-linked immunosorbent assay. Antibodies to IFNα used for capture (22100–1) and detection (32100–1) were from PBL Assay Science (NJ, USA). Data is pooled from two independent experiments. Each symbol represents an individual mouse and the bar indicates the mean. * p < 0.05, Student's unpaired t-test.
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.



