Abstract
Novel typhoid diagnostics currently under development have the potential to improve clinical care, surveillance, and the disease burden estimates that support vaccine introduction. Blood culture is most often used as the reference method to evaluate the accuracy of new typhoid tests; however, it is recognized to be an imperfect gold standard. If no single gold standard test exists, use of a composite reference standard (CRS) can improve estimation of diagnostic accuracy. Numerous studies have used a CRS to evaluate new typhoid diagnostics; however, there is no consensus on an appropriate CRS. In order to evaluate existing tests for use as a reference test or inclusion in a CRS, we performed a systematic review of the typhoid literature to include all index/reference test combinations observed. We described the landscape of comparisons performed, showed results of a meta-analysis on the accuracy of the more common combinations, and evaluated sources of variability based on study quality. This wide-ranging meta-analysis suggests that no single test has sufficiently good performance but some existing diagnostics may be useful as part of a CRS. Additionally, based on findings from the meta-analysis and a constructed numerical example demonstrating the use of CRS, we proposed necessary criteria and potential components of a typhoid CRS to guide future recommendations. Agreement and adoption by all investigators of a standardized CRS is requisite, and would improve comparison of new diagnostics across independent studies, leading to the identification of a better reference test and improved confidence in prevalence estimates.
Introduction
Typhoid fever causes considerable disease burden, with recent estimates at 21.6 million illnesses in 2000 and 26.9 million in 2010 [1,2]. These estimates are extrapolated from limited population-based studies and further compromised by the poor accuracy of current typhoid diagnostics. The most accepted method used for typhoid detection is blood culture [3]. It is desirable to diagnose typhoid fever because of its perfect specificity, but with sensitivity around 50% in most clinical settings, there is much room for improvement [4,5]. New diagnostic tests for typhoid fever are in development which may relieve this shortfall; however, a problem remains in regard to determining the best reference test with which to evaluate new diagnostics [6,7]. Using a reference test with imperfect diagnostic accuracy may cause newer technologies to appear better or worse than they really are, which in turn clouds the evaluation of their utility as a tool to improve disease burden estimates [8]. Additionally, to compare across index tests with statistical rigor, a common reference test should be used.
Lack of a perfect gold standard in diagnostic research is not an uncommon situation, and yet there is no universally accepted solution to the problem [9]. One method to improve diagnostic accuracy when no perfect reference test exists is to develop a composite reference standard (CRS) [10]. A CRS combines more than one imperfect diagnostic test with the goal of increasing diagnostic accuracy (compared to truth: the true presence of infection). If the individual tests in the CRS are highly specific, combining them by declaring CRS positive if either test is positive should give greater sensitivity than either test alone [10]. This would also enable combining multiple types of testing, such as direct detection of the bacteria with culture and immune detection with an antibody-based assay. Compared to other methods such as discrepant resolution and latent class analysis, an ideal consensus CRS has the advantage that it is more clearly defined, independent of the results of the index test, and more straightforward to interpret [10]. Particularly in the context of typhoid diagnostic field evaluations, the consensus CRS approach to addressing imperfect reference tests may be the most feasible and meaningful for the researchers performing the studies.
In order to evaluate tests for use as a reference test or inclusion in a CRS, we conducted a systematic review of the typhoid literature. We described the types of reference tests that are being used to evaluate new typhoid diagnostics, and summarized by meta-analysis the diagnostic accuracy of available index tests when compared to the most common reference test (blood culture), including the evaluation of variability due to study quality. We then discussed how a standardized CRS, rather than a single reference test, may improve the evaluation of new typhoid diagnostics using a constructed numerical example. Finally, based on the results of our systematic review, meta-analysis, and the constructed numerical example, we proposed recommended criteria and potential components of a CRS for consensus-building and discussion. Agreement and adoption of a standardized composite reference method would enable comparison of diagnostic performance data across independent studies, leading to improved confidence in prevalence estimates.
Methods
Search strategy and inclusion criteria
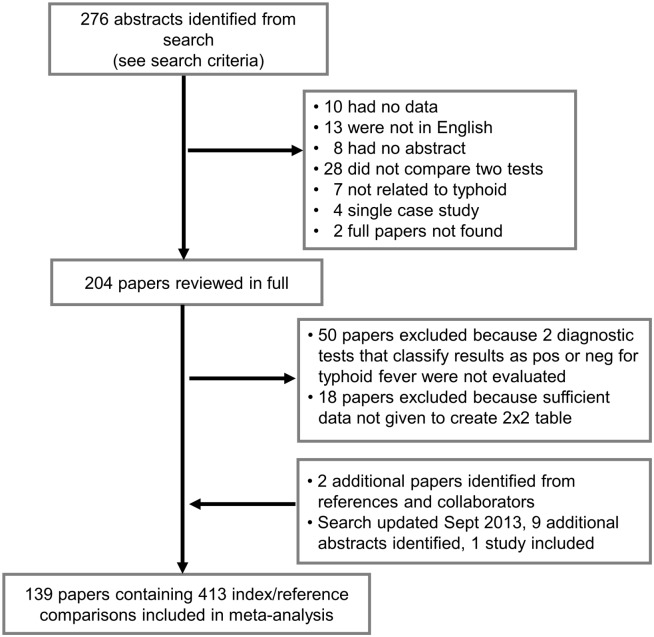
We performed a review and meta-analysis using the PRISMA reporting guidelines (S1 Table) [11]. In May 2013, a detailed search strategy was designed to identify all papers in English that evaluated diagnostic tests for the detection of typhoid fever among humans. If papers considered typhoid fever to include Salmonella typhi and S. paratyphi A, the data were included as such in our review. The following databases were included: Pubmed, EMBASE, and ISI Web of Science (S2 Table). In all, 276 papers were identified from our searches (Fig 1). After reviewing the abstracts, 72 papers were excluded based on the following: had no original data (10), were not in English (13), had no abstract available (8), did not compare two diagnostic tests (28), were not related to typhoid (7), described single case studies (4), and no full paper was found (2). The remaining papers were reviewed in full (204), and further exclusions were made if two diagnostic tests were not evaluated that dichotomized result as positive or negative for typhoid fever (50), or sufficient data were not given to confidently infer all values of the contingency table (18). A diagnosis based on clinical indicators was considered a diagnostic test if classified as positive or negative. Additional papers were identified from references or collaborators (2). The search strategy was updated September 2013 and nine additional abstracts were identified, of which one paper was added to the study. In total, 413 index/reference comparisons from 139 papers were included in the meta-analysis (Fig 1) [12–181].
Fig 1. PRISMA flowchart.
Study flow depicting search strategy, inclusion/exclusion criteria, and summary of systematic review.
Evaluation of studies
Data were extracted from the selected studies with consensus from two investigators and using Microsoft Excel and Access. Many of the included papers reported results for more than one comparison of index and reference tests (S3 Table). Each comparison was classified by the method of detection of the index and reference tests, and broadly grouped in the following six categories: clinical indicators, antibody, antigen, nucleic acid, viable bacteria, or composite. Each index and reference test was specified in as much detail as was given, including specimen type, sample size, and commercial availability (manufacturer and test kit specified). Also recorded were the number of samples analyzed, and the number of true positives, false positives, true negatives, and false negatives for each comparison. If the absolute value for each cell of the 2x2 contingency table was not reported in the paper, it was calculated from reported sensitivities and specificities where possible. Some cohort studies included additional healthy controls as part of their analysis, and that data was excluded from this analysis. If a case-control study analyzed data separately for different control groups, all control groups were combined in this analysis. Where possible, the same antibody test separately evaluating more than one Ig isotype were combined. The data was included separately when not possible to combine.
Each study was evaluated using the QUADAS-2 tool, which assesses the quality of diagnostic accuracy studies [182]. The QUADAS-2 tool includes four domains: patient selection, index test, reference standard, and flow and timing. All domains are evaluated for risk of bias, and the first three domains are also evaluated for concerns regarding applicability (Table 1). Measures of bias or applicability were assessed as potential covariates to adjust for in the meta-regression analysis.
Table 1. Quality assessment of diagnostic accuracy studies.
| Domain | Criteria | Conclusion | Covariate 2 |
|---|---|---|---|
| Patient selection | A consecutive or random sample of patients was enrolled | If no, then risk of bias is high | High compared to low |
| A case-control design was avoided | If no, then risk of bias is high | High compared to low | |
| The study avoided inappropriate exclusions | If no, then risk of bias is high | High compared to low | |
| The included patients were individuals suspected of having typhoid fever and the diagnostics were used to diagnose the patients | If no, then concern of applicability is high | ||
| Index test | A threshold for the test result was pre-specified | If no, then risk of bias is high | High compared to low |
| The test result was interpreted without knowledge of the reference test | If no, then risk of bias is high | High compared to low | |
| The index test aimed to diagnose acute typhoid fever | If no, then concern of applicability is high | ||
| Reference test | The reference standard was likely to correctly classify the target condition of acute typhoid fever 1 (Recorded as “unclear” if the reference test was not a more commonly used test, the methods were not well described, and it was unclear if the results were interpreted without knowledge of the index test) | If no, then risk of bias is high | High compared to low |
| The reference test was interpreted without knowledge of the index test | If no, then risk of bias is high | High compared to low | |
| The reference standard aimed to diagnose acute typhoid fever | If no, then concern of applicability is high | ||
| Flow and timing | There was an appropriate interval between index test and reference test | If no, then risk of bias is high | High compared to low |
| All patients received a reference standard | If no, then risk of bias is high | High compared to low | |
| All patients received the same reference standard | If no, then risk of bias is high | High compared to low | |
| All patients included in the analysis | If no, then risk of bias is high | High compared to low |
Summary of variables included in the QUADAS-2 tool assessing the quality of diagnostic accuracy studies. The criteria determined a study’s risk of bias or concern of applicability. When the domain-specific criteria were not met, the study had a high risk of bias or concern of applicability with respect to that domain. When the domain-specific criteria were all unclear, the risk of bias or concern of applicability was unclear.
1 The currently available tests to detect typhoid fever are not sufficiently accurate; therefore, this question was problematic.
2 “Unclear” = missing.
Analysis and statistical methods
All index/reference combinations were first summarized by type of reference standard used (Fig 2). Index tests were then categorized into five broad groups, based on the detection of nucleic acid, antigen, antibody, viable bacteria, or clinical features (including composite tests). Diagnostic accuracies of individual index tests were analyzed using meta-analysis when particular index/reference combinations were present in more than five comparisons, to increase the relevance of the conclusions. The majority of meta-analyses were based on combinations using blood culture as the reference test. Statistical analyses were performed using STATA® 11.2 (StataCorp, TX, USA) and R version 3.1.3 (https://www.r-project.org/). Summary results were calculated in the meta-analysis using bivariate random effects binomial regression (STATA command: metandi), and listed in the text as follows: summary sensitivity (Sens) (95% Confidence Interval, CI), summary specificity (Spec) (95% CI), and summary diagnostic odds ratio (DOR) (95% CI) [183,184]. Sources of heterogeneity in the summary estimates were investigated in the meta-regression analysis, also using bivariate random effects binomial regression (STATA command: midas) [185]. Potential covariates were transformed into dichotomous variables and evaluated as appropriate for each index test (Table 1) [182]. The hierarchical summary receiver operating characteristic curves were also generated [186].
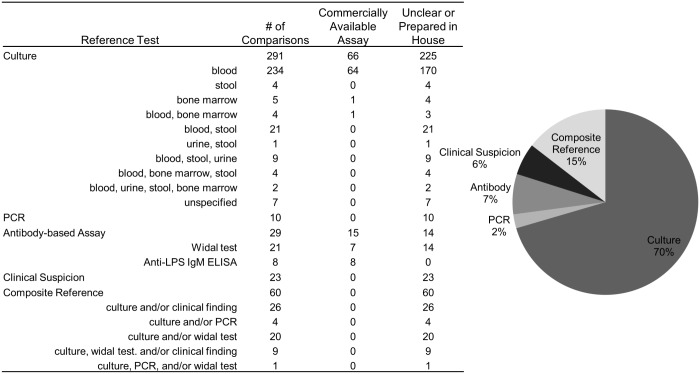
Fig 2. Comparisons by reference test.
Summary of the 139 papers by reference test, including 413 index/reference comparisons. Of the culture reference tests, 80% were blood culture, making up 57% of all reference tests.
Constructed numerical example
To illustrate how the observed sensitivity and specificity of an index test may vary compared to individual reference tests or a CRS, a constructed numerical example was generated with assumptions on disease prevalence and the joint distribution of index and reference tests conditional on disease status. The assessed CRS was fever positive and either test A positive or test B positive, i.e. CRS = ((fever) AND (test A positive) OR (test B positive)). Because test accuracy compared to truth (true presence of infection) is not measurable, and no current reference test is an adequate proxy for truth, assumptions were made regarding the prevalence of disease, as well as true test characteristics of the index test, test A, and test B.
Prevalence of typhoid fever was assumed to be 20%, though many factors vary prevalence estimates, such as participant ages, region, and surveillance method. Studies conducting active community-based surveillance have determined the prevalence of typhoid fever among blood cultures taken to be 2.3%, 2.8%, and 5.0% in five Asian countries, Kenya, and Bangladesh, respectively [187–189]. However, other non-surveillance hospital-based studies have observed the proportion of suspected typhoid patients with positive blood cultures to be 72.8% and 30.4% in Vietnam and sub-Saharan Africa, respectively [86,190]. A prevalence of 20% was selected for illustration purposes. Fever, defined by the World Health Organization (WHO) as ≥37.5°C for ≥3 days, compared to truth was 80% sensitive and 20% specific. Most typhoid fever patients have fever at clinical presentation, but there are many other causes of fever [191]. Additionally, in a recent study, the WHO case definition for suspected typhoid fever demonstrated sensitivity and specificity of 82.6% and 36.3%, respectively [3,169]. Fever is assumed to be independent of all diagnostic tests, conditional on disease. Test A was considered to be blood culture because of its wide appeal, and the sensitivity and specificity of blood culture compared to truth was 50% and 100%, respectively [4,5]. Test B was a hypothetical second test, which was assumed to be 85% sensitive and specific. Test A and test B were independent, conditional on disease. The index test compared to truth was assumed to be 80% sensitive and 90% specific, as the accuracy of a new diagnostic test would probably need to reach these standards [192].
The CRS based on individual tests described above had 74% sensitivity and 88% specificity compared to truth (S1 Text). Two situations were evaluated: (1) independence between index test, test A, and test B, conditional on disease; and (2) dependence between either index test and test A, or index test and test B, conditional on disease. We constructed these situations to demonstrate how a range of conditional dependence may vary the results; however, it is unlikely that technologically the index test would be unrelated to either test A or test B due to related biomarkers or methods for detecting biomarkers. Though the degree of correlation is largely unknown, the minimum and maximum values it can achieve can be determined by the accuracy of the individual tests [193]. Assuming various conditional dependence structures between tests, the following sensitivities and specificities were calculated based on Bayes’ theorem: (1) index test compared to test A; (2) index test compared to test B; and (3) index test compared to CRS (S1 Text). Example R code for computing these values is included in S1 Text as well.
Results
Systematic review
Among the 413 index/reference combinations identified, numerous reference tests were used to evaluate typhoid diagnostic assays. The largest category of reference tests was culture based, accounting for 291 of 413 comparisons, or 70% of all reference tests (Fig 2). Of these, the majority of culture-based reference tests (234) used blood specimens for culture; however, specimen volume, length of culture time, culture media, and sub-culturing techniques varied across studies. Other reference test categories and their corresponding number of comparisons included polymerase chain reaction (PCR)-based assays (10), antibody-based assays (29), clinical suspicion (23), and composite references (60). The composite references identified were variable and many included some clinical assessment along with a laboratory-based diagnostic assay. Only 20% (81/413) of all reference tests were commercially available assays, while other comparisons used reference tests that were developed in house or did not specify how the assay was acquired. Four comparisons were dropped from the meta-analysis because no positives were detected by the reference test (true positives + false negatives = 0). Of the remaining comparisons, 286 had a high risk of bias and 22 had a high concern of applicability in any domain. Other participant characteristics that were initially examined as sources of heterogeneity were later not used in the analysis due to a large proportion of comparisons missing data (176 missing age, 189 missing fever duration, and 294 missing prior antibiotic use).
There were 60 comparisons from 20 studies that evaluated an index test for typhoid detection compared to a CRS. The CRS included the following: culture, PCR, and Widal assay (1); culture and PCR (1); culture and Widal assay (10); and culture and clinical features (9). Exact criteria for a positive reference test result varied from study to study, such as Widal assay titer cutoff, culture specimen and methods, PCR technique, and specifics of clinical features (Fig 2). Reference tests may be combined in a CRS definition by either an “and” or “or” statement. Most of the comparisons evaluated used an “or” statement to combine reference tests (both test A and test B are measured, and a positive test result by either A or B classifies the person as CRS positive).
Meta-analysis
Index tests detecting viable bacteria
From the systematic review, 18 comparisons detected viable bacteria by culture as the index test. This group of assays had considerable variability in methods of culturing, with few studies using automated culture systems (2). The year of publication ranged from 1979 to 2013. The specimens cultured included variable quantities of blood (13), blood clot (1), bone marrow (1), urine (2), and stool (1). Reference tests included cultures using a different specimen (7), such as blood culture compared to bone marrow culture, as well as PCR assays (2), clinical features (6), and composite references combining more than one reference test (3). The only index and reference test combination with at least five comparisons was blood culture compared to bone marrow culture, which gave the following summary results: Sens = 68% (52–81%), Spec = 75% (35–94%), and DOR = 6 (2–27).
Index tests detecting S. typhi antigen
The 66 assays that detected antigen included an assortment of analyte specificity, specimen type, and reference test. The year of publication ranged from 1980 to 2013. The specimens assayed included serum (34), blood culture supernatant or broth (22), and urine (10). The techniques used for antigen detection varied and all but two were in-house preparations. Reference tests included various types of culture (43), antibody-based assays (8), PCR (1), and composite references (14). Of the 66 comparisons, there were no combinations of index and reference tests with five or more comparisons.
Index tests using clinical features or more than one assay
There were three comparisons with index tests that included clinical features and an antibody-based assay, one comparison with only clinical features, one comparison with more than one antibody-based assay, and three comparisons that combined blood culture and an antibody-based assay. These eight comparisons varied in index test attributes as well as reference test attributes. All reference tests included blood culture and some included other reference attributes, such as clinical features (4) and culture of other specimens (1). No combinations of index and reference tests had five or more comparisons.
Index tests detecting S. typhi nucleic acid
Among the 23 comparisons with PCR assay as the index test, 6 were un-nested, 16 were nested, and 1 was a real-time PCR assay. The year of publication ranged from 1993 to 2012. Some of the specimens used were urine (3), stool (1), cultured blood material (2), buffy coat fraction of blood samples (1), and blood (16). Reference tests were most commonly blood culture (16), though some comparisons used another PCR assay (1), clinical features (5), or a composite reference (1). Among the 16 comparisons that evaluated a PCR-based index test to blood culture, the summary results were: Sens = 96% (88–99%), Spec = 87% (69–96%), and DOR = 191 (48–754). (Table 2)
Table 2. Meta-analysis results by study quality.
| Index test | # of comparisons (studies) | Sensitivity (95% CI) | p-value | Specificity (95% CI) | p-value | Diagnostic odds ratio (95% CI) | Any high concern of applicability (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PCR | 16 (16) | 0.96 | (0.88, 0.99) | 0.87 | (0.69, 0.96) | 191 | (48, 754) | 0.06 | ||
| Patient selection bias: Yes | 7 | 0.93 | (0.83, 1.00) | 0.69 | 0.95 | (0.88, 1.00) | 0.04 | |||
| No | 9 | 0.98 | (0.95, 1.00) | 0.76 | (0.52, 1.00) | |||||
| Index test bias: Yes | 1 | 0.4 | (-0.37, 1.00) | 0.02 | 0.93 | (0.67, 1.00) | 0.12 | |||
| No | 11 | 0.96 | (0.91, 1.00) | 0.92 | (0.82, 1.00) | |||||
| Reference test bias: Yes | 1 | 0.99 | (0.95, 1.00) | 0.001 | 0.59 | (-0.37, 1.00) | 0.71 | |||
| No | 11 | 0.97 | (0.92, 1.00) | 0.82 | (0.64, 1.00) | |||||
| Patient flow bias: Yes | 2 | 0.83 | (0.43, 1.00) | 0.49 | 0.94 | (0.75, 1.00) | 0.11 | |||
| No | 14 | 0.97 | (0.94, 1.00) | 0.86 | (0.72, 1.00) | |||||
| Anti-LPS assay | 33 (20) | 0.84 | (0.78, 0.89) | 0.89 | (0.83, 0.93) | 42 | (23, 75) | 0.06 | ||
| Patient selection bias: Yes | 16 | 0.91 | (0.87, 0.95) | 0.25 | 0.91 | (0.86, 0.96) | 0.96 | |||
| No | 10 | 0.77 | (0.68, 0.86) | 0.74 | (0.61, 0.87) | |||||
| Index test bias: Yes | 12 | 0.89 | (0.84, 0.95) | 0.28 | 0.92 | (0.85, 0.98) | 0.17 | |||
| No | 18 | 0.76 | (0.69, 0.84) | 0.89 | (0.82, 0.95) | |||||
| Reference test bias: Yes | 0 | |||||||||
| No | 16 | 0.83 | (0.75, 0.89) | 0.81 | (0.71, 0.88) | |||||
| Patient flow bias: Yes | 6 | 0.89 | (0.79, 0.98) | 0.37 | 0.89 | (0.78, 1.00) | 0.34 | |||
| No | 27 | 0.83 | (0.77, 0.89) | 0.89 | (0.83, 0.94) | |||||
| TUBEX assay | 12 (12) | 0.75 | (0.59, 0.85) | 0.88 | (0.84, 0.92) | 22 | (10, 47) | 0.17 | ||
| Patient selection bias: Yes | 6 | 0.77 | (0.60, 0.94) | 0.94 | 0.91 | (0.86, 0.96) | 0.01 | |||
| No | 6 | 0.71 | (0.51, 0.91) | 0.86 | (0.81, 0.92) | |||||
| Index test bias: Yes | 3 | 0.55 | (0.22, 0.88) | 0.13 | 0.95 | (0.91, 0.98) | 0.07 | |||
| No | 9 | 0.79 | (0.67, 0.91) | 0.85 | (0.80, 0.89) | |||||
| Reference test bias: Yes | 0 | |||||||||
| No | 12 | 0.75 | (0.59, 0.85) | 0.88 | (0.84, 0.92) | |||||
| Patient flow bias: Yes | 5 | 0.67 | (0.44, 0.90) | 0.24 | 0.9 | (0.85, 0.96) | 0.01 | |||
| No | 7 | 0.79 | (0.64, 0.93) | 0.87 | (0.82, 0.92) | |||||
| Anti-S. typhi assay | 13 (9) | 0.75 | (0.65, 0.82) | 0.83 | (0.76, 0.89) | 15 | (8, 27) | 0 | ||
| Patient selection bias: Yes | 7 | 0.79 | (0.68, 0.89) | 0.79 | 0.8 | (0.70, 0.90) | 0.02 | |||
| No | 5 | 0.68 | (0.53, 0.83) | 0.86 | (0.78, 0.95) | |||||
| Index test bias: Yes | 3 | 0.89 | (0.82, 0.97) | 0.7 | 0.77 | (0.61, 0.94) | 0.05 | |||
| No | 10 | 0.69 | (0.61, 0.77) | 0.85 | (0.78, 0.92) | |||||
| Reference test bias: Yes | 0 | |||||||||
| No | 6 | 0.67 | (0.62, 0.71) | 0.87 | (0.79, 0.92) | |||||
| Patient flow bias: Yes | 3 | 0.89 | (0.82, 0.97) | 0.7 | 0.77 | (0.61, 0.94) | 0.05 | |||
| No | 10 | 0.69 | (0.61, 0.77) | 0.85 | (0.78, 0.92) | |||||
| Typhidot assay | 20 (17) | 0.84 | (0.73, 0.92) | 0.8 | (0.67, 0.89) | 22 | (9, 57) | 0.05 | ||
| Patient selection bias: Yes | 9 | 0.84 | (0.68, 0.99) | 0.57 | 0.87 | (0.75, 0.98) | 0.96 | |||
| No | 9 | 0.85 | (0.71, 0.99) | 0.79 | (0.63, 0.95) | |||||
| Index test bias: Yes | 2 | 0.5 | (-0.02, 1.00) | 0.14 | 0.79 | (0.47, 1.00) | 1 | |||
| No | 13 | 0.84 | (0.72, 0.95) | 0.8 | (0.68, 0.92) | |||||
| Reference test bias: Yes | 0 | |||||||||
| No | 14 | 0.78 | (0.62, 0.89) | 0.85 | (0.71, 0.93) | |||||
| Patient flow bias: Yes | 5 | 0.63 | (0.34, 0.91) | 0.02 | 0.88 | (0.74, 1.00) | 0.58 | |||
| No | 15 | 0.89 | (0.81, 0.96) | 0.77 | (0.63, 0.91) | |||||
| Widal assay (any antigen) | 65 (51) | 0.69 | (0.61, 0.75) | 0.83 | (0.77, 0.88) | 11 | (7, 17) | 0.05 | ||
| Patient selection bias: 1 Yes | 29 | |||||||||
| No | 31 | |||||||||
| Index test bias: Yes | 19 | 0.68 | (0.57, 0.79) | 0.24 | 0.88 | (0.81, 0.95) | 0.21 | |||
| No | 34 | 0.65 | (0.57, 0.74) | 0.79 | (0.71, 0.87) | |||||
| Reference test bias: Yes | 0 | |||||||||
| No | 42 | 0.66 | (0.57, 0.73) | 0.84 | (0.76, 0.90) | |||||
| Patient flow bias: Yes | 13 | 0.73 | (0.59, 0.87) | 0.41 | 0.8 | (0.67, 0.93) | 0.04 | |||
| No | 52 | 0.68 | (0.60, 0.75) | 0.84 | (0.78, 0.90) | |||||
Summary diagnostic accuracies of index tests with five or more comparisons and blood culture as the reference test. Meta-analysis performed using bivariate random effects binomial regression.
1 Could not be determined.
Index tests detecting antibodies
Antibody-based index tests were the most abundant, with 293 comparisons, of which 133 were Widal assays. The non-Widal assays ranged in publication year from 1979 to 2013. Analyte specificity varied and included the following antigen-specific antibody responses: flagellin (2), lipopolysaccharide (LPS, 43), LPS and flagellin (1), membrane preparation (3), outer membrane protein (OMP, 50), porins (1), whole S. typhi (20), unspecified Salmonella (2), O-9 analyte (17), Vi antigen (7), O and/or H antigen (10), O, H, and Vi antigen (1), and an unspecified analyte (3). Of the assays using the OMP analyte, 41 were the commercially available kit Typhidot (Reszon Diagnostics International, Malaysia). Additionally, all of the 17 assays using the O-9 analyte were the commercially available kit TUBEX® TF (IDL, Sweden). Specimens used were most often serum, with two assays using lymphocyte culture supernatant, two using blood, one using plasma, and two using saliva. Immunoglobulin (Ig) isotypes evaluated included IgA (7), IgM (53), IgG (28), IgM and IgG together (30), and IgM, IgG, and IgA together (3); 39 studies did not specify the Ig isotype evaluated. Reference tests included PCR (3), Widal assays (10), culture (122), clinical features (1), and composite references (24).
Investigating only comparisons that used blood culture as a reference test, four index analytes were evaluated with five or more comparisons: anti-LPS, anti-S. typhi, TUBEX, and Typhidot. Anti-LPS assays compared to blood culture (n = 33) had the following summary results: Sens = 84% (78–89%), Spec = 89% (83–93%), DOR = 42 (23–75) (Table 2). TUBEX assays compared to blood culture (n = 12) had the following summary results: Sens = 75% (59–85%), Spec = 88% (84–92%), DOR = 22 (10–47) (Table 2). Anti-S. typhi assays compared to blood culture (n = 13) had the following summary results: Sens = 75% (65–82%), Spec = 83% (76–89%), DOR = 15 (8–27) (Table 2). Typhidot assays compared to blood culture (n = 20) had the following summary results: Sens = 84% (73–92%), Spec = 80% (67–89%), DOR = 22 (9–57). (Table 2)
The Widal assays ranged in publication year from 1977 to 2013. Among these assays, the analytes assessed included O antigen alone (58), H antigen alone (31), O and H antigens together (32), and unspecified antigen (12). Titer cutoffs of the Widal assays also varied by study, ranging from 1:20 to 1:640, with many unspecified (25). Reference tests included PCR (3), antibody-based assays (11), clinical features (11), culture (94), and composite references (14). Restricting analysis to comparisons with blood culture as the reference test, the Widal assay summary results were (n = 65): Sens = 69% (61–75%), Spec = 83% (77–88%), DOR = 11 (7–17). (Table 2)
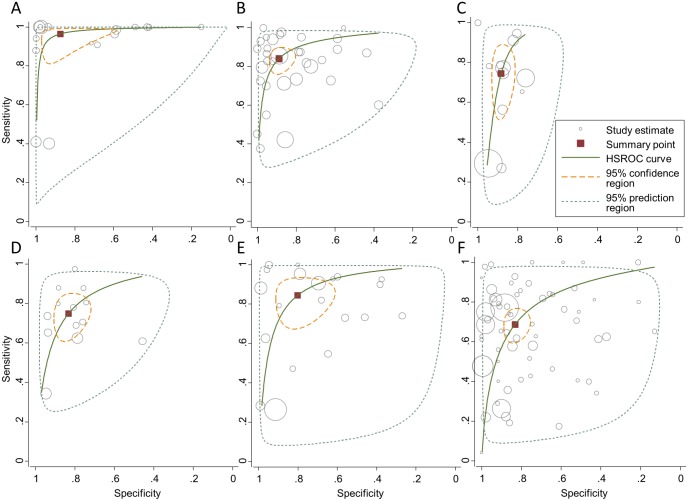
A graphical illustration of sensitivities (y-axis) and specificities (x-axis) corresponding to the above comparisons included in the meta-analysis are presented in Fig 3, including the 95% confidence and prediction regions, and the hierarchical summary receiver operating characteristic curves. Additionally, forest plots of the meta-analysis results are provided in supplementary material (S2 Text). When assessing whether study quality had an effect on observed diagnostic accuracy, studies with patient selection bias had a significantly different specificity compared to studies without this bias for PCR, TUBEX, and anti-S. typhi assays, though the direction of the effect was not consistent across assays. Patient flow bias significantly affected specificity for TUBEX, anti-S. typhi, and Widal assays and sensitivity for Typhidot assays. Index test bias significantly affected sensitivity for PCR and specificity for anti-S. typhi assays. Finally, risk of reference test bias was unclear for many studies, based on the defined criteria, hampering interpretation of observed effect on diagnostic accuracy. (Table 2)
Fig 3. Meta-analysis results.
Graphical illustration of sensitivities (y-axis) and specificities (x-axis) corresponding to comparisons included in the meta-analysis: PCR-based assays (A), anti-LPS assays (B), TUBEX® assays (C), anti-S. typhi assays (D), Typhidot assays (E), Widal assays (F). Meta-analysis was performed using bivariate random effects binomial regression (STATA command: metandi). Sizes of individual study estimates (grey circle) represent sample size. Summary point (red square), hierarchical summary receiver operating characteristic curves (green line), 95% confidence regions (yellow dashed line), and 95% prediction regions (grey dashed line) are depicted.
Constructed numerical example
At a prevalence of 20% with a 50% sensitive and 100% specific test A, when the index test and test A are conditionally independent, the index test compared to test A provides an unbiased estimate of the true sensitivity of the index test, but underestimates specificity. When the index test is conditionally dependent on test A among subjects declared as “diseased” with a correlation of 0.4, the index test compared to test A appears to overestimate sensitivity considerably. Specificity is still underestimated, but to a lesser extent compared to the conditionally independent scenario. (Table 3)
Table 3. Constructed numerical example.
| All tests independent conditional on disease status | Index test conditionally dependent on test A among diseased (correlation = 0.4) and independent of test B | Index test conditionally dependent on test B among both diseased and non-diseased (correlation = 0.4) and independent of test A | Index test conditionally dependent on test B among both diseased and non-diseased (correlation = 0.7) and independent of test A | |||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| Index test compared to test A | 80% | 82.2% | 96.0% | 84.0% | NA | NA | NA | NA |
| Index test compared to test B | 51.0% | 87.0% | NA | NA | 66.8% | 93.5% | 78.6% | 98.3% |
| Index test compared to CRS | 52.5% | 85.2% | 53.2% | 85.4% | 65.6% | 89.4% | 75.4% | 92.6% |
Assumed sensitivity and specificity of the three tests: index test, 80% and 90%; test A, 50% and 100%; test B, 85% and 85%. Comparing the index test to a CRS = (fever) AND ((test A positive) OR (test B positive)). Fever, test A, and test B are independent conditional on disease status. Index test is independent of fever conditional on disease status.
For test B with 85% sensitivity and 85% specificity, when the index test and test B are conditionally independent, the index test compared to test B underestimates sensitivity considerably. Specificity is only slightly underestimated. Note that the underestimation of sensitivity using test B as the reference results partially from the imperfect specificity of test B and the good sensitivity of the index test. A portion of subjects declared as diseased by test B are actually non-diseased and will tend to be declared as “non-diseased” by the index test, making the index test appear less sensitive than it actually is. Increasing the correlation between the index test and test B to 0.4 among diseased and non-diseased, the underestimation in sensitivity is decreased while specificity is slightly overestimated. When the correlation is increased to 0.7, the sensitivity estimate of the index test using test B as the reference is closer to the true sensitivity of the index test, with overestimation in specificity further increased.
When all tests are conditionally independent, results comparing the index test to the CRS appear similar to those comparing the index test to test B, with a slightly larger sensitivity estimate and smaller specificity estimate. When the index test is conditionally dependent on test A among diseased with a correlation of 0.4 and conditionally independent of test B, a slight increase in sensitivity and decrease in specificity are observed. If the index test is instead conditionally dependent on test B with a correlation of 0.4 among diseased and non-diseased and conditionally independent of test A, the index test compared to the CRS has an observed sensitivity closer to that using test B as the reference, with the observed specificity approximating true specificity. When the correlation is increased to 0.7, the index test compared to the CRS will slightly underestimate sensitivity and approximate the true specificity. This observed sensitivity and specificity are closest to truth among all comparisons, in the sense that the absolute bias is less than 5% for both sensitivity and specificity.
In S4 Table, we also explored results varying disease prevalence from 5% to 70%. Disease prevalence alters the interpretation of the observed performance of the index test relative to the reference test. There are some general patterns in the data: the specificity of the index test differs from truth with less magnitude for lower prevalence values, for most combinations of reference test and its correlation with index test explored; and, the sensitivity of the index test differs from truth with less magnitude at higher prevalence values, for most combinations of reference test and its correlation with index test explored. For some combinations of reference test and its correlation with index test, there are, however, switches in the directionality of the differences as prevalence changes (see S4 Table). Moreover, when disease prevalence is low (5%), using a reference test with imperfect sensitivity (50%) but perfect specificity gives an observed sensitivity and specificity fairly close to their true value when the index test is independent of the reference test conditional on disease status, not necessitating the use of a CRS. However, for larger prevalence, a reference test with low sensitivity can lead to severe bias in observed specificity, which may be alleviated with the use of a CRS. Interested readers can use the R code presented in S1 Text to explore the impact of parameter settings on the comparison between observed and true performance of the index test.
Discussion
Based on our systematic review, numerous reference tests have been used to evaluate new typhoid diagnostics, with blood culture being the most commonly used test. Most studies included fever as an inclusion criterion, and further classified patients as having typhoid fever if fever reached a duration and/or temperature cutoff, which varied by study. A clinical criterion such as fever, in combination with another reference test, is a composite reference, though often it was not shown as such. Additionally, there were many studies that clearly stated their use of a composite reference, demonstrating willingness to use and general acceptance of composite reference standards by typhoid researchers. In the 21 studies that stated use of a CRS as the reference method, however, no two studies employed an identical CRS. The individual tests included in the CRS varied and were not well standardized, causing the resulting CRS to also vary and muddle comparisons across studies.
To evaluate the diagnostic accuracy of common index tests, a single reference test was used for comparison. Blood culture, the most common reference test used because of its perfect specificity, has unacceptably low sensitivity for many populations. Culturing more blood may improve the sensitivity of the test, as one setting demonstrated a sensitivity of 87.5% when using automated blood culture with 10 ml of blood [194]. Another study among children older than 60 days observed that the proportion of blood culture positives increased with each ml of blood cultured [195]. Though culturing 10 ml of blood may improve the diagnostic accuracy of blood culture, it may not be a feasible volume to obtain from patients, especially children, who are most impacted by the disease.
The meta-analysis provided a systematic process without necessitating assumptions to evaluate the accuracy of common index tests; however, the accuracy measures determined in the meta-analysis are still biased by the inaccuracy of the reference test. Antibody-based assays may be informative but standardization is essential. The available data are too varied in assay methods, Ig isotypes examined, and interpretation of results to form strong conclusions about a particular test’s diagnostic accuracy. More extensive evaluation of specific assays with few comparison studies may highlight promising tools. Additionally, there is the issue of regional variation in antibody responses. Healthy populations in regions with highly endemic typhoid fever have greater disease-specific antibody titers compared to regions with low or little typhoid fever, requiring diagnostic titer cutoffs to vary by region [7,194]. Based on our findings, PCR demonstrates promising results so far; however, fewer studies have been performed and these methods are still being modified and validated. Finally, study quality significantly impacted measures of sensitivity and specificity, though not to the same degree across assays. Statistical methods accounting for imperfect reference tests, such as latent class analysis, may help inform the selection of the CRS components, as well as its validation [9]. Latent class analysis offers additional benefits to dealing with an imperfect reference test compared to a CRS; however, the straightforward nature of calculating a CRS is preferable for routine practice.
In the constructed numerical example we considered combining blood culture, which has low sensitivity and perfect specificity, along with fever and another test with good sensitivity and specificity to generate a CRS that has improved sensitivity over blood culture at the cost of some specificity. Though actual performance of a CRS cannot be determined by this method, it explores how a CRS may compare to standalone reference tests in the evaluation of a new index test. We observed that the performance of the index test compared to the CRS depends on the correlation between the index test and the components of the CRS, which should be taken into account when interpreting the observed sensitivity and specificity of an index test relative to a CRS. Additionally, a CRS using an “or” statement will increase the reference test positives (true positives and false negatives) compared to using a single component of the CRS. A small increase in reference test positives will have a greater effect on observed sensitivity than specificity when the absolute number of reference test positives is much less than the number of reference test negatives, such as in low-prevalence studies. Additionally, high prevalence settings may benefit more from use of a CRS instead of a single imperfect test as reference.
The goal of this work is to start the conversation about the best components for a typhoid CRS and encourage agreement and adoption of a standardized composite reference for evaluating new typhoid diagnostics by all investigators. A standardized CRS will enable rigorous comparison of diagnostic accuracy data across studies, which is often difficult because of varying study designs and reference standards. We acknowledge the difficulties in standardizing a composite reference within the context of scarce resources. Strong advocacy by typhoid opinion leaders will be required to translate recommendations into consensus, and consensus into consistent action by the community. In order to validate the diagnostic accuracy of a new test, results from multiple studies in diverse regions will be needed. Additionally, use of a CRS would lead to improved confidence in prevalence estimates, which may help guide typhoid vaccination efforts. While a new CRS for typhoid may still be imperfect compared to diagnostic truth, there is much to gain from the adoption of a standardized composite reference.
In addition to the findings above, which are based on reviewing the literature, conducting a meta-analysis, and exploring a constructed numerical example, we think there are a number of considerations that are also important. First, one agreed upon composite reference should be adopted by all investigators to enable the leveraging of multiple studies in rigorous meta-analysis. That CRS should not include new technologies that are candidates for evaluation by the CRS, as this biases accuracy measures [10]. For example, newer technologies such as PCR may demonstrate superior diagnostic accuracy to the tests included in the CRS; however, studies evaluating these technologies are few, and their inclusion in the current CRS would prevent further validation and potential adoption in the future.
To achieve reproducibility, individual reference test components in the CRS need to be standardized. Clinical signs used should match the WHO definition of suspected typhoid fever: ≥37.5°C for ≥3 days. This feature may be considered a screening criteria or a component of the CRS using an AND rule (fever positive AND positive based on some algorithm of other tests), depending on study design. The important issue is that CRS positive cases also meet the WHO definition for suspected typhoid fever. Also, if blood culture is used, it is necessary to specify blood volume and media or culture system, at a minimum. Including blood culture is recommended because of its widespread use, 100% specificity, and ability to isolate the organism for further investigation, such as drug resistance. Additionally, a commercially available antibody-based assay, which is technologically feasible in many settings, is more likely to generate reproducible results through use of a standardized product and protocol, though issues with performance variability can still arise. Only two commercially available antibody-based assays had been evaluated in a sufficient number of studies to be summarized in our meta-analysis, TUBEX TF and Typhidot. In a recent meta-analysis comparing the same two tests, the TUBEX assay showed comparable summary sensitivity and specificity as this study (69% and 88%, respectively), while sufficient studies were not available to evaluate Typhidot by the same metrics [196].Of note: the current manufacturer of TyphiDot (Reszon Diagnostics International Sdn. Bhd., Selangor, Malaysia) has only been making this test since ca. 2010, and many of the studies that established the diagnostic accuracy of this product were performed with the tests made by the previous manufacturer (Malaysian Bio-Diagnostic Research, Sdn. Bhd, Bangi, Malaysia). To our knowledge, there is no published equivalence data that establishes continuity in performance after the change in manufacturing site, which may warrant additional evaluation studies.
Finally, studies should use a prospective cohort design and clearly specify inclusion and exclusion criteria. Patient selection bias significantly impacted the observed specificity of several evaluated index tests (PCR, TUBEX, anti-S. typhi assays). Covariates such as age, fever duration, and prior antibiotic use may be important to consider, though there were insufficient data to evaluate these variables in this study. Ultimately, the proposed CRS should be viewed as a dynamic diagnostic tool. Evaluation and revision as newer technologies are validated and demonstrate improved diagnostic accuracy is encouraged, with the ultimate goal of identifying a robust gold standard.
Strengths of this study include its thorough review of all available published literature evaluating two diagnostic tests for the detection of typhoid fever. In addition, rigorous statistical techniques were utilized to summarize the diagnostic accuracy of similar comparisons across studies. Limitations in this work arose from the diversity of diagnostic tests used by different investigators, and how those tests were performed. As a result, many comparisons could not be included in the meta-analysis or had to be summarized in broader groups. Incomplete description of methods and confounding factors also limited our ability to assess heterogeneity across studies. Finally, this analysis was based on published literature only; however, publication bias was not detected in the meta-analysis, as assessed by Deeks’ funnel plot asymmetry test (p>0.05) [197].
Better diagnostic tests to detect typhoid fever are needed to improve disease burden estimates and potentially accelerate the adoption of new typhoid vaccines where they are needed most [198,199]. For this to happen, standardization, consensus, and broad adoption of a single gold standard based on a composite reference are required so that new technologies can be confidently judged for their diagnostic accuracy. Additionally, uniform reporting of diagnostic test accuracy study results conforming to the STARD guidelines and QUADAS-2 tool should remain the standard to enable facile comparison of studies [182,200]. Necessary next steps include a broad discussion by stakeholders of the merits of a CRS to build consensus, and selection of the CRS components, followed by validation of the CRS in a well-designed clinical study. The work presented in this paper is an important initial step, and combined with statistical analyses such as latent class analysis, may further increase confidence in using a CRS. Ultimately, using a CRS as an improved gold standard for typhoid fever will contribute to an increased awareness of the real global cost of the typhoid epidemic.
Supporting Information
(DOC)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was made possible by a grant from the Bill and Melinda Gates Foundation (grant #OPP1063918, www.gatesfoundation.org). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Buckle G, Walker C, Black R (2012) Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2: 010401 10.7189/jogh.02.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82: 346–353. [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (2003) The diagnosis, treatment, and prevention of typhoid fever.
- 4. Gilman RH, Terminel M, Levine MM, Hernandez-Mendoza P, Hornick RB (1975) Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet 1: 1211–1213. [DOI] [PubMed] [Google Scholar]
- 5. Wain J, Pham VB, Ha V, Nguyen NM, To SD, Walsh AL, et al. (2001) Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J Clin Microbiol 39: 1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker S, Favorov M, Dougan G (2010) Searching for the elusive typhoid diagnostic. BMC Infect Dis 10: 45 10.1186/1471-2334-10-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parry CM, Wijedoru L, Arjyal A, Baker S (2011) The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther 9: 711–725. 10.1586/eri.11.47 [DOI] [PubMed] [Google Scholar]
- 8. Whiting PF, Rutjes AW, Westwood ME, Mallett S (2013) A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol 66: 1093–1104. 10.1016/j.jclinepi.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 9. Reitsma JB, Rutjes AW, Khan KS, Coomarasamy A, Bossuyt PM (2009) A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 62: 797–806. 10.1016/j.jclinepi.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 10. Alonzo TA, Pepe MS (1999) Using a combination of reference tests to assess the accuracy of a new diagnostic test. Stat Med 18: 2987–3003. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdel Wahab MF, Haseeb AN, Hamdy HS, Awadalla YA (1996) Comparative study between paratyphoid A and typhoid fever cases. J Egypt Public Health Assoc 71: 539–551. [PubMed] [Google Scholar]
- 13. Abdoel TH, Pastoor R, Smits HL, Hatta M (2007) Laboratory evaluation of a simple and rapid latex agglutination assay for the serodiagnosis of typhoid fever. Trans R Soc Trop Med Hyg 101: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 14. Abraham G, Teklu B, Gedebu M, Selassie GH, Azene G (1981) Diagnostic value of the Widal test. Trop Geogr Med 33: 329–333. [PubMed] [Google Scholar]
- 15. Akoh JA (1991) Relative sensitivity of blood and bone marrow cultures in typhoid fever. Trop Doct 21: 174–176. [DOI] [PubMed] [Google Scholar]
- 16. Ali A, Haque A, Sarwar Y, Mohsin M, Bashir S, Tariq A (2009) Multiplex PCR for differential diagnosis of emerging typhoidal pathogens directly from blood samples. Epidemiol Infect 137: 102–107. 10.1017/S0950268808000654 [DOI] [PubMed] [Google Scholar]
- 17. Almogren A, Shakoor Z, Adam MH, Gadelrab MO, Musa HA (2012) Modifications influencing Widal test reactivity in a novel microplate assay. Pol J Microbiol 61: 137–142. [PubMed] [Google Scholar]
- 18. Ambati SR, Nath G, Das BK (2007) Diagnosis of typhoid fever by polymerase chain reaction. Indian J Pediatr 74: 909–913. [DOI] [PubMed] [Google Scholar]
- 19. Andrews JR, Prajapati KG, Eypper E, Shrestha P, Shakya M, Pathak KR, et al. (2013) Evaluation of an electricity-free, culture-based approach for detecting typhoidal Salmonella bacteremia during enteric fever in a high burden, resource-limited setting. PLoS Negl Trop Dis 7: e2292 10.1371/journal.pntd.0002292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anusha R, Ganesh R, Lalitha J (2011) Comparison of a rapid commercial test, Enterocheck WB((R)), with automated blood culture for diagnosis of typhoid fever. Ann Trop Paediatr 31: 231–234. 10.1179/1465328111Y.0000000030 [DOI] [PubMed] [Google Scholar]
- 21. Aquino RL, Lansang MA, Quimpo VS, Sombrero LT, Saniel MC (1991) Evaluation of a single Widal test in the diagnosis of enteric fever. Southeast Asian J Trop Med Public Health 22: 375–379. [PubMed] [Google Scholar]
- 22. Aziah I, Ravichandran M, Ismail A (2007) Amplification of ST50 gene using dry-reagent-based polymerase chain reaction for the detection of Salmonella typhi. Diagn Microbiol Infect Dis 59: 373–377. [DOI] [PubMed] [Google Scholar]
- 23. Bakr WM, El Attar LA, Ashour MS, El Toukhy AM (2011) The dilemma of widal test—which brand to use? a study of four different widal brands: a cross sectional comparative study. Ann Clin Microbiol Antimicrob 10: 7 10.1186/1476-0711-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Banchuin N, Appassakij H, Sarasombath S, Manatsathit S, Rungpitarangsi B, Komolpit P, et al. (1987) Detection of Salmonella typhi protein antigen in serum and urine: a value for diagnosis of typhoid fever in an endemic area. Asian Pac J Allergy Immunol 5: 155–159. [PubMed] [Google Scholar]
- 25. Begum Z, Hossain MA, Musa AK, Shamsuzzaman AK, Mahmud MC, Ahsan MM, et al. (2009) Comparison between DOT EIA IgM and Widal Test as early diagnosis of typhoid fever. Mymensingh Med J 18: 13–17. [PubMed] [Google Scholar]
- 26. Beig FK, Ahmad F, Ekram M, Shukla I (2010) Typhidot M and Diazo test vis-a-vis blood culture and Widal test in the early diagnosis of typhoid fever in children in a resource poor setting. Braz J Infect Dis 14: 589–593. [DOI] [PubMed] [Google Scholar]
- 27. Bhutta ZA, Mansurali N (1999) Rapid serologic diagnosis of pediatric typhoid fever in an endemic area: a prospective comparative evaluation of two dot-enzyme immunoassays and the Widal test. Am J Trop Med Hyg 61: 654–657. [DOI] [PubMed] [Google Scholar]
- 28. Buck RL, Escamilla J, Sangalang RP, Cabanban AB, Santiago LT, Ranoa CP, et al. (1987) Diagnostic value of a single, pre-treatment Widal test in suspected enteric fever cases in the Philippines. Trans R Soc Trop Med Hyg 81: 871–873. [DOI] [PubMed] [Google Scholar]
- 29. Cardona-Castro N, Gotuzzo E, Rodriguez M, Guerra H (2000) Clinical application of a dot blot test for diagnosis of enteric fever due to Salmonella enterica serovar typhi in patients with typhoid fever from Colombia and Peru. Clin Diagn Lab Immunol 7: 312–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casanueva V, Cid X, Cavicchioli G, Oelker M, Cofre J, Chiang MT (1992) Serum adenosine deaminase in the early diagnosis of typhoid fever. Pediatr Infect Dis J 11: 828–830. [DOI] [PubMed] [Google Scholar]
- 31. Castonguay-Vanier J, Davong V, Bouthasavong L, Sengdetka D, Simmalavong M, Seupsavith A, et al. (2013) Evaluation of a simple blood culture amplification and antigen detection method for diagnosis of Salmonella enterica serovar typhi bacteremia. J Clin Microbiol 51: 142–148. 10.1128/JCM.02360-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaicumpa W, Thin-Inta W, Khusmith S, Tapchaisri P, Echeverria P, Kalambaheti T, et al. (1988) Detection with monoclonal antibody of Salmonella typhi antigen 9 in specimens from patients. J Clin Microbiol 26: 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chart H, Cheesbrough JS, Waghorn DJ (2000) The serodiagnosis of infection with Salmonella typhi. J Clin Pathol 53: 851–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaudhry R, Laxmi BV, Nisar N, Ray K, Kumar D (1997) Standardisation of polymerase chain reaction for the detection of Salmonella typhi in typhoid fever. J Clin Pathol 50: 437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheesbrough JS, Taxman BC, Green SDR, Mewa FI, Numbi A (1997) Clinical definition for invasive Salmonella infection in African children. Pediatr Infect Dis J 16: 277–283. [DOI] [PubMed] [Google Scholar]
- 36. Chew SK, Cruz MS, Lim YS, Monteiro EH (1992) Diagnostic value of the Widal test for typhoid fever in Singapore. J Trop Med Hyg 95: 288–291. [PubMed] [Google Scholar]
- 37. Chitkara YK, Urquhart AE (1979) Fluorescent Vi antibody test in the screening of typhoid carriers. Am J Clin Pathol 72: 87–89. [DOI] [PubMed] [Google Scholar]
- 38. Choo KE, Razif AR, Oppenheimer SJ, Ariffin WA, Lau J, Abraham T (1993) Usefulness of the Widal test in diagnosing childhood typhoid fever in endemic areas. J Paediatr Child Health 29: 36–39. [DOI] [PubMed] [Google Scholar]
- 39. Choo KE, Oppenheimer SJ, Ismail AB, Ong KH (1994) Rapid serodiagnosis of typhoid fever by dot enzyme immunoassay in an endemic area. Clin Infect Dis 19: 172–176. [DOI] [PubMed] [Google Scholar]
- 40. Choo KE, Davis TM, Ismail A, Tuan Ibrahim TA, Ghazali WN (1999) Rapid and reliable serological diagnosis of enteric fever: comparative sensitivity and specificity of Typhidot and Typhidot-M tests in febrile Malaysian children. Acta Trop 72: 175–183. [DOI] [PubMed] [Google Scholar]
- 41. Choo KE, Davis TM, Henry RL, Chan LP (2001) Serum C-reactive protein concentrations in Malaysian children with enteric fever. J Trop Pediatr 47: 211–214. [DOI] [PubMed] [Google Scholar]
- 42. Chow CB, Wang PS, Cheung MW, Yan WW, Leung NK (1987) Diagnostic value of the Widal test in childhood typhoid fever. Pediatr Infect Dis J 6: 914–917. [DOI] [PubMed] [Google Scholar]
- 43. Coovadia YM, Singh V, Bhana RH, Moodley N (1986) Comparison of passive haemagglutination test with Widal agglutination test for serological diagnosis of typhoid fever in an endemic area. J Clin Pathol 39: 680–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Das S, Rajendran K, Dutta P, Saha TK, Dutta S (2013) Validation of a new serology-based dipstick test for rapid diagnosis of typhoid fever. Diagn Microbiol Infect Dis 76: 5–9. 10.1016/j.diagmicrobio.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 45. Devrim I, Ergunay K, Kara A, Tezer H, Yigitkanl I, Cengiz AB, et al. (2008) The comparison of cultures, widal agglutination test and polymerase chain reaction as a diagnostic tool in typhoid fever. Cent Eur J Med 3: 470–474. [Google Scholar]
- 46. Dong B, Galindo CM, Shin E, Acosta CJ, Page AL, Wang M, et al. (2007) Optimizing typhoid fever case definitions by combining serological tests in a large population study in Hechi City, China. Epidemiol Infect 135: 1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duthie R, French GL (1990) Comparison of methods for the diagnosis of typhoid fever. J Clin Pathol 43: 863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dutta S, Sur D, Manna B, Sen B, Deb AK, Deen JL, et al. (2006) Evaluation of new-generation serologic tests for the diagnosis of typhoid fever: data from a community-based surveillance in Calcutta, India. Diagn Microbiol Infect Dis 56: 359–365. [DOI] [PubMed] [Google Scholar]
- 49. el Fattah MM, Hassan EM, Shaheen H, Awad RA, Girgis N (1991) Evaluation of enzyme-linked immunosorbent assay for detection of Salmonella typhi antigen and antibodies. J Egypt Public Health Assoc 66: 145–157. [PubMed] [Google Scholar]
- 50. Fadeel MA, Crump JA, Mahoney FJ, Nakhla IA, Mansour AM, Reyad B, et al. (2004) Rapid diagnosis of typhoid fever by enzyme-linked immunosorbent assay detection of Salmonella serotype typhi antigens in urine. Am J Trop Med Hyg 70: 323–328. [PubMed] [Google Scholar]
- 51. Fadeel MA, House BL, Wasfy MM, Klena JD, Habashy EE, Said MM, et al. (2011) Evaluation of a newly developed ELISA against Widal, TUBEX-TF and Typhidot for typhoid fever surveillance. J Infect Dev Ctries 5: 169–175. Article. [DOI] [PubMed] [Google Scholar]
- 52. Gasem MH, Smits HL, Goris MG, Dolmans WM (2002) Evaluation of a simple and rapid dipstick assay for the diagnosis of typhoid fever in Indonesia. J Med Microbiol 51: 173–177. [DOI] [PubMed] [Google Scholar]
- 53. Gopalakrishnan V, Sekhar WY, Soo EH, Vinsent RA, Devi S (2002) Typhoid fever in Kuala Lumpur and a comparative evaluation of two commercial diagnostic kits for the detection of antibodies to Salmonella typhi. Singapore Med J 43: 354–358. [PubMed] [Google Scholar]
- 54. Guerra-Caceres JG, Gotuzzo-Herencia E, Crosby-Dagnino E, Miro-Quesada M, Carrillo-Parodi C (1979) Diagnostic value of bone marrow culture in typhoid fever. Trans R Soc Trop Med Hyg 73: 680–683. [DOI] [PubMed] [Google Scholar]
- 55. Gupta A, My Thanh NT, Olsen SJ, Sivapalasingam S, My Trinh TT, Phuong Lan NT, et al. (2006) Evaluation of community-based serologic screening for identification of chronic Salmonella typhi carriers in Vietnam. Int J Infect Dis 10: 309–314. [DOI] [PubMed] [Google Scholar]
- 56. Gupta AK, Rao KM (1981) A simple bactericidal antibody test for sero-diagnosis of typhoid fever. J Immunol Methods 42: 121–125. [DOI] [PubMed] [Google Scholar]
- 57. Handojo I, Dewi R (2000) The diagnostic value of the ELISA-Ty test for the detection of typhoid fever in children. Southeast Asian J Trop Med Public Health 31: 702–707. [PubMed] [Google Scholar]
- 58. Handojo I, Edijanto SP, Probohoesodo MY, Mahartini NN (2004) Comparison of the diagnostic value of local Widal slide test with imported Widal slide test. Southeast Asian J Trop Med Public Health 35: 366–370. [PubMed] [Google Scholar]
- 59. Haque A, Ahmed J, Qureshi JA (1999) Early detection of typhoid by polymerase chain reaction. Ann Saudi Med 19: 337–340. [DOI] [PubMed] [Google Scholar]
- 60. Haque A, Ahmed N, Peerzada A, Raza A, Bashir S, Abbas G (2001) Utility of PCR in diagnosis of problematic cases of typhoid. Jpn J Infect Dis 54: 237–239. [PubMed] [Google Scholar]
- 61. Hassanein F, Mostafa FM, Elbehairy F, Hammam HM, Allam FA, El-Rehaiwy M, et al. (1975) Study of the pattern of Widal test in infants and children; II. Pattern of Widal test in children with enteric fevers. An attempt to define the diagnostic titer for upper Egypt. Gaz Egypt Paediatr Assoc 23: 173–180. [PubMed] [Google Scholar]
- 62. Hatta M, Goris MG, Heerkens E, Gooskens J, Smits HL (2002) Simple dipstick assay for the detection of Salmonella typhi-specific IgM antibodies and the evolution of the immune response in patients with typhoid fever. Am J Trop Med Hyg 66: 416–421. [DOI] [PubMed] [Google Scholar]
- 63. Hatta M, Mubin H, Abdoel T, Smits HL (2002) Antibody response in typhoid fever in endemic Indonesia and the relevance of serology and culture to diagnosis. Southeast Asian J Trop Med Public Health 33: 742–751. [PubMed] [Google Scholar]
- 64. Hatta M, Smits HL (2007) Detection of Salmonella typhi by nested polymerase chain reaction in blood, urine, and stool samples. Am J Trop Med Hyg 76: 139–143. [PubMed] [Google Scholar]
- 65. Heiba I, Girgis NI, Farid Z (1989) Enzyme-linked immunosorbent assays (ELISA) for the diagnosis of enteric fever. Trop Geogr Med 41: 213–217. [PubMed] [Google Scholar]
- 66. Herath HM (2003) Early diagnosis of typhoid fever by the detection of salivary IgA. J Clin Pathol 56: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hernandez-Velarde R, Sanchez-Castillo J, az-Godinez MC, Munoz-Hernandez O (1980) Utilization of the enzyme linked immunosorbent assay technique (ELISA) for the diagnosis of typhoid fever. Arch Invest Med (Mex) 11: 267–271. [PubMed] [Google Scholar]
- 68. Hirschl A, Stanek G, Rotter ML, Chau PY, Niemetz AH (1985) Antibody response to somatic antigen of Salmonella typhi in areas endemic and non-endemic for typhoid fever. Infect Control 6: 110–114. [DOI] [PubMed] [Google Scholar]
- 69. Hoffman SL, Flanigan TP, Klaucke D, Leksana B, Rockhill RC, Punjabi NH, et al. (1986) The Widal slide agglutination test, a valuable rapid diagnostic test in typhoid fever patients at the Infectious Diseases Hospital of Jakarta. Am J Epidemiol 123: 869–875. [DOI] [PubMed] [Google Scholar]
- 70. Hosoglu S, Geyik MF, Akalin S, Ayaz C, Kokoglu OF, Loeb M (2006) A simple validated prediction rule to diagnose typhoid fever in Turkey. Trans R Soc Trop Med Hyg 100: 1068–1074. [DOI] [PubMed] [Google Scholar]
- 71. Hosoglu S, Bosnak V, Akalin S, Geyik MF, Ayaz C (2008) Evaluation of false negativity of the Widal test among culture proven typhoid fever cases. J Infect Dev Ctries 2: 475–478. [PubMed] [Google Scholar]
- 72. House D, Wain J, Ho VA, Diep TS, Chinh NT, Bay PV, et al. (2001) Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol 39: 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. House D, Chinh NT, Diep TS, Parry CM, Wain J, Dougan G, et al. (2005) Use of paired serum samples for serodiagnosis of typhoid fever. J Clin Microbiol 43: 4889–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ismail TF, Smits H, Wasfy MO, Malone JL, Fadeel MA, Mahoney F (2002) Evaluation of dipstick serologic tests for diagnosis of brucellosis and typhoid Fever in egypt. 40: 3509–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Itah AY, Akpan CJ (2004) Correlation studies on Widal agglutination reaction and diagnosis of typhoid fever. Southeast Asian J Trop Med Public Health 35: 88–91. [PubMed] [Google Scholar]
- 76. Jackson AA, Ismail A, Ibrahim TA, Kader ZS, Nawi NM (1995) Retrospective review of dot enzyme immunoassay test for typhoid fever in an endemic area. Southeast Asian J Trop Med Public Health 26: 625–630. [PubMed] [Google Scholar]
- 77. Jesudason M, Esther E, Mathai E (2002) Typhidot test to detect IgG & IgM antibodies in typhoid fever. Indian J Med Res 116: 70–72. [PubMed] [Google Scholar]
- 78. Jesudason MV, Sridharan G, Mukundan S, John TJ (1994) Vi-specific latex agglutination for early and rapid detection of Salmonella serotype typhi in blood cultures. Diagn Microbiol Infect Dis 18: 75–78. [DOI] [PubMed] [Google Scholar]
- 79. Jesudason MV, Sridharan G, Arulselvan R, Babu PG, John TJ (1998) Diagnosis of typhoid fever by the detection of anti-LPS & anti-flagellin antibodies by ELISA. Indian J Med Res 107: 204–207. [PubMed] [Google Scholar]
- 80. Jesudason MV, Sivakumar S (2006) Prospective evaluation of a rapid diagnostic test Typhidot for typhoid fever. Indian J Med Res 123: 513–516. [PubMed] [Google Scholar]
- 81. Jindal N, Aggarwal IB, Arora A, Arora S (1992) Diagnostic accuracy of IgM antibody detection in typhoid by a modification in Widal test. Indian J Med Res 95: 224–226. [PubMed] [Google Scholar]
- 82. John TJ, Sivadasan K, Kurien B (1984) Evaluation of passive bacterial agglutination for the diagnosis of typhoid fever. J Clin Microbiol 20: 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kalhan R, Kaur I, Singh RP, Gupta HC (1998) Rapid diagnosis of typhoid fever. Indian J Pediatr 65: 561–564. [DOI] [PubMed] [Google Scholar]
- 84. Kariuki S, Mwituria J, Munyalo A, Revathi G, Onsongo J (2004) Typhoid is over-reported in Embu and Nairobi, Kenya. Afr J Health Sci 11: 103–110. [PubMed] [Google Scholar]
- 85. Kawano RL, Leano SA, Agdamag DM (2007) Comparison of serological test kits for diagnosis of typhoid fever in the Philippines. J Clin Microbiol 45: 246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Keddy KH, Sooka A, Letsoalo ME, Hoyland G, Chaignat CL, Morrissey AB, et al. (2011) Sensitivity and specificity of typhoid fever rapid antibody tests for laboratory diagnosis at two sub-Saharan African sites. Bull World Health Organ 89: 640–647. 10.2471/BLT.11.087627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Khanam F, Sheikh A, Sayeed MA, Bhuiyan MS, Choudhury FK, Salma U, et al. (2013) Evaluation of a Typhoid/Paratyphoid Diagnostic Assay (TPTest) Detecting Anti-Salmonella IgA in Secretions of Peripheral Blood Lymphocytes in Patients in Dhaka, Bangladesh. PLoS Negl Trop Dis 7: e2316 10.1371/journal.pntd.0002316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khoharo H, Shuaib A, Fatima Q (2009) Prospective evaluation of a rapid diagnostic test Dot EIA (Typhidot) for typhoid fever. JDUHS 3: 127–131. [Google Scholar]
- 89. Khoharo HK (2011) A comparative study of the typhidot (Dot-EIA) and Widal tests in blood culture positive cases of typhoid fever. Trop Doct 41: 136–138. 10.1258/td.2011.100406 [DOI] [PubMed] [Google Scholar]
- 90. Kim JM, Kim E, Chong Y, Hong CS (1989) Diagnostic usefulness of Vi-indirect fluorescent antibody test(Vi-IFAT) for typhoid fever—a prospective study. Yonsei Med J 30: 65–71. [DOI] [PubMed] [Google Scholar]
- 91. Koeleman JG, Regensburg DF, van KF, MacLaren DM (1992) Retrospective study to determine the diagnostic value of the Widal test in a non-endemic country. Eur J Clin Microbiol Infect Dis 11: 167–170. [DOI] [PubMed] [Google Scholar]
- 92. Korbsrisate S, Thanomsakyuth A, Banchuin N, McKay S, Hossain M, Sarasombath S (1999) Characterization of a phase 1-d epitope on Salmonella typhi flagellin and its role in the serodiagnosis of typhoid fever. Asian Pac J Allergy Immunol 17: 31–39. [PubMed] [Google Scholar]
- 93. Kulkarni ML, Rego SJ (1994) Value of single Widal test in the diagnosis of typhoid fever. Indian Pediatr 31: 1373–1377. [PubMed] [Google Scholar]
- 94. Kumar A, Arora V, Bashamboo A, Ali S (2002) Detection of Salmonella typhi by polymerase chain reaction: implications in diagnosis of typhoid fever. Infect Genet Evol 2: 107–110. [DOI] [PubMed] [Google Scholar]
- 95. Kumar G, Pratap CB, Mishra OP, Kumar K, Nath G (2012) Use of urine with nested PCR targeting the flagellin gene (fliC) for diagnosis of typhoid fever. J Clin Microbiol 50: 1964–1967. 10.1128/JCM.00031-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Levine MM, Grados O, Gilman RH, Woodward WE, Solis-Plaza R, Waldman W (1978) Diagnostic value of the Widal test in areas endemic for typhoid fever. Am J Trop Med Hyg 27: 795–800. [DOI] [PubMed] [Google Scholar]
- 97. Ley B, Mtove G, Thriemer K, Amos B, von SL, Hendriksen I, et al. (2010) Evaluation of the Widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hdospital in Tanzania and a comparison with previous studies. BMC Infect Dis 10: 180 10.1186/1471-2334-10-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ley B, Thriemer K, Ame SM, Mtove GM, von SL, Amos B, et al. (2011) Assessment and comparative analysis of a rapid diagnostic test (Tubex(R)) for the diagnosis of typhoid fever among hospitalized children in rural Tanzania. BMC Infect Dis 11: 147 10.1186/1471-2334-11-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lim PL, Fok YP (1987) Detection of group D salmonellae in blood culture broth and of soluble antigen by tube agglutination using an O-9 monoclonal antibody latex conjugate. J Clin Microbiol 25: 1165–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lim PL, Tam FC, Cheong YM, Jegathesan M (1998) One-step 2-minute test to detect typhoid-specific antibodies based on particle separation in tubes. J Clin Microbiol 36: 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Malla T, Malla KK, Thapalial A, Shaw C (2007) Enteric fever: a retrospective 6-year analysis of 82 paediatric cases in a teaching hospital. Kathmandu Univ Med J (KUMJ) 5: 181–187. [PubMed] [Google Scholar]
- 102. Massi MN, Shirakawa T, Gotoh A, Bishnu A, Hatta M, Kawabata M (2003) Rapid diagnosis of typhoid fever by PCR assay using one pair of primers from flagellin gene of Salmonella typhi. J Infect Chemother 9: 233–237. [DOI] [PubMed] [Google Scholar]
- 103. Massi MN, Shirakawa T, Gotoh A, Bishnu A, Hatta M, Kawabata M (2005) Quantitative detection of Salmonella enterica serovar Typhi from blood of suspected typhoid fever patients by real-time PCR. Int J Med Microbiol 295: 117–120. [DOI] [PubMed] [Google Scholar]
- 104. Mathai E, Jesudason MV (1989) Coagglutination test in the diagnosis of typhoid fever. Indian J Med Res 89: 287–289. [PubMed] [Google Scholar]
- 105. Mekara Y, Maneekarn N, Vithayasai V, Makonkawkeyoon S (1990) Determination of antibody from typhoid patients against lipopolysaccharide and protein antigens of Salmonella typhi. Asian Pac J Allergy Immunol 8: 95–101. [PubMed] [Google Scholar]
- 106. Mishra M, Thakar YS, Chande C, Tankhiwale NS, Saoji AM (1998) Rapid detection of enteric fever by coagglutination and countercurrent immunoelectrophoresis. Indian J Pathol Microbiol 41: 391–396. [PubMed] [Google Scholar]
- 107. Mukherjee C, Malik A, Khan HM (1993) Rapid diagnosis of typhoid fever by co-agglutination in an Indian hospital. J Med Microbiol 39: 74–77. [DOI] [PubMed] [Google Scholar]
- 108. Munoz O, Hernandez-Velarde R, Garduno-Rodriguez G, Gonzalez-Arroyo S, Gutierrez G (1979) Detection of antibodies to Salmonella "O" antigens in typhoid fever by counterimmunoelectrophoresis. II. Assessment in patients with typhoid fever and in a healthy population. Arch Invest Med (Mex) 10: 33–38. [PubMed] [Google Scholar]
- 109. Murphy JR (1993) Diarrhoeal disease: current concepts and future challenges. Rapid detection of enteric infections by measurement of enteric pathogen-specific antibody secreting cells in peripheral blood. Trans R Soc Trop Med Hyg 87 Suppl 3: 27–30. [DOI] [PubMed] [Google Scholar]
- 110. Nagarajan AG, Karnam G, Lahiri A, Allam US, Chakravortty D (2009) Reliable means of diagnosis and serovar determination of blood-borne Salmonella strains: quick PCR amplification of unique genomic loci by novel primer sets. J Clin Microbiol 47: 2435–2441. 10.1128/JCM.00327-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Naheed A, Ram PK, Brooks WA, Mintz ED, Hossain MA, Parsons MM, et al. (2008) Clinical value of Tubex and Typhidot rapid diagnostic tests for typhoid fever in an urban community clinic in Bangladesh. Diagn Microbiol Infect Dis 61: 381–386. 10.1016/j.diagmicrobio.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 112. Nakhla I, El MH, Mansour A, Klena JD, Hassan K, Sultan Y, et al. (2011) Validation of the Dri-Dot Latex agglutination and IgM lateral flow assays for the diagnosis of typhoid fever in an Egyptian population. Diagn Microbiol Infect Dis 70: 435–441. 10.1016/j.diagmicrobio.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 113. Nandagopal B, Sankar S, Lingesan K, Appu KC, Padmini B, Sridharan G, et al. (2010) Prevalence of Salmonella typhi among patients with febrile illness in rural and peri-urban populations of Vellore district, as determined by nested PCR targeting the flagellin gene. Mol Diagn Ther 14: 107–112. 10.2165/11534350-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 114. Nandakumar KS, Palanivel V, Muthukkaruppan V (1993) Diagnosis of typhoid fever: detection of Salmonella typhi porins-specific antibodies by inhibition ELISA. Clin Exp Immunol 94: 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Narayanappa D, Sripathi R, Jagdishkumar K, Rajani HS (2010) Comparative study of dot enzyme immunoassay (Typhidot-M) and Widal test in the diagnosis of typhoid fever. Indian Pediatr 47: 331–333. [DOI] [PubMed] [Google Scholar]
- 116. Nardiello S, Pizzella T, Russo M, Galanti B (1983) ELISA determination of IgM anti-LPS in the early phase of typhoid fever. Boll Ist Sieroter Milan 62: 372–375. [PubMed] [Google Scholar]
- 117. Nardiello S, Pizzella T, Russo M, Galanti B (1984) Serodiagnosis of typhoid fever by enzyme-linked immunosorbent assay determination of anti-Salmonella typhi lipopolysaccharide antibodies. J Clin Microbiol 20: 718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nga TV, Karkey A, Dongol S, Thuy HN, Dunstan S, Holt K, et al. (2010) The sensitivity of real-time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infect Dis 10: 125 10.1186/1471-2334-10-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nguyen NQ, Tapchaisri P, Chongsa-nguan M, Cao VV, Doan TT, Sakolvaree Y, et al. (1997) Diagnosis of enteric fever caused by Salmonella spp. in Vietnam by a monoclonal antibody-based dot-blot ELISA. Asian Pac J Allergy Immunol 15: 205–212. [PubMed] [Google Scholar]
- 120. Ngwu BA, Agbo JA (2003) Typhoid fever: clinical diagnosis versus laboratory confirmation. Niger J Med 12: 187–192. [PubMed] [Google Scholar]
- 121. Nizami SQ, Bhutta ZA, Siddiqui AA, Lubbad L (2006) Enhanced detection rate of typhoid fever in children in a periurban slum in Karachi, Pakistan using polymerase chain reaction technology. Scand J Clin Lab Invest 66: 429–436. [DOI] [PubMed] [Google Scholar]
- 122. Nugraha J, Marpaung FR, Tam FC, Lim PL (2012) Microbiological culture simplified using anti-O12 monoclonal antibody in TUBEX test to detect Salmonella bacteria from blood culture broths of enteric fever patients. PLoS One 7: e49586 10.1371/journal.pone.0049586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Olsen SJ, Pruckler J, Bibb W, Nguyen TM, Tran MT, Sivapalasingam S, et al. (2004) Evaluation of rapid diagnostic tests for typhoid fever. Bull World Health Organ 42: 1885–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Omuse G, Kohli R, Revathi G (2010) Diagnostic utility of a single Widal test in the diagnosis of typhoid fever at Aga Khan University Hospital (AKUH), Nairobi, Kenya. Trop Doct 40: 43–44. 10.1258/td.2009.090109 [DOI] [PubMed] [Google Scholar]
- 125. Ong LY, Pang T, Lim SH, Tan EL, Puthucheary SD (1989) A simple adherence test for detection of IgM antibodies in typhoid. J Med Microbiol 29: 195–198. [DOI] [PubMed] [Google Scholar]
- 126. Pandya M, Pillai P, Deb M (1995) Rapid diagnosis of typhoid fever by detection of Barber protein and Vi antigen of Salmonella serotype typhi. J Med Microbiol 43: 185–188. [DOI] [PubMed] [Google Scholar]
- 127. Pang T, Puthucheary SD (1983) Significance and value of the Widal test in the diagnosis of typhoid fever in an endemic area. J Clin Pathol 36: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pastoor R, Hatta M, Abdoel TH, Smits HL (2008) Simple, rapid, and affordable point-of-care test for the serodiagnosis of typhoid fever. Diagn Microbiol Infect Dis 61: 129–134. 10.1016/j.diagmicrobio.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 129. Prakash P, Mishra OP, Singh AK, Gulati AK, Nath G (2005) Evaluation of nested PCR in diagnosis of typhoid fever. J Clin Microbiol 43: 431–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pu SJ, Huang HS (1985) Diagnostic value of a single Widal test. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 18: 256–263. [PubMed] [Google Scholar]
- 131. Purwaningsih S, Handojo I, Prihatini, Probohoesodo Y (2001) Diagnostic value of dot-enzyme-immunoassay test to detect outer membrane protein antigen in sera of patients with typhoid fever. Southeast Asian J Trop Med Public Health 32: 507–512. [PubMed] [Google Scholar]
- 132. Qadri A, Ghosh S, Prakash K, Kumar R, Moudgil KD, Talwar GP (1990) Sandwich enzyme immunoassays for detection of Salmonella typhi. J Immunoassay 11: 251–269. [DOI] [PubMed] [Google Scholar]
- 133. Quiroga T, Goycoolea M, Tagle R, Gonzalez F, Rodriguez L, Villarroel L (1992) Diagnosis of typhoid fever by two serologic methods. Enzyme-linked immunosorbent assay of antilipopolysaccharide of Salmonella typhi antibodies and Widal test. Diagn Microbiol Infect Dis 15: 651–656. [DOI] [PubMed] [Google Scholar]
- 134. Rahman M, Siddique AK, Tam FC, Sharmin S, Rashid H, Iqbal A, et al. (2007) Rapid detection of early typhoid fever in endemic community children by the TUBEX O9-antibody test. Diagn Microbiol Infect Dis 58: 275–281. [DOI] [PubMed] [Google Scholar]
- 135. Rai GP, Zachariah K, Shrivastava S (1989) Comparative efficacy of indirect haemagglutination test, indirect fluorescent antibody test and enzyme linked immunosorbent assay in serodiagnosis of typhoid fever. J Trop Med Hyg 92: 431–434. [PubMed] [Google Scholar]
- 136. Rai GP, Zachariah K, Shrivastava S (1989) Detection of typhoid fever by Widal and indirect fluorescent antibody (IFA) tests. A comparative study. J Hyg Epidemiol Microbiol Immunol 33: 331–336. [PubMed] [Google Scholar]
- 137. Rao PS, Prasad SV, Arunkumar G, Shivananda PG (1999) Salmonella typhi VI antigen co-agglutination test for the rapid diagnosis of typhoid fever. Indian J Med Sci 53: 7–9. [PubMed] [Google Scholar]
- 138. Rasaily R, Dutta P, Saha MR, Mitra U, Bhattacharya SK, Manna B, et al. (1993) Value of a single Widal test in the diagnosis of typhoid fever. Indian J Med Res 97: 104–107. [PubMed] [Google Scholar]
- 139. Rockhill RC, Rumans LW, Lesmana M, Dennis DT (1980) Detection of Salmonella typhi D, Vi, and d antigens, by slide coagglutination, in urine from patients with typhoid fever. J Clin Microbiol 11: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sadallah F, Brighouse G, Del GG, Drager-Dayal R, Hocine M, Lambert PH (1990) Production of specific monoclonal antibodies to Salmonella typhi flagellin and possible application to immunodiagnosis of typhoid fever. J Infect Dis 161: 59–64. [DOI] [PubMed] [Google Scholar]
- 141. Saha SK, Ruhulamin M, Hanif M, Islam M, Khan WA (1996) Interpretation of the Widal test in the diagnosis of typhoid fever in Bangladeshi children. Ann Trop Paediatr 16: 75–78. [DOI] [PubMed] [Google Scholar]
- 142. Sanchez-Jimenez MM, Cardona-Castro N (2004) Validation of a PCR for diagnosis of typhoid fever and salmonellosis by amplification of the hilA gene in clinical samples from Colombian patients. J Med Microbiol 53: 875–878. [DOI] [PubMed] [Google Scholar]
- 143. Senewiratne B, Senewiratne K (1977) Reassessment of the Widal test in the diagnosis of typhoid. Gastroenterology 73: 233–236. [PubMed] [Google Scholar]
- 144. Shaheen HI, Girgis NI, Rodier GR, Kamal KA (1995) Evaluation of the response of human humoral antibodies to Salmonella typhi lipopolysaccharide in an area of endemic typhoid fever. Clin Infect Dis 21: 1012–1013. [DOI] [PubMed] [Google Scholar]
- 145. Sharma M, Datta U, Roy P, Verma S, Sehgal S (1997) Low sensitivity of counter-current immuno-electrophoresis for serodiagnosis of typhoid fever. J Med Microbiol 46: 1039–1042. [DOI] [PubMed] [Google Scholar]
- 146. Shehabi AA (1981) The value of a single Widal test in the diagnosis of acute typhoid fever. Trop Geogr Med 33: 113–116. [PubMed] [Google Scholar]
- 147. Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, Ahmed D, et al. (2009) Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol 2009/09/11: 1587–1594. 10.1128/CVI.00311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shukla S, Chitnis DS (1997) Haemagglutination system for the simultaneous detection of LPS and anti LPS antibodies of S.typhi. Indian J Med Sci 51: 265–269. [PubMed] [Google Scholar]
- 149. Shukla S, Patel B, Chitnis DS (1997) 100 years of Widal test & its reappraisal in an endemic area. Indian J Med Res 105: 53–57. [PubMed] [Google Scholar]
- 150. Siba V, Horwood PF, Vanuga K, Wapling J, Sehuko R, Siba PM, et al. (2012) Evaluation of serological diagnostic tests for typhoid fever in Papua New Guinea using a composite reference standard. Clin Vaccine Immunol 19: 1833–1837. 10.1128/CVI.00380-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sippel J, Bukhtiari N, Awan MB, Krieg R, Duncan JF, Karamat KA, et al. (1989) Indirect immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays (ELISAs) and IgM capture ELISA for detection of antibodies to lipopolysaccharide in adult typhoid fever patients in Pakistan. J Clin Microbiol 27: 1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sippel JE, Hanafy HM, Diab AS, Prato C, Arroyo R (1987) Serodiagnosis of typhoid fever in paediatric patients by anti-LPS ELISA. Trans R Soc Trop Med Hyg 81: 1022–1026. [DOI] [PubMed] [Google Scholar]
- 153. Sivadasan K, Kurien B, John TJ (1984) Rapid diagnosis of typhoid fever by antigen detection. Lancet 1: 134–135. [DOI] [PubMed] [Google Scholar]
- 154. Smith SI, Odunukwe NN, Niemogha MT, Ahmed AO, Efienemokwu CA, Otuonye MN, et al. (2004) Diagnostic methods for typhoid fever in Nigeria. Br J Biomed Sci 61: 179–181. [DOI] [PubMed] [Google Scholar]
- 155. Smith SI, Bamidele M, Fowora M, Goodluck HT, Omonigbehin EA, Akinsinde KA, et al. (2011) Application of a point-of-care test for the serodiagnosis of typhoid fever in Nigeria and the need for improved diagnostics. J Infect Dev Ctries 5: 520–526. [DOI] [PubMed] [Google Scholar]
- 156. Somerville PC, Lewis M, Koornhof HJ, Alberts M, Alberts HW, Raymond R (1981) The Widal test in the diagnosis of typhoid fever in the transvaal. S Afr Med J 59: 851–854. [PubMed] [Google Scholar]
- 157. Song JH, Cho H, Park MY, Na DS, Moon HB, Pai CH (1993) Detection of Salmonella typhi in the blood of patients with typhoid fever by polymerase chain reaction. J Clin Microbiol 31: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Srivastava L, Srivastava VK (1986) Serological diagnosis of typhoid fever by enzyme-linked immunosorbent assay (ELISA). Ann Trop Paediatr 6: 191–194. [DOI] [PubMed] [Google Scholar]
- 159. Sultana S, Hossain MA, Alam MA, Paul SK, Kabir MR, Hoque SM, et al. (2012) Comparative study of immunochromatographic assay (IgM) and widal test for early diagnosis of typhoid fever. Mymensingh Med J 21: 600–604. [PubMed] [Google Scholar]
- 160. Sundararaj T, Ilango B, Subramanian S (1983) A study on the usefulness of counter immuno-electrophoresis for the detection of Salmonella typhi antigen in the sera of suspected cases of enteric fever. Trans R Soc Trop Med Hyg 77: 194–197. [DOI] [PubMed] [Google Scholar]
- 161. Sushma K, Seemanthini D, Anjana V, Paranthaaman R (2011) Typhidot (IgM) as a reliable and rapid diagnostic test for typhoid fever. Ann Trop Med Public Health 4: 42–44. [Google Scholar]
- 162. Taiwo SS, Fadiora SO, Oparinde DP, Olowe OA (2007) Widal agglutination titres in the diagnosis of typhoid fever. West Afr J Med 26: 97–101. [PubMed] [Google Scholar]
- 163. Tam FC, Wang M, Dong B, Leung DT, Ma CH, Lim PL (2008) New rapid test for paratyphoid a fever: usefulness, cross-detection, and solution. Diagn Microbiol Infect Dis 62: 142–150. 10.1016/j.diagmicrobio.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 164. Tang SW, Abubakar S, Devi S, Puthucheary S, Pang T (1997) Induction and characterization of heat shock proteins of Salmonella typhi and their reactivity with sera from patients with typhoid fever. Infect Immun 65: 2983–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tantivanich S, Chongsanguan M, Sangpetchsong V, Tharavanij S (1984) A simple and rapid diagnostic test for typhoid fever. Southeast Asian J Trop Med Public Health 15: 317–322. [PubMed] [Google Scholar]
- 166. Tanyigna K, Ogor J (2008) The sensitivity of Diazo test in the diagnosis of enteric fevers. Afr J Cln Exper Microbiol 9: 115–118. [Google Scholar]
- 167. Tanyigna KB, Bello CS, Okeke N, Onwukeme KE (2001) Comparison of blood, bone marrow aspirate, stool and urine cultures in the diagnosis of enteric fever. Niger J Med 10: 21–24. [PubMed] [Google Scholar]
- 168. Thevanesam V (1992) An evaluation of the SAT in the diagnosis of typhoid. Ceylon Med J 37: 48–51. [PubMed] [Google Scholar]
- 169. Thriemer K, Ley BB, Ame SS, Deen JL, Pak GD, Chang NY, et al. (2012) Clinical and epidemiological features of typhoid fever in Pemba, Zanzibar: assessment of the performance of the WHO case definitions. PLoS One 7: e51823 10.1371/journal.pone.0051823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tsang RS, Chau PY, Lam SK, La Brooy JT, Rowley D (1981) Antibody response to the lipopolysaccharide and protein antigens of Salmonella typhi during typhoid infection. I. Measurement of serum antibodies by radioimmunoassay. Clin Exp Immunol 46: 508–514. [PMC free article] [PubMed] [Google Scholar]
- 171. Vaishnavi C, Kaur S, Singh K (2006) Evaluation of an in-house rapid diagnostic method for detection of Salmonella enterica serovar Typhi in fecal specimens. Trop Gastroenterol 27: 19–21. [PubMed] [Google Scholar]
- 172. Verdugo-Rodriguez A, Gam LH, Devi S, Koh CL, Puthucheary SD, Calva E, et al. (1993) Detection of antibodies against Salmonella typhi outer membrane protein (OMP) preparation in typhoid fever patients. Asian Pac J Allergy Immunol 11: 45–52. [PubMed] [Google Scholar]
- 173. Wain J, Diep TS, Bay PV, Walsh AL, Vinh H, Duong NM, et al. (2008) Specimens and culture media for the laboratory diagnosis of typhoid fever. J Infect Dev Ctries 2: 469–474. [DOI] [PubMed] [Google Scholar]
- 174. Weeramanthri TS, Corrah PT, Mabey DC, Greenwood BM (1989) An evaluation of the Widal test in the diagnosis of enteric fever. J Trop Med Hyg 92: 315–316. [PubMed] [Google Scholar]
- 175. West B, Richens JE, Howard PF (1989) Evaluation in Papua New Guinea of a urine coagglutination test and a Widal slide agglutination test for rapid diagnosis of typhoid fever. Trans R Soc Trop Med Hyg 83: 715–717. [DOI] [PubMed] [Google Scholar]
- 176. Willke A, Ergonul O, Bayar B (2002) Widal test in diagnosis of typhoid fever in Turkey. Clin Diagn Lab Immunol 9: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yan M, Tam FC, Kan B, Lim PL (2011) Combined rapid (TUBEX) test for typhoid-paratyphoid A fever based on strong anti-O12 response: design and critical assessment of sensitivity. PLoS One 6: e24743 10.1371/journal.pone.0024743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yap YF, Puthucheary SD (1998) Typhoid fever in children—a retrospective study of 54 cases from Malaysia. Singapore Med J 39: 260–262. [PubMed] [Google Scholar]
- 179. Youssef FG, Daba AS, Kabeil SS, Parker TM (2010) A comparative study of blood culture and antibody response with the duration of illness in diagnosis of typhoid fever. Aust J Basic Appl Sci 609–614. [Google Scholar]
- 180. Zaka-ur-Rab Z, Abqari S, Shahab T, Islam N, Shukla I (2012) Evaluation of salivary anti-Salmonella typhi lipopolysaccharide IgA ELISA for serodiagnosis of typhoid fever in children. Arch Dis Child 97: 236–238. 10.1136/adc.2011.300622 [DOI] [PubMed] [Google Scholar]
- 181. Zuniga J, Lillo L, Shin JJ, Machavarapu RL, Quiroga T, Goycoolea M, et al. (2005) Salmonella enterica serovar Typhi O:1,9,12 polysaccharide-protein conjugate as a diagnostic tool for typhoid fever. J Clin Microbiol 43: 4545–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011/10/19: 529–536. [DOI] [PubMed] [Google Scholar]
- 183. Harbord RM, Whiting P (2009) Metandi: meta-analysis of diagnostic accuracy using heirarchical logistic regression In: Sterne JAC, editors. Meta-analysis in Stata: an updated collection from the Stata journal. College Station, TX: Stata Press; pp. 211–229. [Google Scholar]
- 184. Zhou Xiao hua, McClish Donna K., and Obuchowski Nancy A. (2011) Statistical methods in diagnostic medicine. Hoboken, NJ: Wiley. [Google Scholar]
- 185.Dwamena BA (2007) Midas: Stata module for meta-analytical integration of diagnostic accuracy studies.
- 186. Rutter CM, Gatsonis CA (2001) A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 20: 2865–2884. 10.1002/sim.942 [pii]. [DOI] [PubMed] [Google Scholar]
- 187. Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, et al. (2012) Population-Based Incidence of Typhoid Fever in an Urban Informal Settlement and a Rural Area in Kenya: Implications for Typhoid Vaccine Use in Africa. PLoS One 7: e29119 10.1371/journal.pone.0029119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Naheed A, Ram PK, Brooks WA, Hossain MA, Parsons MB, Talukder KA, et al. (2010) Burden of typhoid and paratyphoid fever in a densely populated urban community, Dhaka, Bangladesh. Int J Infect Dis 14: E93–E99. 10.1016/j.ijid.2009.11.023 [DOI] [PubMed] [Google Scholar]
- 189. Ochiai RL, Acosta CJ, novaro-Holliday MC, Baiqing D, Lhattacharya SK, Agtini MD, et al. (2008) A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 86: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, et al. (1998) Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998/06/10: 1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ (2002) Typhoid fever. N Engl J Med 2002/11/29: 1770–1782. [DOI] [PubMed] [Google Scholar]
- 192.Herrick T, Hawkins K, Golden A, Lee A, Gerns Storey HL, et al. (2013) Product attributes for a novel typhoid surveillance tool. Annual Conference of the American Society of Tropical Medicine and Hygiene. Abstract.
- 193. Prentice RL (1988) Correlated binary regression with covariates specific to each binary observation. Biometrics 44: 1033–1048. [PubMed] [Google Scholar]
- 194. Darton TC, Blohmke CJ, Pollard AJ (2014) Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol 2013/12/07: 7–17. 10.1097/MOG.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 195. Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, et al. (2005) Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005/01/07: 39–47. [DOI] [PubMed] [Google Scholar]
- 196. Thriemer K, Ley B, Menten J, Jacobs J, van den Ende J (2013) A systematic review and meta-analysis of the performance of two point of care typhoid fever tests, Tubex TF and Typhidot, in endemic countries. PLoS One 8: e81263 10.1371/journal.pone.0081263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005/08/09: 882–893. [DOI] [PubMed] [Google Scholar]
- 198. Crump JA, Mintz ED (2010) Global Trends in Typhoid and Paratyphoid Fever. Clin Infect Dis 50: 241–246. Article. 10.1086/649541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. DeRoeck D, Clemens JD, Nyamete A, Mahoney RT (2005) Policymakers' views regarding the introduction of new-generation vaccines against typhoid fever, shigellosis and cholera in Asia. Vaccine 2005/03/23: 2762–2774. [DOI] [PubMed] [Google Scholar]
- 200. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003/01/04: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.