Abstract
Tilia is an ecologically and economically important genus in the family Malvaceae. However, there is no complete plastid genome of Tilia sequenced to date, and the taxonomy of Tilia is difficult owing to frequent hybridization and polyploidization. A well-supported interspecific relationships of this genus is not available due to limited informative sites from the commonly used molecular markers. We report here the complete plastid genome sequences of four Tilia species determined by the Illumina technology. The Tilia plastid genome is 162,653 bp to 162,796 bp in length, encoding 113 unique genes and a total number of 130 genes. The gene order and organization of the Tilia plastid genome exhibits the general structure of angiosperms and is very similar to other published plastid genomes of Malvaceae. As other long-lived tree genera, the sequence divergence among the four Tilia plastid genomes is very low. And we analyzed the nucleotide substitution patterns and the evolution of insertions and deletions in the Tilia plastid genomes. Finally, we build a phylogeny of the four sampled Tilia species with high supports using plastid phylogenomics, suggesting that it is an efficient way to resolve the phylogenetic relationships of this genus.
Introduction
Tilia L. (basswood or lime-tree) is a genus of the family Malvaceae in the order Malvales, which contains 23 species of deciduous tree disjunctly distributed in the temperate area across Asia, Europe and North America [1–3]. Trees of Tilia are ecologically and economically important. Most species are the dominant element in the broad-leaved temperate forests, and many species are used for timber, honey resources and cultivated worldwide for ornamental purpose.
Traditionally Tilia has been placed in its own family Tiliaceae [4, 5]. However, molecular evidence strongly supported the polyphyly of the traditionally circumscribed Tiliaceae [6]. A clade of Tilioideae was resolved and Tilia was closely related to the genera Craigia and Mortoniodendron in the family of Malvaceae [7, 8]. Within the Malvaceae family, Tilia is a distinct genus characterized by woody habit and paddle-shaped bracts of flowers. However, the taxonomy of Tilia is difficult and controversial due to limited taxonomic characters and frequently occurred hybridization [3], and species relationships within the genus are poorly known. Previous molecular studies mainly focused on population genetic analyses of some Tilia species employing plastid PCR-RFLP markers [9], random amplified polymorphic DNA (RAPD) markers [10–12], microsatellite markers [13]. Recent molecular phylogenetic studies have used chloroplast regions (rpL32-trnL and ndhF-rpL32) or ribosomal internal transcribed spacer (ITS) to reconstruct the phylogeny of selected species in Tilia, but without satisfaction [14, 15]. An expanded attempt of seven plastid regions and three low-copy nuclear regions on comprehensively sampled taxa did also not well resolve interspecific relationships of Tilia [16]. The age of Tilia trees when they begin to flower and produce seed ranges from six to 40 years old [3]. The slow nucleotide substitution rates of Tilia may be attributed to their long generation times.
The recent availability of the next-generation sequencing techniques has enabled generating large amounts of sequence data at relatively low cost [17–19]. Whole plastid genome is increasingly used for phylogenetic analyses and has proven to be effective in resolving difficult phylogenetic relationships [20–22]. Plastid genomes of angiosperms are well known to be highly conserved [23, 24]. They usually have a circular structure of two copies of large inverted repeats (IR) separated by small (SSC) and large (LSC) single-copy regions [25]. With a size ranging from 120 to 160 kb in general, they also exhibit highly conserved gene content and order [23, 24].
The number of sequenced plant plastid genomes increased rapidly during last decade due to the establishment of the next-generation sequencing techniques. Within the Malvaceae family, however, there are only two genera Gossypium [26, 27] and Theobroma [28] having their plastid genomes sequenced to date. At present, there is no complete plastid genome from Tilia sequenced despite of its ecological and economic importance.
To better understand the evolution of plastid genome and explore the potential of phylogenomics basing on plastid genome sequence to clarify interspecific relationships in Tilia, four representative Tilia species were sequenced using next-generation Illumina sequencing-by-synthesis technology. The main purposes of this study are to (1) gain insights into the structure of plastid genome of Tilia as well as in Malvaceae; and (2) explore the feasibility of plastid phylogenomics in reconstructing a solid interspecific relationships of the long-lived tree genus Tilia.
Materials and Methods
Plant material
Four species were chosen as representatives of Tilia for plastid genome sequencing. Studied species are commonly found in the public woodland where they are native (Table 1) and no specific collecting permits required for sampling. T. amurensis is listed in the China’s protected species Grade II, but it is not require permit to collect a few twigs and leaves for vouchers and DNA extraction. Healthy and fresh leaves were collected from a single individual for DNA extraction. The voucher specimens of sampled species were all deposited at the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN) (Table 1).
Table 1. Sequencing information for the four Tilia species used in this study.
| Species | Voucher No. | Locality | GenBank numbers | Clean reads | Mean coverage |
|---|---|---|---|---|---|
| T. amurensis | 11CS2872 | Harbin, Heilongjiang, China | KT894772 | 5,021,460 | 282x |
| T. mandshurica | 11CS2873 | Harbin, Heilongjiang, China | KT894773 | 10,712,702 | 602x |
| T. oliveri | 12CS5580 | Wushan, Chongqing, China | KT894774 | 13,141,606 | 739x |
| T. paucicostata | 13CS6898 | Zhongdian, Yunnan, China | KT894775 | 5,074,944 | 285x |
DNA extraction and template amplification
Total genomic DNA was extracted from ~100 mg of leaf material using a modified CTAB method [29, 30], and their quality was assessed by agarose gel electrophoresis. We amplified the entire plastid genome using long-range PCR and 9 primer pairs as described in Yang et al. 2014 [30]. Briefly, amplification was performed using Takara PrimeSTAR GXL DNA polymerase (TAKARA BIO INC.) in 25-μl reaction mixtures with 30–100 ng of DNA template. The PCR amplification conditions were the same as those of Yang et al. 2014 [30]. All amplifications were successful and amplicon DNA concentrations were determined by visual approximation using gel electrophoresis. Subsequently, the 9 long-ranged PCR products were pooled together in roughly equal mass mixtures for genome sequencing.
Illumina sequencing, assembly, and annotation
Pooled amplified plastid DNAs (6 μg) were sheared for short-insert (500 bp) sequencing libraries construction according to the manufacturer’s instructions (Illumina). The 90 bp paired-end reads were generated on an Illumina Hiseq 2000 at BGI Shenzhen, China. Illumina raw reads were first quality trimmed using NGS QC Tool Kit [31] (cut-off value for percentage of read length = 80 and for Phred quality score = 30). The plastid genomes from the filtered clean reads were assembled using the CLC Genomics Workbench v. 6.5 (CLC Bio) de novo assembly program at hash length of 63 and with a minimum contig length of 1 kb. The assembled contigs were analyzed by a BLAST of the nucleotide database at the NCBI (http://www.ncbi.nlm.nih.gov/), and these aligning to the published plastid DNA sequences were collected for genome finishing. The annotation of completed plastid genomes was carried out using the program DOGMA [32]. We then manually adjusted the start and stop codons and intron/exon boundaries if necessary. The annotated GenBank files were used to draw the circular plastid genome maps using the OrganellarGenomeDRAW (OGDRAW) [33].
Comparison of Tilia plastid genome with other Malvaceae genera
The complete plastid genomes of Gossypium hirsutum (GenBank NC_007944) and Theobroma cacao (GenBank HQ336404) were downloaded from NCBI for comparison. The general plastid genome characters were compared between these two and our sequenced four Tilia species. Any large structural events such as gene order rearrangement and IR expansion/contraction were recorded. To investigate the difference in genome size between Gossypium and Tilia, the plastid DNA sequences of G. hirsutum and T. mandshurica were partitioned into genes, introns and intergenic spacers and then were compared separately.
Sequence analysis for Tilia species
The plastid genome sequences of four Tilia species and G. hirsutum were aligned using MAFFT v. 7.215 [34] in the default sets and manually adjusted in MEGA 5.0 [35]. Sequence divergence between the four Tilia plastid genome sequences was calculated as uncorrected p-distance using MEGA 5.0. The percentage of variable characters for each noncoding region with an aligned length >200 bp in the genome was calculated as described in Zhang et al [17]. We scored the number of transitional and transversional substitutions among the four Tilia plastid genome sequences and decided the direction of substitutions using G. hirsutum as outgroup. The indels among them were polarized into insertions and deletions in the same way.
Phylogenetic inference
We downloaded the plastid genome sequences of two species of Brassicaceae, Arabidopsis thaliana (GenBank NC_000932) and Brassica napus (GenBank NC_016734) as outgroups. The ingroup taxa included one Theobroma species, two Gossypium species (the second being G. herbaceum with GenBank NC_016734) and four Tilia species sequenced here. The orientation of the SSC regions from two Brassicaceae species and Theobroma cacao were manually reversed for alignment. The alignment of nine plastid genomes with one IR region removed was conducted with MAFFT v. 7.215 [34] in the default sets, followed by manual adjustments in MEGA 5.0 [35]. Phylogenetic analysis using maximum likelihood (ML) method was performed using RAxML v. 8.0.20 [36]. Both the unpartitioned and partitioned ML analyses were performed with the dataset dividing into three partitions corresponding to the LSC, SSC and IR region of the plastid genome. The ML tree was constructed with the combined rapid bootstrap of 500 replicates and search for the best tree in a single run under the GTR + G model as suggested in the RAxML manual. Bayesian analysis was performed using MrBayes v. 3.2 [37] with the GTR + I +G model in the unpartitioned way. The Markov chain Monte Carlo (MCMC) algorithm was run for two million generations with trees sampled very 100 generations. The convergence was reached with the average standard deviation of split frequencies (ASDFs) following 0.01. The first 25% of trees generated were discarded as burn-in and the remaining trees were used to build majority-rule consensus tree. To test the rate of evolution of Tilia relative to other Malvaceae species we applied Tajima relative rate test [38] on the plastid genome sequence alignment. The relative rates of evolution between each of the four Tilia species and G. hirsutum were evaluated using the Theobroma cacao as outgroup.
Results
Sequencing of Tilia plastid genomes
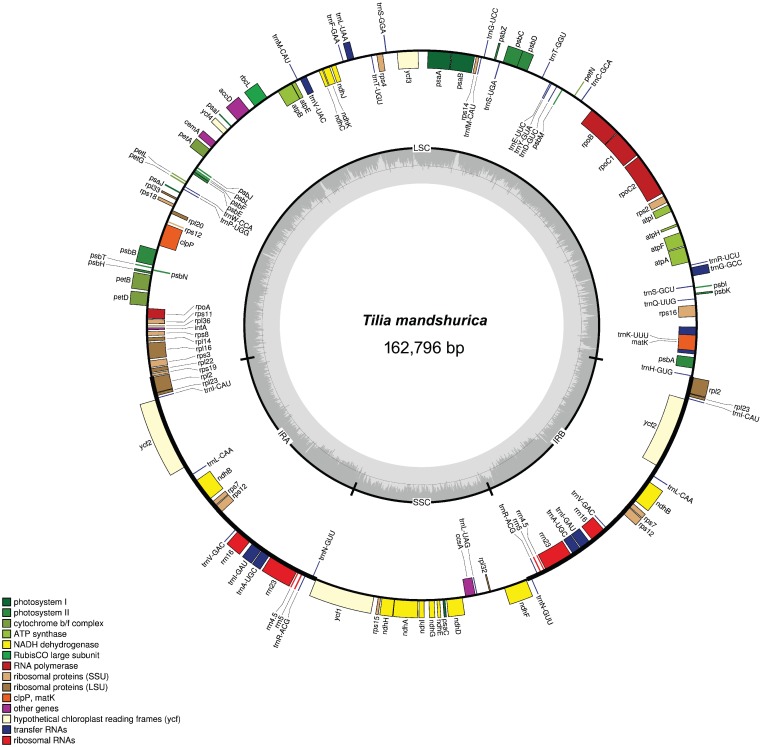
Illumina 90-bp paired-end sequencing of long-rang PCR amplified plastid DNA generated 5,021,460–13,141,606 clean reads for the four sampled Tilia species, with an average sequencing depth from 282× to 739× (Table 1). Using the combination of de novo and reference-guided assembly, we obtained the complete plastid nucleotide sequences for all four species. The determined nucleotide sequences of the four plastid genomes range narrowly from 162,653 bp in Tilia paucicostata to 162,796 bp in Tilia mandshurica (Table 2). They all have a genome structure resembling those of the vast majority of angiosperms, consisting of a pair of IRs separated by LSC and SSC, and the gene map of T. mandshurica plastid genome is presented in Fig 1 as a representative. The four genomes encode an identical set of 130 genes, of which 113 are unique and 17 are duplicated in the IR regions (Table 2), and the arrangements of these 130 genes in them are totally collinear. The 113 unique genes include 79 protein-coding genes, 30 tRNA genes and 4 rRNA genes. They also have an identical GC content of 36.5% that is similar to other angiosperms plastid genomes [23, 24].
Table 2. Comparison of Malvaceae plastid genomes sampled in this study.
| Gossypium hirsutum | Theobroma cacao | Tilia amurensis | Tilia mandshurica | Tilia oliveri | Tilia paucicostata | |
|---|---|---|---|---|---|---|
| Size (bp) | 160,301 | 160,604 | 162,715 | 162,796 | 162,734 | 162,653 |
| LSC (bp) | 88,816 | 89,395 | 91,124 | 91,127 | 91,095 | 91,139 |
| SSC (bp) | 20,269 | 20,187 | 20,397 | 20,371 | 20,381 | 20,380 |
| IR (bp) | 25,608 | 25,511 | 25,597 | 25,649 | 25,629 | 25,567 |
| Number of protein-coding genes a | 84 (6) | 85 (6) | 85 (6) | 85 (6) | 85 (6) | 85 (6) |
| Number of tRNA genes a | 37 (7) | 37 (7) | 37 (7) | 37 (7) | 37 (7) | 37 (7) |
| Number of rRNA genes a | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) | 8 (4) |
| GC content (%) | 37.3 | 36.9 | 36.5 | 36.5 | 36.5 | 36.5 |
aThe numbers in parenthesis indicate the genes duplicated in the IR regions.
Fig 1. Circular gene map of the plastid genome of Tilia mandshurica.
Genes drawn within the circle are transcribed clockwise, while those drawn outside are transcribed counterclockwise. Genes are color-coded according to their functional groups.
Comparisons with other Malvaceae plastid genomes
The comparisons of general genomic features between Gossypium hirsutum [26] as a representative species from genus Gossypium, Theobroma cacao [28] and four sequenced Tilia species were presented in Table 2. All plastid genomes except for G. hirsutum share identical complements of coding genes with similar order. The infA gene in the plastid genome of genus Gossypium was inferred to be pseudogene due to the presence of frameshift indels [26]. In addition, the GC content of Malvaceae species is almost the same (Table 2).
All plastid genomes of Malvaceae possess a typical quadripartite structure of angiosperms. However, the SSC region in the assembled plastid genomes of Gossypium and Tilia is in the reverse orientation relative to Theobroma and most other angiosperms. The four Tilia species have the same IR and SSC boundary with 36-bp sequence of the ycf1 gene extending into the IR regions. The ycf1 gene also extended into the IR regions with 98-bp sequence duplication in G. hirsutum. However, this gene is wholly confined in the SSC region in Theobroma cacao while the ndhF gene had 6-bp sequence duplicated in the IR regions.
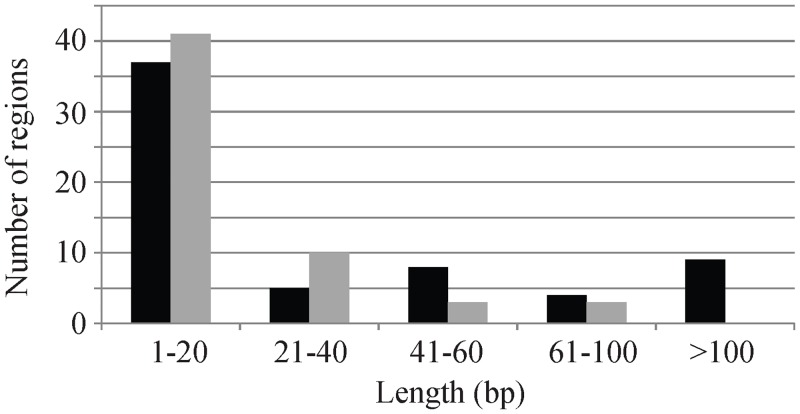
In terms of genome size, the plastid genome of Tilia (162,653–162,796 bp) is slightly larger than those of Gossypium (159,039–160,433 bp) and Theobroma (160,604 bp). We selected T. mandshurica and G. hirsutum as representative species to investigate the trend toward increased genome size in Tilia. The whole plastid genome of T. mandshurica is 2495 bp larger than that of G. hirsutum, and all three regions LSC, SSC and IR of T. mandshurica are larger with LSC (2311 bp) accounting for most variation in genome size (Table 2). Among the 78 common unique protein-coding genes between these two genomes there are 11 genes with difference in length. Four genes (atpI, ccsA, rbcL, and ycf1) are larger in T. mandshurica while seven genes (accD, matK, ndhF, petB, rpl22, rpoA, and rpoC2) are larger in G. hirsutum, accounting for 63 bp and 180 bp of the variation in genome size respectively. On the other hand, the majority of noncoding (intergenic and intron) regions (120 of 153) show variations in length. The number of noncoding regions that are larger is similar in T. mandshurica and G. hirsutum (63 versus 47) (Fig 2). Nevertheless, the 9 regions with length difference above 100 bp are all those larger in T. mandshurica than G. hirsutum. These 9 regions are ycf3-trnS-GGA, ndhC-trnV-UAC, trnH-GUG-psbA, trnT-UGU-trnL-UAA, trnR-UCC-atpA, psbZ-trnG-UCC, atpB-rbcL, trnC-GCA-petN, and trnK-UUU-rps16. These length differences are mainly caused by the large insertions in the T. mandshurica (or deletions in G. hirsutum) plastid genome and there are 8 indels (insertions or deletions) in all larger than 100 bp. The total length of these indels is 1990 bp, explaining ~80% of variation in genome size.
Fig 2. The number of noncoding regions with different sizes between the plastid genomes of Tilia mandshurica and Gossypium hirsutum.
The black bar indicates the regions are larger in T. mandshurica, while the gray bar indicates the regions are larger in G. hirsutum.
Sequence divergence of Tilia plastid genomes
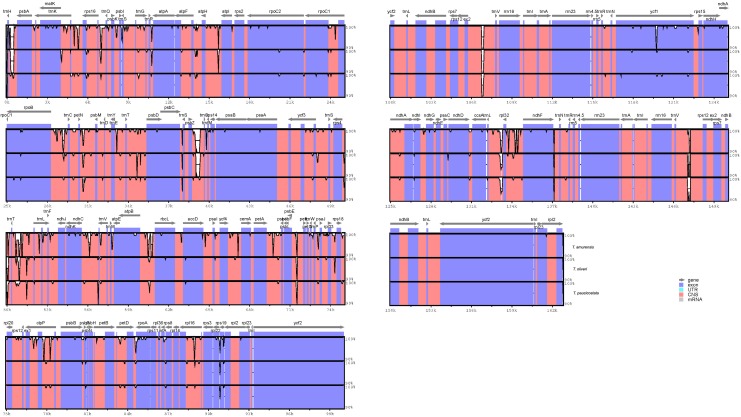
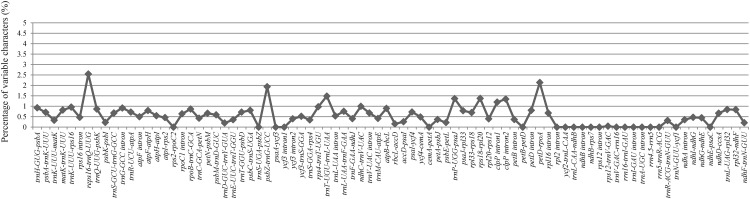
We plotted sequence identity among the four Tilia plastid genomes using the mVISTA software [39] with T. mandshurica as a reference. A genome-wide alignment reveals globally high sequence similarity (> 90% identity) among them (Fig 3). The overall sequence divergence estimated by p-distance among the four genomes was only 0.0013. The pairwise p-distance between the four species ranged from 0.0004 to 0.0021, and the T. amurensis has a somewhat larger sequence divergence from the others within the genus. We also compared the sequence divergence among the different noncoding regions in the four Tilia species. Among the 84 noncoding regions, the percentage of variation ranged from 0 to 2.55%, and there were no mutational hotspots identified (Fig 4).
Fig 3. mVISTA percent identity plot comparing the four Tilia plastid genomes with T. mandshurica as a reference.
Vertical scale indicates the percentage of identity ranging from 70% to 100%. Coding regions are in blue and noncoding regions are in pink.
Fig 4. Percentage of variable characters in aligned noncoding regions of the four Tilia plastid genomes.
These regions are oriented according to their locations in the plastid genome.
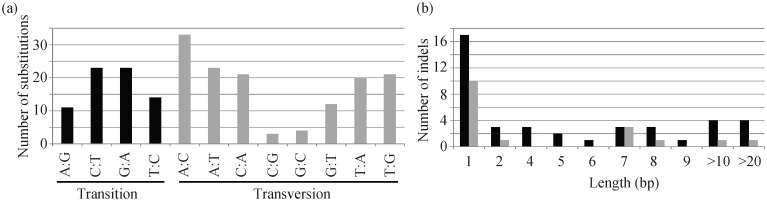
We further investigated the pattern of sequence divergence in these four plastid genomes. Using G. hirsutum as an outgroup, we only considered these substitutions for which the direction could be unambiguously identified and there were a total of 208 substitutions determined in the whole genome (Fig 5A). Among them, there were 71 transitions and 137 transversions and the transition/transversion ratio (Ts/Tv = 0.52) was nearly identical to the 1:2 ratio expected from equal rates of transition and transversion. More specifically, we observed a significant excess of A to C and lack of G to C and C to G transversions relative to all other substitutions (Fig 5A). The insertions or deletions in plastid genomes of Tilia were also inferred with G. hirsutum as an outgroup. The number of insertions (41) is much larger than the number of deletions (17) (Fig 5B). And about 41% of the total insertions and 59% of the total deletions are of size 1 bp. The longest insertion and deletion was 76 and 21 bp, respectively. In addition, 52 of these 58 indels were associated with tandem repeats.
Fig 5. Nucleotide sequence variations identified in the four Tilia plastid genomes.
(A) The nucleotide substitution patterns in the plastid genomes. (B) The length distribution of insertions (black) and deletions (gray) in the plastid genomes.
Phylogenetic analyses of Tilia
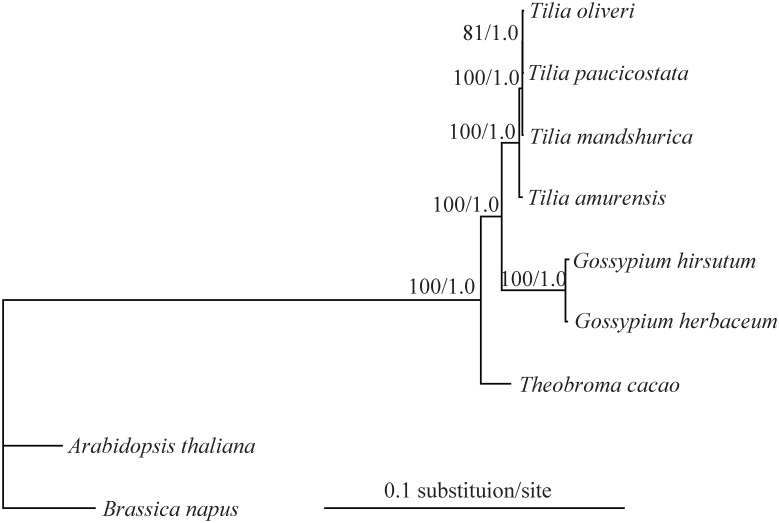
The data matrix for phylogenetic analyses contained the whole plastid genome with one IR region removed for nine taxa, including six species of Malvaceae and two outgroups from Brassicaceae. The data set comprised of 145,679 nucleotide positions with 3,377 informative sites for the ingroup taxa. However, there were only 20 informative sites for the four Tilia species. Unpartitioned ML analyses resulted in a fully resolved tree with 5 of the 6 nodes supported by 100% bootstrap values (Fig 6). ML analyses partitioned by the three plastid genomic regions (LSC, SSC, and IR) yielded an identical topology with the same 5 nodes 100% supported (data not shown). The remaining one received 81% and 82% bootstrap values from unpartitoned and partitioned analyses, respectively. In Bayesian analysis, the identical topology was obtained with a posterior probability (PP) of 1.0 for all nodes (Fig 6). T. amurensis was sister to the other three sampled Tilia species, and T. mandshurica was then sister to the grouping of T. oliveri and T. paucicostata (Fig 6).
Fig 6. Maximum likelihood phylogeny of the seven Malvaceae species based on the complete plastid genome sequences.
The numbers associated with the nodes are bootstrap support and posterior probability values.
As can been seen in Fig 6, the branch length leading to Tilia and especially those among species were extremely short. This indicated that Tilia likely had a slow rate of evolution relative to Gossypium. To explore the rate heterogeneity existed between Gossypium and Tilia, we applied Tajima relative rate test [38] on the sequence alignments of whole plastid genome. This test showed that all the four sampled Tilia species were evolving significantly slower than the Gossypium (p < 0.001; Table 3).
Table 3. Results of Tajima relative rate test.
| Ingroup1 | Ingroup2 | Outgroup | Identical sites | Divergent sites | Ingroup1 specific a | Ingroup2 specific | Outgroup specific | Chi-square statistic | p-value | Slow |
|---|---|---|---|---|---|---|---|---|---|---|
| Gossypium hirsutum | Tilia mandshurica | Theobroma cacao | 125,627 | 113 | 2456 | 725 | 1801 | 941.96 | < 0.001 | Tilia mandshurica |
| Gossypium hirsutum | Tilia paucicostata | Theobroma cacao | 125,624 | 112 | 2458 | 727 | 1801 | 940.77 | < 0.001 | Tilia paucicostata |
| Gossypium hirsutum | Tilia amurensis | Theobroma cacao | 125,670 | 104 | 2452 | 694 | 1813 | 982.38 | < 0.001 | Tilia amurensis |
| Gossypium hirsutum | Tilia oliveri | Theobroma cacao | 125,625 | 112 | 2451 | 720 | 1804 | 944.93 | < 0.001 | Tilia oliveri |
a These nucleotide sites were identical in ingroup2 and outgroup but not in ingroup1, and the same idea applies for ingroup2 and outgroup specific.
Discussion
Comparison of plastid genome within Malvaceae
In this study, we determined the complete plastid genome sequences from four Tilia species using Illumina sequencing technology. In addition to Gossypium and Theobroma [26–28], Tilia is the third genus within the family Malvaceae to have its complete plastid genome sequenced. All of the four Tilia plastid genomes possess a typical quadripartite structure of angiosperms with a pair of inverted repeats dividing the whole genome into two single copy regions (Fig 1). In comparison to Gossypium and Theobroma, no significant structural reconfigurations such as inversions or gene relocations were detected in the four Tilia plastid genomes. The six plastid genomes analyzed here are rather conserved, and only with minor variations in the junctions between the SSC and IRs regions, which are usually different within the same family [40–42]. The four Tilia plastid genomes have the same boundary between the SSC and IRs regions with 36-bp sequence of the ycf1 gene duplicated in IRs and the length of duplication is 98 bp in G. hirsutum. In the Theobroma cacao plastid genome, it is the ndhF gene rather than ycf1 extending into the IRs. However, the SSC region in the Tilia plastid genomes was assembled to being in the reverse orientation relative to Theobroma and majority of angiosperms while identical to Gossypium. The SSC region could exist in two orientations in plastid genomes [43, 44] and this result does not reflect any differences in gene order in these genomes.
In terms of gene content, the Tilia plastid genomes share the same set of 85 protein genes with Theobroma rather than Gossypium (Table 2), although Tilia has a closer relationship to Gossypium (Fig 6). The infA gene that has become a pseudogene in Gossypium has intact reading frame and can be functional in Tilia. This result indicates that pseudogenization of infA independently occurred in the Gossypium lineage. In terms of genome size, the Tilia plastid genomes show a trend toward increased size within Malvaceae. We divided the whole plastid genome into genes and intergenic spacers and compared their lengths between Tilia and Gossypium, finding that the variation in genome size is mostly due to length differences in the noncoding regions (Fig 2). Furthermore, a few large indels (> 100 bp) instead of many small indels can be responsible for ~80% of the total length difference between these two genera.
Molecular evolution of Tilia plastid genome sequences
In addition to the rather conserved evolution of genome structure, the genetic divergence is extremely low among the four Tilia plastid genomes, and there is no mutation hotspot region identified across the genome (Figs 3 and 4) as in other angiosperms [17, 45, 46]. Within the four species, the T. amurensis is slight divergent relative to the others. Among a total of 208 substitutions identified among the four Tilia plastid genomes (Fig 5A), the transition/transversion ratio of 0.52 is very close to the expected 1:2 ratio in considering equal rates of transition and transversion. The rate of transitions is not significantly elevated as demonstrated recently in many other plant plastid genomes [22, 27, 47]. However, there are a significant excess of A to C and lack of G to C and C to G transversions among the eight types of transversions (Fig 5A). The underlying mechanism under this phenomenon would require more studies to clarify in the future.
In addition to substitutions, the indels are another important class of genetic variation [48–50]. The insertions occur much more frequently than the deletions in the Tilia plastid genomes (Fig 5B), consistent with the trend toward larger genome size within Malvaceae. Approximately half of the total indels are of size 1 bp and mainly occur on the homopolymer regions, and almost entirely of the remaining indels > 1 bp are associated with tandem repeats. This result indicates that these indels very likely originated as mutation events formed by slipped strand mispairing [51].
Using plastid phylogenomics to resolve phylogeny of Tilia
As suggested by the low sequence divergence observed among the four Tilia plastid genomes, the slowdown in evolutionary rates may occur in this long-lived tree genus. Relative rate test does demonstrate a reduced rate of evolution in the Tilia relative to its relative genus Gossypium (Table 3). The slow evolutionary rate in Tilia can be largely attributed to its long generation times [3]. Although there are a few phylogenetic informative sites contained in the Tilia plastid genomes, the phylogeny of the four sampled Tilia species is well resolved (Fig 6). As previous studies employing multi nuclear and plastid DNA regions have failed to reconstruct the phylogeny of Tilia [14, 16], our successful reconstruction of phylogeny for the four Tilia species sampled here indicates that plastid phylogenomics holds promise in resolving the interspecific relationships of this ecologically and economically important genus.
Acknowledgments
We wish to thank Prof. C. Donald Pigott for his support to this study, to Prof. Ting-Shuang Yi for his valuable comments on the manuscript. We are very grateful to Prof. Jun-Bo Yang for his help during the experiments.
Data Availability
Four Tilia plastid genomes sequenced in current study are available in NCBI database. The Genbank numbers are KT894772, KT894773, KT894774, KT894775.
Funding Statement
This work was supported by grants from the National Key Basic Research Program of China (No. 2014CB954100) and Kunming Institute of Botany, Chinese Academy of Sciences (No. 2014KIB02) to DZL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009; 161: 105–121. [Google Scholar]
- 2. Mabberley DJ. Mabberley’s plant-book: a portable dictionary of plants, their classifications, and uses. 3rd ed Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 3. Pigott CD. Lime-trees and basswoods. A biological monograph of the genus Tilia. Cambridge: Cambridge University Press; 2012. [Google Scholar]
- 4. Engler A. Syllabus der Pflanzenfamilien. Berlin: Borntraeger; 1921. [Google Scholar]
- 5. Cronquist A. An intergrated system of classification of flowering plants. New York: Columbia University Press; 1981. [Google Scholar]
- 6. Bayer C, Fay MF, de Bruijn AY, Savolainen V, Morton CM, Kubitzki K, et al. Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: a combined analysis of plastid atpB and rbcL sequences. Bot J Linn Soc. 1999; 129: 267–303 [Google Scholar]
- 7. Alverson WS, Whitlock BA, Nyffeler R, Bayer C, Baum DA. Phylogenetic analysis of the core Malvales based on sequences of ndhF. Am J Bot. 1999; 86: 1474–1486. [PubMed] [Google Scholar]
- 8. Nyffeler R, Clemens Bayer, Alverson WS, Yen A, Whitlock BA, Chase MW, et al. Phylogenetic analysis of the Malvadendrina clade (Malvaceae s.l.) based on plastid DNA sequences. Org Divers Evol, 2005, 5(2): 109–123 [Google Scholar]
- 9. Fineschi S, Salvini D, Taurchini D, Carnevale S, Vendramin GG. Chloroplast DNA variation of Tilia cordata (Tiliaceae). Can J For Res. 2003; 33: 2503–2508. [Google Scholar]
- 10. Liesebach H, Sinkó Z. A contribution to the systematics of the genus Tilia with respect to some hybrids by RAPD analysis. Dendrobiology. 2008; 59: 13–22 [Google Scholar]
- 11. Filiz E, Birbilener S, Ozyigit II, Kulac S, Oruc FCS. Assessment of genetic variations of silver lime (Tilia tomentosa Moench.) by RAPD markers in urban and forest ecosystems, Biotechnol Biotechnol Equip. 2015; 29(4): 631–636 [Google Scholar]
- 12. Hosseinzadeh Colagar A, Yusefi M, Zarei M, Yousefzadeh A. Assessment of genetic diversity of Tilia rubra DC. by RAPD analysis in the Hyrcanian forests, North of Iran. Pol J Ecol. 2013; 61: 341–348 [Google Scholar]
- 13. Logan SA, Phuekvilai P, Wolff K. Ancient woodlands in the limelight: delineation and genetic structure of ancient woodland species Tilia cordata and Tilia platyphyllos (Tiliaceae) in the UK. Tree Genet. Genomes. 2015; 11: 52 [Google Scholar]
- 14.McCarthy D. Systematics and phylogeography of the genus Tilia in North America. PhD Thesis, University of Illinois. 2012.
- 15. Yousefzadeh H, Hosseinzadeh Colagar A, Tabari M, Sattarian A, Assadi M. Utility of the ITS region sequence and structure for molecular identification of the Tilia species from Hyrcanian forest, Iran. Plant Syst Evol. 2012; 298: 947–961 [Google Scholar]
- 16.Phuekvilai P. Relicts, Refugia and Reticulation: A study of population history, hybrids and phylogeny in the long-lived flowering tree genus Tilia. PhD Thesis, Newcastle University. 2014.
- 17. Zhang YJ, Ma PF, Li DZ. High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLoS ONE. 2011; 6(5): e20596 10.1371/journal.pone.0020596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruhsam M, Rai HS, Mathews S, Ross TG, Graham SW, Raubeson LA, et al. Does complete plastid genome sequencing improve species discrimination and phylogenetic resolution in Araucaria? Mol Ecol Resour. 2015. 10.1111/1755-0998.12375 [DOI] [PubMed] [Google Scholar]
- 19. Qiao J, Cai M, Yan G, Wang N, Li F, Chen B, et al. High-throughput multiplex cpDNA resequencing clarifies the genetic diversity and genetic relationships among Brassica napus, Brassica rapa and Brassica oleracea . Plant Biotechnol J. 2015; 10.1111/pbi.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009; 7: 84 10.1186/1741-7007-7-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma PF, Zhang YX, Zeng CX, Guo ZH, Li DZ. Chloroplast phylogenomic analyses resolve deep-level relationships of an intractable bamboo tribe Arundinarieae (Poaceae). Syst Biol. 2014. 63(6): 933–950. 10.1093/sysbio/syu054 [DOI] [PubMed] [Google Scholar]
- 22. Carbonell-Caballero J, Alonso R, Ibañez V, Terol J, Talon M, Dopazo J. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus . Mol Biol Evol. 2015; 32(8): 2015–2035 10.1093/molbev/msv082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raubeson LA, Jansen RK. Chloroplast genomes of plants In: Henry RJ, editor. Diversity and evolution of plants-genotypic and phenotypic variation in higher plants. Wallingford (UK): CABI publishing; 2005. pp. 45–68. [Google Scholar]
- 24. Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011; 76: 273–297. 10.1007/s11103-011-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bendich AJ. Circular chloroplast chromosomes: the grand illusion. Plant Cell. 2004; 16: 1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee SB, Kaittanis C, Jansen RK, Hostetler JB, Tallon LJ, Town CD, et al. The complete chloroplast genome sequence of Gossypium hirsutum: organization and phylogenetic relationships to other angiosperms. BMC Genomics. 2006; 7: 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Q, Xiong G, Li P, He F, Huang Y, Wang K, et al. Analysis of complete nucleotide sequences of 12 Gossypium chloroplast genomes: origin and evolution of Allotetraploids. PLoS ONE. 2012; 7(8): e37128 10.1371/journal.pone.0037128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jansen RK, Saski C, Lee SB, Hansen AK, Daniell H. Complete plastid genome sequences of three Rosids (Castanea, Prunus, Theobroma): evidence for at least two independent transfers of rpl22 to the nucleus. Mol Biol Evol. 2011; 28:835–847. 10.1093/molbev/msq261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bul. 1987; 19: 11–15. [Google Scholar]
- 30. Yang JB, Li DZ, Li HT. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Mol Ecol Resour. 2014; 14: 1024–1031. 10.1111/1755-0998.12251 [DOI] [PubMed] [Google Scholar]
- 31. Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE. 2012; 7: e30619 10.1371/journal.pone.0030619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004; 20(17): 3252–3255 [DOI] [PubMed] [Google Scholar]
- 33. Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013; 41(Web Server issue): W575–581. 10.1093/nar/gkt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002; 30(14): 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28(10): 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014; 30 (9):1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 2012; 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tajima F. Simple methods for testing the molecular evolutionary clock hypothesis. Genetics. 1993; 135: 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004; 32(Web Server issue): W273–W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saski C, Lee SB, Daniell H, Wood TC, Tomkins J, Kim HG, et al. Complete chloroplast genome sequence of Glycine max and comparative analyses with other legume genomes. Plant Mol Biol. 2005; 59: 309–322 [DOI] [PubMed] [Google Scholar]
- 41. Davis JI, Soreng RJ. Migration of endpoints of two genes relative to boundaries between regions of the plastid genome in the grass family (Poaceae). Am J Bot. 2010; 97(5): 874–892. 10.3732/ajb.0900228 [DOI] [PubMed] [Google Scholar]
- 42. Sun Y-x, Moore MJ, Meng A-p, Soltis PS, Soltis DE, Li J-q, et al. Complete plastid genome sequencing of Trochodendraceae reveals a significant expansion of the inverted repeat and suggests a Paleogene divergence between the two extant species. PLoS ONE. 2013; 8(4): e60429 10.1371/journal.pone.0060429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palmer JD. Chloroplast DNA exists in two orientations. Nature. 1983; 301: 92–93 [Google Scholar]
- 44. Martin G, Baurens FC, Cardi C, Aury JM, D'Hont A. The complete chloroplast genome of banana (Musa acuminata, Zingiberales): insight into plastid monocotyledon evolution. PLoS One. 2013; 8(6): e67350 10.1371/journal.pone.0067350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diekmann K, Hodkinson TR, Wolfe KH, van den Bekerom R, Dix PJ, Barth S. Complete chloroplast genome sequence of a major allogamous forage species, perennial ryegrass (Lolium perenne L.). DNA Res. 2009; 16(3):165–176. 10.1093/dnares/dsp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greiner S, Wang X, Rauwolf U, Silber MV, Mayer K, Meurer J, et al. The complete nucleotide sequences of the five genetically distinct plastid genomes of Oenothera, subsection Oenothera: I. sequence evaluation and plastome evolution. Nucleic Acids Res. 2008; 36(7): 2366–2378. 10.1093/nar/gkn081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim HT, Kim KJ. Chloroplast genome differences between Asian and American Equisetum arvense (Equisetaceae) and the origin of the hypervariable trnY-trnE intergenic spacer. PLoS ONE. 2014; 9(8): e103898 10.1371/journal.pone.0103898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Britten RJ, Rowen L, Williams J, Cameron RA. Majority of divergence between closely related DNA samples is due to indels. Proc Natl Acad Sci USA. 2003; 100(8): 4661–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anzai T, Shiina T, Kimura N, Yanagiya K, Kohara S, Shigenari A, et al. Comparative sequencing of human and chimpanzee MHC class I regions unveils insertions/deletions as the major path to genomic divergence. Proc Natl Acad Sci USA. 2003; 100(13): 7708–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ogurtsov AY, Sunyaev S, Kondrashov AS. Indel-based evolutionary distance and mouse-human divergence. Genome Res. 2004; 14(8): 1610–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987; 4(3): 203–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Four Tilia plastid genomes sequenced in current study are available in NCBI database. The Genbank numbers are KT894772, KT894773, KT894774, KT894775.