Summary
Flight maneuvers require rapid sensory integration to generate adaptive motor output. Bats achieve remarkable agility with modified forelimbs that serve as airfoils while retaining capacity for object manipulation. Wing sensory inputs provide behaviorally relevant information to guide flight; however, components of wing sensory-motor circuits have not been analyzed. Here, we elucidate the organization of wing innervation in an insectivore, the big brown bat, Eptesicus fuscus. We demonstrate that wing sensory innervation differs from other vertebrate forelimbs, revealing a peripheral basis for the atypical topographic organization reported for bat somatosensory nuclei. Furthermore, the wing is innervated by an unusual complement of sensory neurons poised to report airflow and touch. Finally, we report that cortical neurons encode tactile and airflow inputs with sparse activity patterns. Together, our findings identify neural substrates of somatosensation in the bat wing and imply that evolutionary pressures giving rise to mammalian flight led to unusual sensorimotor projections.
Introduction
Insectivorous bats perform complex aerial maneuvers to catch prey mid-flight. These dynamic flyers are capable of executing quick altitude drops and directional changes. Such agile flight is unmatched by gliding mammals and existing aircraft technologies. Bat wings have evolved not only for flight, like avian wings, but also for object manipulation, such as pup handling and capturing insects. This range of functions is possible because bat wings have more than 20 degrees of freedom in their independently movable joints, allowing them to readily adjust wing shape, or camber (Riskin et al., 2008). This wing flexibility is unique among flying animals, and provides an advantage when maneuvering during flight.
To fly, bats rely on rapid integration of sensory inputs to guide adaptive motor outputs. The contribution of hearing and vision to bat flight behaviors is established (Horowitz et al., 2004; Simmons et al., 1979); however, the role of touch has been largely overlooked since the discovery of echolocation (Chadha et al., 2011; Sterbing-D’Angelo et al., 2011; Zook, 2006). As bats flap their wings, they produce complex aerodynamic trails (Hedenstrom et al., 2007; Hubel et al., 2010). The air flowing over the wing stimulates microscopic hairs distributed over its dorsal and ventral surfaces. Hair follicles, a uniquely mammalian adaptation, are innervated by touch receptors that report hair deflection to the central nervous system. Depilating wing hairs from two echolocating bat species (E. fuscus and Carollia perspicillata) changed their flight behaviors, including turn angles and flight speeds (Sterbing-D’Angelo et al., 2011). These findings indicate that bats use tactile feedback from their forelimb-derived wings to inform motor output during flight.
Bats are not the only mammals in which somatosensory inputs guide skilled forelimb movements. Recent studies have defined proprioceptive circuits that control reaching and grasping behaviors in rodents (Azim et al., 2014; Bui et al., 2013; Esposito et al., 2014). In primates, cutaneous input from the hand is essential for manipulating objects (Witney et al., 2004). For example, tactile feedback is used for adjusting grip strength to prevent slip (Augurelle et al., 2003). Similarly, inputs from wing touch receptors might guide adjustments of a bat’s wing camber, akin to grip, when changing directions or adjusting to wind patterns. Although behavioral evidence establishes a role for wing tactile inputs in bat flight (Sterbing-D’Angelo et al., 2011), little is known about the identities and organization of somatosensory circuit components in bat wings.
Peripheral neurons are the inputs and outputs of vertebrate sensorimotor circuits. Somatosensory neurons of dorsal root ganglia (DRG) transduce tactile, temperature and proprioceptive information. Motor neurons, whose cell bodies are located in the ventral spinal cord, send motor commands to muscles. During development, motor and sensory neurons extend from the same spinal level to innervate individual body segments. This process is the basis of dermatome and myotome maps (Landmesser, 2001; Wang and Scott, 2002). Mammalian forelimbs are usually innervated by neurons from cervical segment 5 (C5) through the first thoracic segment (T1), but there is interspecies variability: rat forelimb innervation extends from C4–T2, whereas dolphin fins are innervated from levels C4–8 (Strickler, 1978; Takahashi et al., 2003). This somatotopic organization, in which adjacent anatomical areas are represented near each other, is set up in the periphery and preserved through multiple relays in the central nervous system (Florence et al., 1989; Kandel et al., 2012; Xu and Wall, 1999). Peripheral sensory innervation density determines the size of central representations; therefore, high-acuity skin areas are magnified in cortical maps.
In Chiroptera, however, somatotopic maps are atypical, displaying discontinuous representations of body areas and large forelimbs (Calford et al., 1985; Chadha et al., 2011; Martin, 1993). This suggests that peripheral innervation patterns of the forelimb might differ between bats and other vertebrate species. Shoulder musculature that generates the bat’s wing beat has been shown to arise from C5–T1 (Ryan et al., 1997; Tokita et al., 2012), but sensory innervation of the wing has not been analyzed. To investigate the organization of sensorimotor elements in bat wings, we performed anatomical and functional studies in E. fuscus, an echolocating insectivore that displays agile flight.
Results
Peripheral organization of wing sensorimotor circuitry
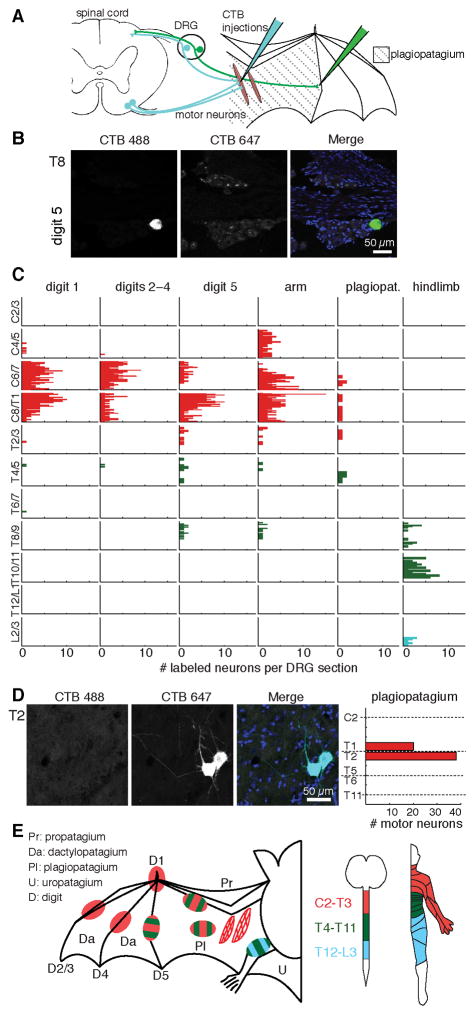
We hypothesized that bats have unique sensorimotor circuitry that reflects the wing membrane’s unusual ontogeny, deriving from the forelimb bud, trunk and hindlimb. Atypical organization of peripheral innervation should be most evident in the plagiopatagium because it develops through fusion of the forelimb bud and a flank-derived primordium (Weatherbee et al., 2006). The plagiopatagium is the largest part of the wing skin membrane, spanning the area between the fifth digit and body (Figure 1A). We performed anterograde neuronal tracing using subcutaneous injections of fluorescent Cholera toxin B (CTB). Focal injections in different wing sites labeled tens to hundreds of DRG neurons (Figure S1). Notably, labeling from individual injections was found in cervical, mid- and lower-thoracic DRGs (Figures 1B–C and S1). Labeling from digits 1–4 appeared at cervical and upper thoracic levels as observed in other mammalian species. For areas surrounding the plagiopatagium, however, some labeled neurons localized to mid-thoracic DRGs. Labeling from T3–T8 accounted for 4% of DRG neurons innervating the arm, 6% of DRG neurons in digit 5, and 18% of DRG neurons at plagiopatagial sites. Injections in plagiopatagial areas near the hindlimb also revealed atypical innervation, from T8–T11.
Figure 1. Bat wing neuronal tracing reveals atypical somatosensory-motor innervation.
(A) Schematic of neuronal tracing approach.
(B) T8 DRG section from bat wing injected at digit 5 with CTB Alexa-488 (green). Merged images shows DAPI-stained nuclei (blue).
(C) Histograms show the number of neurons labeled at each spinal level from all injections (≤1.5 μl per injection). Each column shows labeling from a separate wing site (N=2–3 injections per site from 2–3 bats). See also Figure S1. Color key in panel E.
(D) Motor neurons in upper thoracic spinal cord were labeled by injection of CTB Alexa-647 into plagiopatagial muscles. Merged image shows DAPI-stained nuclei (blue). Right, motor neuron quantification (N=6 injections in 2 bats). Dashed lines indicate transection levels of dissected spinal cords (see Supplemental Methods).
(E) Dermatome and myotome maps. Left, injection sites colored according to spinal level of innervation. Motor pools are represented by hatched areas. Middle: spinal level color key. Right, map of corresponding human dermatomes.
Plagiopatagial muscles tune stiffness of the wing membrane during flight (Cheney et al., 2014). These muscles, which are unusual because they lack bone insertions, derive from forelimb levels (Tokita et al., 2012). To identify spinal motor neurons that innervate the plagiopatagium, we targeted CTB injections to intramembranous muscles. Focal CTB injections showed that >98% of labeled motor neurons extended from levels T1–T3 to innervate plagiopatagial muscles (Figure 1D). By contrast, sensory neurons labeled by the same plagiopatagial injections extended from C6 through T5 (Figure 1C). Thus, the sensory innervation of the wing extends from a broader segmental range than the motor innervation, and arises from lower levels than other mammalian forelimbs (Figure 1E). Together, these findings support the hypothesis that the ontogeny of the bat wing, arising from the fusion of the forelimb and plagiopatagial buds, gives rise to atypical innervation patterns in the wing.
Identification of sensory receptors that innervate bat wings
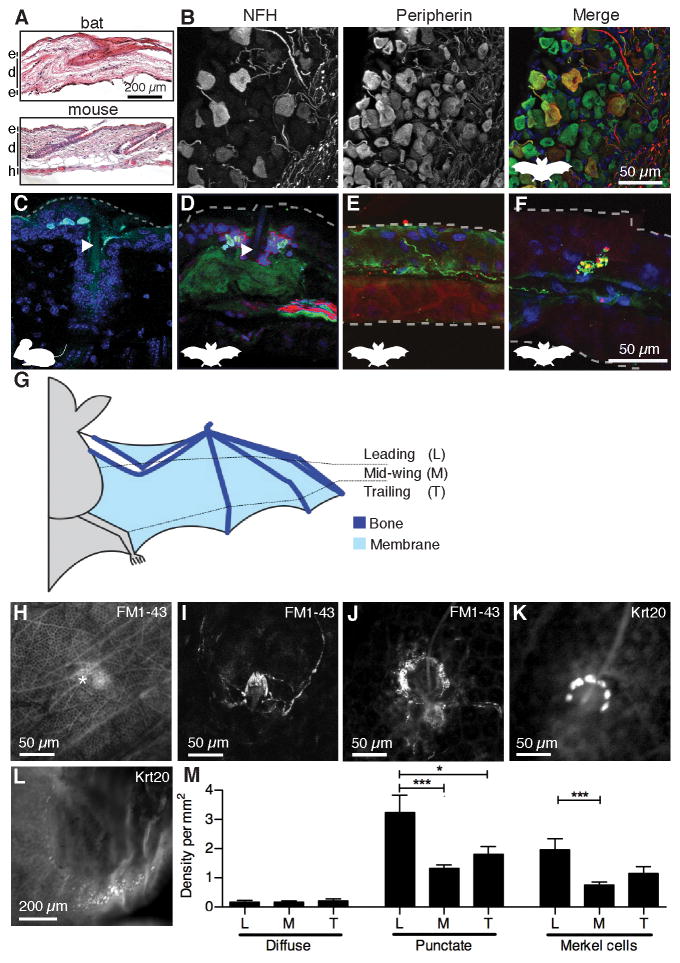
We next asked whether the repertoire of somatosensory receptors in wing skin differs from other mammalian limbs. Mammalian forelimbs are replete with morphologically diverse tactile receptors in hairy and glabrous (thick, hairless) skin, some of which have also been reported in bat wings (Ackert, 1914; Yin et al., 2009; Zook, 2006). Bat wing skin is thin, with two epidermal layers sandwiching the dermis (Swartz et al., 1996). The wing membrane has been proposed to be glabrous skin due to its lack of coat hair (Makanya and Mortola, 2007; Quay, 1970). Histological analysis revealed that the wing membrane in E. fuscus bears two defining features of hairy skin: hair follicles and thin epidermis. These two features are similar in bat wing membrane and mouse hairy skin, although follicle density differs (Figure 2A). Thus, we conclude that the wing membrane comprises hairy skin.
Figure 2. An unusual repertoire of touch receptors innervates bat wings.
(A) Skin histology of bat wing and mouse limb [epidermis (e), dermis (d), hypodermis (h)].
(B) Bat DRG labeled with antibodies against neurofilament H (NFH; red) and peripherin (green). DAPI (blue) labeled nuclei. Labeling and colors apply to B–F. See also Figure S2A.
(C–F) Immunohistochemistry of mouse limb (C) and bat wing skin (D–F). Dashed lines denote skin surfaces. (C) Keratin 8 (Krt8) antibodies (cyan) labeled mouse Merkel cells adjacent to a guard hair (arrowhead). (D) Krt20 antibodies (cyan) labeled bat Merkel cells around a wing hair (arrowhead). (E) Free nerve ending. (F) Knob-like ending. Scale applies to C–F.
(G) Schematic of wing areas.
(H–J) In vivo FM1-43 injections labeled (H) diffuse endings (asterisk) (I) lanceolate endings and (j) sensory neurons similar to mouse Merkel-cell afferents (see also Figure S2B–D).
(K–L) Merkel cells were surveyed using whole-mount Krt20 immunostaining of 12 wing areas (see Figure S2E). Merkel cells were found near hairs (K) and along fingertips (L).
(M) Sensory ending density at wing areas defined in (G). [N=4 wings from four bats (diffuse and punctate), N=4 wings from three bats (Merkel cells)]. Punctate endings and Merkel cells were unevenly distributed across wing areas (One-way ANOVA; P=0.0004 and P=0.002, respectively). Asterisks denote significance between groups by Bonferroni’s multiple comparison test. ***P≤0.001, **P≤0.01, *P≤0.05. Bars: mean ± SEM. See also Figure S2E–H.
We compared sensory endings in bat wing and mouse hairy skin by staining for Neurofilament H (NFH; a conserved marker of myelinated afferents) and peripherin, which is preferentially expressed in small diameter DRG neurons in rodents but appeared to be uniformly expressed in bat DRG neurons (Figures 2B and S2). We first examined Merkel cell-neurite complexes, which are innervated by myelinated afferents that report sustained pressure and contribute to shape discrimination (Johnson et al., 2000). In other mammals, Merkel cell-neurite complexes localize to areas of high tactile acuity, including fingerpads, whisker follicles, and touch domes surrounding guard (or tylotrich) hair follicles (Figure 2C). In bat wings, Merkel cells were likewise associated with hair follicles and innervated by NFH-positive neurons (Figure 2D). The bat epidermis was also innervated by NFH-negative free nerve endings (Figure 2E), which mediate nociception and thermoreception in rodent and human skin (Basbaum et al., 2009). Along with these conserved sensory endings, we observed NFH-positive neurons with unusual knob-like endings (Figure 2F). These structures resembled end-knobs described in 1914 in bat wing (Ackert, 1914) and Krause end-bulbs, which are proposed to respond to high force levels in glabrous skin of other mammals (Munger and Ide, 1988). These end organs have not been reported in the hairy skin; therefore, these data reveal that an usual combination of sensory receptors innervate bat wings.
We next analyzed how touch receptors are distributed across the wing to provide sensory feedback for behaviors such as food handling, pup cradling or flight (Figure 2G). In vivo injections of fluorescent FM1-43 were used to visualize sensory neurons (Figure 2H–J) and Keratin 20 (Krt20) antibodies to stain Merkel cells in whole mount (Figure 2K–L; Lesniak et al., 2014; Meyers et al., 2003). Three sensory receptor types were distinguished by FM1-43 labeling. We observed bright patches, ~50 μm in diameter, termed diffuse endings (Figures 2H & S2B). These endings were sparse but enriched in inter-digit membranes (Figures 2M & S2). Hair follicles, which were innervated by lanceolate endings visible at high magnification (Figure 2I), were marked by intense staining, termed punctate endings. Bat lanceolate endings appear similar to rapidly adapting low-threshold mechanoreceptors that report hair movement in mice (Figure S2C; Abraira and Ginty, 2013). Punctate hair receptors were enriched along leading wing edges and were more dense over bones than between digits (Figures 2M & S2). Finally, superficial sensory arbors formed crescents around some hair follicles (Figure 2J). These afferents were comparable to those that innervate Merkel cells in other species (Figure S2D). Consistent with this observation, Merkel-cell clusters were usually situated near hair follicles, and were distributed across the wing in a pattern similar to that of punctate hair receptors (Figures 2K & 2M). Although Merkel cells associate with only ~2% of rodent hair follicles (Li et al., 2011), almost half (47%) of all wing hairs were juxtaposed to Merkel cells. Thus, many wing hairs are dually innervated by lanceolate endings and Merkel-cell afferents, which serve as parallel sensory inputs to report hair movement. High Merkel-cell densities were sometimes also observed along digit tips and at knuckles, indicating these receptors are clustered at phalanges (Figure 2L). Thus, this systematic survey reveals a differential distribution of sensory endings across the wing.
Cortical representation of tactile inputs
To determine how wing sensory inputs are encoded in cortex, we performed in vivo single-unit recordings from neurons in primary somatosensory cortex (SI; Figure 3A). When wings were stimulated with spatially restricted (<1 cm2), 40-ms air puffs, neuronal response duration varied from 1–50 ms and displayed sparse firing patterns (Figure 3B–D), typically 1–3 spikes per stimulus (Figure 3E–F). Tactile responses to calibrated monofilaments were also tested in ten airflow-sensitive sites. We observed a high correspondence of responses to airflow and light touch (Figure 3C), suggesting that airflow and tactile stimulation activated common neural pathways. Airflow responses were similar under Isoflurane (Figure 3B) and Ketamine-Xylazine anesthesia (Figure 3D), indicating that sparse firing is not an epiphenomenon of Isoflurane anesthesia. Analysis of airflow-evoked responses showed decreased onset latency, but little change in spike counts, as a function of stimulus intensity (Figure S3). These findings suggest that bat SI cortex uses a sparse temporal onset code to guide wing adjustments during fast, dynamic flight.
Figure 3. SI neuronal response to airflow is encoded by onset latency rather than spike times.
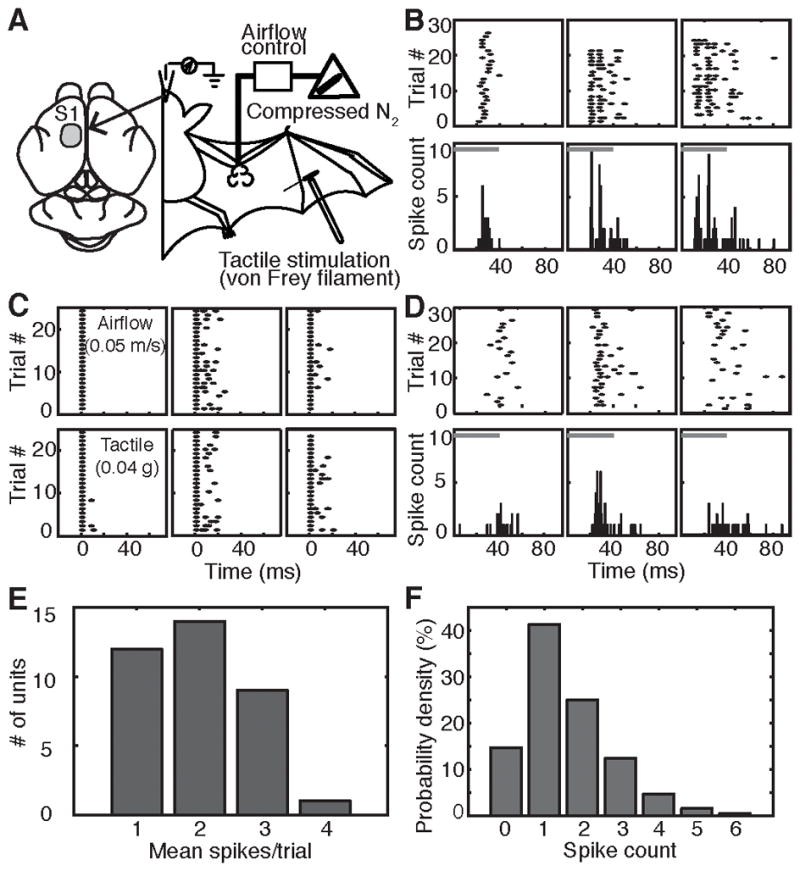
(A) Schematic of in vivo neurophysiological recordings.
(B) Raster plots (top) and post-stimulus time histograms (PSTH, bottom, 1-ms bins) of single-unit responses from three example neurons. Gray bars: stimulus duration.
(C) Responses of three neurons to airflow (top) and tactile stimulation (bottom). Responses were aligned to the first post-stimulus spike.
(D) Airflow responses of three representative neurons recorded under ketamine-xylazine anesthesia.
(E) Distribution of mean spikes/trial across all neurons (N=35) and stimulus conditions. See also Figure S3.
(F) Distribution of number of spikes elicited by air puffs for sampled neurons (N=35) pooled across all stimuli.
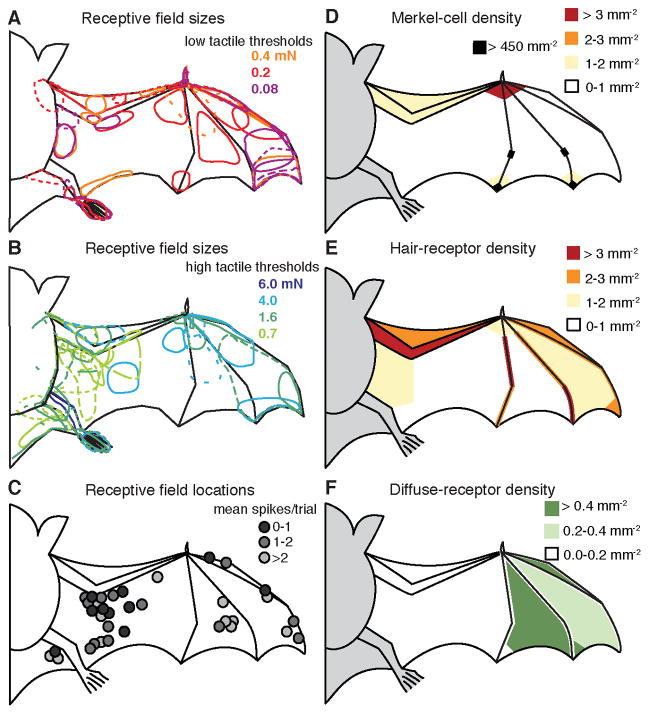
Summary maps of SI receptive fields and receptor distributions provide insights into somatosensory specializations that have evolved in the bat’s multifunctional forelimbs to guide motor behaviors (Figure 4). The receptive fields of SI neurons, which varied from punctate on the thumb to large on wing membranes, might reflect innervation of multiple hair follicles by single afferents (Li et al., 2011), multiunit responses or cortical integration of many sensory neurons. Multiunit SI responses to tactile stimuli (Figure 4A–B; Chadha et al., 2011) and airflow-sensitive single-unit responses (Figure 4C) were distributed across wing locations; however, studies of airflow over flying bats’ wings reveal dramatic differences in airflow patterns at different wing sites. For example, large vortices on the leading edge are important to enhance lift (Muijres et al., 2008). Notably, SI units with the lowest thresholds, comparable to the human fingertip, were prominent along the leading edge (Figure 4C), where hair-associated receptors and Merkel cells were anatomically enriched (Figure 4D–E). Merkel cells were most abundant on phalanges (Figure 4D). Diffuse endings were enriched in the dactylopatagium wing membrane (Figure 4F) in a pattern complementary to hair receptors. These distribution maps reveal wing regions that are functionally and anatomically specialized for tactile inputs.
Figure 4. Response properties of SI cortical neuron receptive fields and peripheral receptor densities.
(A–B) Receptive field sizes and response thresholds for multiunit SI neurons responding to tactile stimulation. Colors correspond to von Frey thresholds.
(C) Receptive field locations for air-puff sensitive single units. Grayscale indicates mean spikes per trial.
(D–F) Density maps of anatomical sensory endings.
Discussion
The anatomical and functional analysis presented here sets up a system that can be used to discover paradigms for how coherent neural circuits form in appendages that derive from multiple embryonic regions. Our observations demonstrate that the evolutionary progression that gave rise to the bat wing membrane has resulted in atypical somatosensory inputs, which have been co-opted to enhance flight control (Sterbing-D’Angelo et al., 2011). Consistent with this notion, mixed cranial and cervical motor projections innervate the propatagium, which evolved independently in birds, bats and gliding mammals (Chickering and Sokoloff, 1996; Thewissen and Babcock, 1991). Thus, vertebrate nervous systems have flexibly adapted to accommodate anatomical specializations for flight.
Our findings suggest that the ontogeny of the wing gives rise to the development of unusual tactile circuitry. Whereas the segmental organization of motor neurons is similar to other mammalian forelimbs, sensory innervation by mid- to lower-thoracic DRGs has not been reported in dermatome maps. This expanded innervation is not simply due to the enlarged size of the wing. Instead, mammals with larger forearms typically have larger sensory ganglia at brachial levels, rather than an extended innervation range. For example, in proportion to their body size, primates have larger forelimbs than rodents, yet spinal levels innervating forelimbs in these species are similar: C4–T2 in rats (Angelica-Almeida et al., 2013; Takahashi and Nakajima, 1996) and C5–T1 in humans (Bromberg, 2014). The innervation of the bat forelimb extends beyond this range by six segmental levels. Moreover, focal injections demonstrated that a localized region of the wing can be innervated by DRG neurons distributed over 11 spinal levels. By contrast, small tracer applications in rodent limbs labeled neurons from 3–6 spinal levels (Bacskai et al., 2013; Takahashi et al., 2003). We hypothesize that mid- and lower thoracic innervation in the bat derives from the trunk in development. During development, forelimb proprioceptors require motor neuron outgrowth to find their peripheral targets but cutaneous neurons do not (Swanson and Lewis, 1986). Thus, it is possible that the observed thoracic innervation in bat represents cutaneous neurons from the trunk that grow to reach local targets during development, whereas motor and proprioceptive neurons extend from upper thoracic levels (Bacskai et al., 2013; Ryan et al., 1997; Tokita et al., 2012).
Our results also lend insight into the discontinuous organization of gracile and cuneate nuclei reported in Chiroptera (Martin, 1993). Unlike other mammals, somatotopic representations in brainstem nuclei of the flying fox do not preserve spatial relationships of peripheral tissues. Instead, representations of the body’s surface are organized into bands that intermingle in the trunk, plagiopatagium, hindlimb and digits. Most notably, the back, abdomen and side representations split the plagiopatagium representation into two parts. The observation that mid- and lower thoracic DRGs innervate all of these body sites suggests a peripheral basis for the unusual topography in bat gracile and cuneate nuclei. Future studies of brainstem nuclei in E. fuscus and other bat species are needed to evaluate this hypothesis and to determine whether organizational principles are conserved among flying mammals.
Interestingly, thalamic and cortical regions are organized somatotopically in E. fuscus and other bats, although the forelimb representation is rotated compared with other mammals (Calford et al., 1985; Chadha et al., 2011; Manger et al., 2001). Our results indicate the somatotopic maps in the bat’s higher brain areas cannot be explained by peripheral innervation patterns. Instead, somatotopic maps are likely to be refined through central mechanisms that control projection patterns between brainstem and thalamic relays (Manger et al., 2001; Martin, 1993).
Along with neuronal specializations, wing evolution has resulted in unusual skin features. For example, Merkel cells were juxtaposed to almost half of wing hair follicles. By contrast, Merkel cells in the mouse coat selectively associate with guard hairs, which are the least prevalent hair type. We propose that the evolutionary loss of drag-inducing coat hairs on the bat wing can account for both the sparse distribution of wing hair follicles and high percentage associated with Merkel cells. Another unusual feature is that hair follicles appeared in all wing areas, including the ventral thumb, a region that is covered with glabrous skin in other mammals. Developmental studies of bat wings indicate that negative regulators of Bone Morphogenetic Protein (BMP) signaling during limb formation provide an anti-apoptotic signal that results in interdigital webbing (Weatherbee et al., 2006). In mice, inhibiting BMP signaling triggers ectopic hair growth on glabrous skin (Mayer et al., 2008); therefore, the anti-apoptotic mechanisms that govern wing membrane formation might also account for its unusual hair localization.
Hair-follicle receptors are proposed to serve as biosensors to detect changes in boundary-layer airflow and provide feedback to prevent stall (Dickinson, 2010; Sterbing-D’Angelo et al., 2011). Detection of hair deflection is consistent with the function of lanceolate endings in other species, but our findings suggest an unconventional role in the context of flight: airflow sensing. In mice, different hair follicle types are innervated by distinct receptor complements; therefore, individual hairs serve as units of multimodal tactile integration (Li et al., 2011). Similarly, we found that some hair follicles were associated with both lanceolate endings and Merkel cells. Interestingly, mouse hair receptors with overlapping receptive fields form columnar projections in the dorsal horn of the spinal cord (Abraira and Ginty, 2013). Defining the projections of wing tactile receptors and the circuitry by which they impinge on the motor system are important areas for future investigations.
The distribution of sensory endings across the wing indicates that tactile specializations could support distinct sensory-guided behaviors. For example, Merkel cells were concentrated on the phalanges, where they could provide information about surface features during climbing and food handling. This is consistent with their role in encoding object features in other mammals (Johnson et al., 2000). Based on the enrichment of diffuse endings in skin membranes, we propose that these receptors detect skin stretch and changes in wing camber. The identity of diffuse endings was not discernable from in vivo labeling; however, based on location and size, we hypothesize that they correspond to end-knobs observed in cryosections. The localization of end-knob receptors in hairy skin might be a specialization of the wing membrane, which is subjected to turbulent forces during flight (Muijres et al., 2008). DRG recordings are needed to confirm the functional identities of the bat wing’s somatosensory receptors.
Recordings from supragranular SI cortex revealed that tactile information produced by air puff stimulation of the wing surface is carried by a sparse temporal “onset-only” code, with little change in spike counts as a function of stimulus intensity. The tactile stimulation a bat encounters during flapping flight is complex and contains high-frequency fluctuations. Our data indicates that spike timing might play a role in processing of such spectrally complex, high-frequency stimuli. Under this type of stimulation, SI activity in the bat must be regarded as rapidly-adapting, despite the presence of Merkel cell-neurite complexes, which are slowly adapting receptors in other mammals (Iggo and Muir, 1969; Woodbury and Koerber, 2007). This does not necessarily indicate that these receptors fail to contribute to the response observed in SI. In the rodent whisker system, rapidly and slowly adapting inputs converge on second order neurons, suggesting pre-cortical processing (Sakurai et al., 2013). Moreover, tactile features that activate both rapidly and slowly adapting inputs are represented by sparse temporal encoding in the somatosensory cortex of awake, behaving rodents (Jadhav et al., 2009; Szwed et al., 2003). Finally, sparse coding in upper layers of the cortex is found across many sensory stimulus types and recording conditions, so this could reflect our electrode position in the cortex (Olshausen and Field, 2004). The concordance between cortical response profiles to touch and air puffs indicate that these stimuli are processed by the same neuronal pathways in bats. Thus, although the peripheral organization of somatosensory inputs differs in bats and rodents, some cortical encoding mechanisms appear to be conserved. An intriguing question for future study is how these somatosensory signals are integrated with motor circuits to guide behaviors that underlie the bat’s diverse repertoire of forelimb-dependent functions.
Although the evolution of flight has proven to be an advantageous adaptation for Chiroptera, an open question is whether the wing’s tactile receptors provide a selective advantage in flight. Chiroptera represents about 20% of all mammalian species, which provides rich fodder for comparing the behavioral consequences and functional organization of wing sensorimotor circuitry across species and ecological niches. Such future studies are needed to understand the evolutionary benefits of the bat wing’s somatosensory specializations.
Experimental procedures
All procedures complied with the Institutional Animal Care and Use Committees of the University of Maryland and Columbia University Medical Center. Extended methods include details of immunohistochemistry, in vivo neuronal labeling, cortical electrophysiology and data analysis. Statistical comparisons were made with ANOVA or Student’s two-tail t tests as noted.
Supplementary Material
Acknowledgments
Thanks to Dr. Thomas Jessell for discussions, Wei Xian for technical support, and the Catherine Carr lab for access to their facilities. Funding was provided by AFOSR grant FA95501210109 (to SSD and CFM) and NIH/NINDS grant R01NS073119 (to EAL and G. J. Gerling). LD was supported by T32HD007430. Histology and microscopy was performed with support from the Columbia Skin Disease Research Center (P30AR044535).
Footnotes
Author contributions
KLM mapped sensory and motor innervation (Figs. 1 and S1), performed histochemical studies and mapped fluorescent sensory endings (Figs. 2, S2 and 4D–F). MC performed cortical recordings and mapped air-puff sensitive units (Figs. 3, S3 and 4C). LAD participated in dermatome mapping and analysis (Figs. 1 and S1). SJSD performed cortical recordings and mapped tactile receptive fields (Figs 3, S3 and 4A–4C). CFM, EAL, and SJSD supervised the project. All authors designed experiments and contributed to data analysis and interpretation. KLM took the lead on writing the manuscript and all authors contributed, as well as edited the manuscript.
The authors declare no conflict of interest.
References
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackert JE. Innervation of the integument of chiroptera. Journal of Morphology. 1914;25:301–343. [Google Scholar]
- Angelica-Almeida M, Casal D, Mafra M, Mascarenhas-Lemos L, Martins-Ferreira J, Ferraz-Oliveira M, Amarante J, Goyri-O’Neill J. Brachial plexus morphology and vascular supply in the wistar rat. Acta medica portuguesa. 2013;26:243–250. [PubMed] [Google Scholar]
- Augurelle AS, Smith AM, Lejeune T, Thonnard JL. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J Neurophysiol. 2003;89:665–671. doi: 10.1152/jn.00249.2002. [DOI] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508:357–363. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai T, Fu Y, Sengul G, Rusznak Z, Paxinos G, Watson C. Musculotopic organization of the motor neurons supplying forelimb and shoulder girdle muscles in the mouse. Brain structure & function. 2013;218:221–238. doi: 10.1007/s00429-012-0396-3. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg MB. UpToDate. Watham, MA: 2014. Brachial plexus syndromes: Anatomy. [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron. 2013;78:191–204. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Graydon ML, Huerta MF, Kaas JH, Pettigrew JD. A variant of the mammalian somatotopic map in a bat. Nature. 1985;313:477–479. doi: 10.1038/313477a0. [DOI] [PubMed] [Google Scholar]
- Chadha M, Moss CF, Sterbing-D’Angelo SJ. Organization of the primary somatosensory cortex and wing representation in the Big Brown Bat, Eptesicus fuscus. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2011;197:89–96. doi: 10.1007/s00359-010-0590-9. [DOI] [PubMed] [Google Scholar]
- Cheney JA, Konow N, Middleton KM, Breuer KS, Roberts TJ, Giblin EL, Swartz SM. Membrane muscle function in the compliant wings of bats. Bioinspiration & biomimetics. 2014;9:025007. doi: 10.1088/1748-3182/9/2/025007. [DOI] [PubMed] [Google Scholar]
- Chickering JG, Sokoloff AJ. Innervation of propatagial musculature in a flying squirrel, Glaucomys volans (Rodentia, Sciuridae) Brain, behavior and evolution. 1996;47:1–7. doi: 10.1159/000113224. [DOI] [PubMed] [Google Scholar]
- Dickinson BT. Hair receptor sensitivity to changes in laminar boundary layer shape. Bioinspiration & biomimetics. 2010;5:16002. doi: 10.1088/1748-3182/5/1/016002. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Somatotopic organization of inputs from the hand to the spinal gray and cuneate nucleus of monkeys with observations on the cuneate nucleus of humans. J Comp Neurol. 1989;286:48–70. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]
- Hedenstrom A, Johansson LC, Wolf M, von Busse R, Winter Y, Spedding GR. Bat flight generates complex aerodynamic tracks. Science. 2007;316:894–897. doi: 10.1126/science.1142281. [DOI] [PubMed] [Google Scholar]
- Horowitz SS, Cheney CA, Simmons JA. Interaction of vestibular, echolocation, and visual modalities guiding flight by the big brown bat, Eptesicus fuscus. Journal of vestibular research : equilibrium & orientation. 2004;14:17–32. [PubMed] [Google Scholar]
- Hubel TY, Riskin DK, Swartz SM, Breuer KS. Wake structure and wing kinematics: the flight of the lesser dog-faced fruit bat, Cynopterus brachyotis. The Journal of experimental biology. 2010;213:3427–3440. doi: 10.1242/jeb.043257. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Wolfe J, Feldman DE. Sparse temporal coding of elementary tactile features during active whisker sensation. Nat Neurosci. 2009;12:792–800. doi: 10.1038/nn.2328. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol. 2000;17:539–558. doi: 10.1097/00004691-200011000-00002. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5. United States: McGraw-Hill; 2012. [Google Scholar]
- Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2001;19:175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Lesniak DR, Marshall KL, Wellnitz SA, Jenkins BA, Baba Y, Rasband MN, Gerling GJ, Lumpkin EA. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. eLife. 2014;3:e01488. doi: 10.7554/eLife.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makanya AN, Mortola JP. The structural design of the bat wing web and its possible role in gas exchange. Journal of anatomy. 2007;211:687–697. doi: 10.1111/j.1469-7580.2007.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger PR, Rosa MG, Collins R. Somatotopic organization and cortical projections of the ventrobasal complex of the flying fox: an “inverted” wing representation in the thalamus. Somatosensory & motor research. 2001;18:19–30. doi: 10.1080/08990220020002079. [DOI] [PubMed] [Google Scholar]
- Martin RL. Representation of the body surface in the gracile, cuneate, and spinal trigeminal nuclei of the little red flying fox (Pteropus scapulatus) J Comp Neurol. 1993;335:334–342. doi: 10.1002/cne.903350304. [DOI] [PubMed] [Google Scholar]
- Mayer JA, Foley J, De La Cruz D, Chuong CM, Widelitz R. Conversion of the nipple to hair-bearing epithelia by lowering bone morphogenetic protein pathway activity at the dermal-epidermal interface. The American journal of pathology. 2008;173:1339–1348. doi: 10.2353/ajpath.2008.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muijres FT, Johansson LC, Barfield R, Wolf M, Spedding GR, Hedenstrom A. Leading-edge vortex improves lift in slow-flying bats. Science. 2008;319:1250–1253. doi: 10.1126/science.1153019. [DOI] [PubMed] [Google Scholar]
- Munger BL, Ide C. The structure and function of cutaneous sensory receptors. Arch Histol Cytol. 1988;51:1–34. doi: 10.1679/aohc.51.1. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Quay WB. Histology of the para-anal sebaceous glandular organs of the bat Eonycteris spelaea (Chiroptera: Pteropidae) The Anatomical record. 1970;166:189–197. doi: 10.1002/ar.1091660207. [DOI] [PubMed] [Google Scholar]
- Riskin DK, Willis DJ, Iriarte-Diaz J, Hedrick TL, Kostandov M, Chen J, Laidlaw DH, Breuer KS, Swartz SM. Quantifying the complexity of bat wing kinematics. Journal of theoretical biology. 2008;254:604–615. doi: 10.1016/j.jtbi.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Cushman J, Baier C. Organization of forelimb motoneuron pools in two bat species (Eptesicus fuscus and Myotis lucifugus) Acta anatomica. 1997;158:121–129. [PubMed] [Google Scholar]
- Sakurai K, Akiyama M, Cai B, Scott A, Han BX, Takatoh J, Sigrist M, Arber S, Wang F. The organization of submodality-specific touch afferent inputs in the vibrissa column. Cell reports. 2013;5:87–98. doi: 10.1016/j.celrep.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JA, Fenton MB, O’Farrell MJ. Echolocation and pursuit of prey by bats. Science. 1979;203:16–21. doi: 10.1126/science.758674. [DOI] [PubMed] [Google Scholar]
- Sterbing-D’Angelo S, Chadha M, Chiu C, Falk B, Xian W, Barcelo J, Zook JM, Moss CF. Bat wing sensors support flight control. Proc Natl Acad Sci U S A. 2011;108:11291–11296. doi: 10.1073/pnas.1018740108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler TL. Myology of the shoulder of Pontoporia blainvillei, including a review of the literature on shoulder morphology in the cetacea. The American journal of anatomy. 1978;152:419–431. doi: 10.1002/aja.1001520310. [DOI] [PubMed] [Google Scholar]
- Swanson GJ, Lewis J. Sensory nerve routes in chick wing buds deprived of motor innervation. Journal of embryology and experimental morphology. 1986;95:37–52. [PubMed] [Google Scholar]
- Swartz SM, Groves MD, Kim HD, Walsh WR. Mechanical properties of bat wing membrane skin: aerodynamic and mechanical functions. Journal of Zoology. 1996;239:357–378. [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron. 2003;40:621–630. doi: 10.1016/s0896-6273(03)00671-8. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003;462:29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- Thewissen JG, Babcock SK. Distinctive cranial and cervical innervation of wing muscles: new evidence for bat monophyly. Science. 1991;251:934–936. doi: 10.1126/science.2000493. [DOI] [PubMed] [Google Scholar]
- Tokita M, Abe T, Suzuki K. The developmental basis of bat wing muscle. Nature communications. 2012;3:1302. doi: 10.1038/ncomms2298. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA. Development of “normal” dermatomes and somatotopic maps by “abnormal” populations of cutaneous neurons. Developmental biology. 2002;251:424–433. doi: 10.1006/dbio.2002.0824. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Behringer RR, Rasweiler JJt, Niswander LA. Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc Natl Acad Sci U S A. 2006;103:15103–15107. doi: 10.1073/pnas.0604934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witney AG, Wing A, Thonnard JL, Smith AM. The cutaneous contribution to adaptive precision grip. Trends in neurosciences. 2004;27:637–643. doi: 10.1016/j.tins.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. J Comp Neurol. 2007;505:547–561. doi: 10.1002/cne.21517. [DOI] [PubMed] [Google Scholar]
- Xu J, Wall JT. Functional organization of tactile inputs from the hand in the cuneate nucleus and its relationship to organization in the somatosensory cortex. J Comp Neurol. 1999;411:369–389. [PubMed] [Google Scholar]
- Yin J, Wang H, Racey P, Zhang S. Distribution and ultrastructure of Merkel cell of the fishing bat (Myotis ricketti) Science in China Series C, Life sciences / Chinese Academy of Sciences. 2009;52:802–806. doi: 10.1007/s11427-009-0118-0. [DOI] [PubMed] [Google Scholar]
- Zook JM. Somatosensory Adaptations of Flying Mammals. In: Kaas JH, editor. Evolution of Nervous Systems. Oxford: Academic Press; 2006. pp. 215–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.