Abstract
The work presented here reports the use of long lifetime (>1 μs) BSA Au clusters as a cellular/tissue, time gated, intensity imaging probe. By collecting the emission signal 50 ns post excitation, one can off-gate the intense auto-fluorescence background, thereby greatly enhancing the clarity/specificity in fluorescence imaging.
Introduction
Near infrared (NIR) imaging is one of the most widespread optical imaging techniques in biomedical sciences. Fluorescence imaging modalities, particularly NIR fluorescence imaging, has several advantages over other imaging techniques, such as sensitivity and multiplex detection capabilities. Current NIR imaging heavily relies upon NIR organic dyes. Utilizing organic dyes remains problematic due to several reasons such as rapid photo-bleaching, small stokes shift between excitation and emission, and partial spectral overlap with auto-fluorescence. Moreover, organic fluorophores have their fluorescence lifetimes in the range of 1–5 ns, close to auto-fluorescence lifetime, making it difficult to separate the emission signal of the fluorophore from the auto-fluorescence of the cell. Auto-fluorescence from biological samples is one of the biggest hurdles in fluorescence experiments. It is not only difficult to separate the auto-fluorescence from the organic dye spectrally, but temporally as well, since the lifetime of auto-fluorescence is similar to the lifetime of routinely used organic fluorophores (fluorescein and rhodamine). Rich et al., in their recent report, explained different methods to overcome issues with auto-fluorescence and the disadvantages associated with them, by showing how a long fluorescence lifetime organic dye can be used to remove auto-fluorescence background1. Hence, there is a fundamental need to develop new imaging probes for emission intensity and lifetime imaging applications.
With rapid growth and development in biomedical nanotechnology, synthesis and development of luminescent nanomaterials is gaining increasing attention among the scientific community 2–4. Despite the superior optical properties of quantum dots, they are of limited use due to several disadvantages. First, quantum dots emitting in visible region tend to overlap with the auto-fluorescence spectra. Second, quantum dots with NIR emission are large in size and can introduce perturbations in the targeted cell. Third, toxicity due to toxic core materials used in synthesis further limits its usage 5, 6. Lastly, despite good photo stability, quantum dots show photo-blinking which affects the emission signal during imaging. Recently, a newer class of nanobio probe protein protected metal nanoclusters has gained increasing attention due to reasonably strong and tunable fluorescence in visible and NIR region. Proteins with differing molecular weight and amino acid sequences have been used to synthesize these metal nanoclusters. Namely, bovine serum albumin (BSA), human serum albumin (HSA), lysozyme, trypsin and the ferritin family of proteins have been used to synthesize metal nanoclusters7–11. Among these BSA Au clusters appear to be most researched preparation due to its optical properties12–17 and biosensing applications7, 18–21.
We are interested in cellular imaging applications of these BSA Au nanoclusters. There are several reports mentioning the use of BSA Au clusters as emission intensity based imaging probes. Koyakutty et al. showed that folic acid conjugated BSA Au clusters can internalize into oral and breast cancer cells through interaction with folate receptor 22. Koyakutty et al., in another report, showed that BSA Au clusters conjugated with antibodies against CD33 myeloid antigen, were specifically taken up by leukemia cells over expressing CD33 myeloid antigens thus enabling their use in targeted flow cytometry and imaging of cancer cells23. Chen et al. simultaneously conjugated folic acid and doxorubicin to BSA Au nanoclusters and used them for in vivo tracking and cancer therapy24. Methionine conjugated BSA Au clusters were also used similarly as doxorubicin conjugated BSA Au clusters to treat and image cancer cells in vivo 25. Irudayaraj et al. conjugated BSA Au clusters with Herceptin to show that it can be used simultaneously as an imaging and treatment modality 26.
Furthermore, Qing et al. reported the use of BSA Au clusters for in vivo imaging of mice tumor xenograft models and showed that BSA Au clusters accumulate in the tumor through enhanced permeability and retention (EPR) effect27.
Despite the numerous reports on the use of BSA Au clusters as an imaging probe due to its emission intensity, there are no reports on its use as a fluorescence lifetime and time gated intensity imaging probe to the best of our knowledge. There are several publications reporting the microsecond lifetime of BSA Au clusters 14, 16, 28. This long lifetime is several hundred fold longer than the auto-fluorescence lifetime (~7 ns)29, 30 and can be effectively used to off-gate cellular auto-fluorescence by time gated imaging. The approach, referred to as “time gating”, entails discarding all detected photons after each excitation pulse until the point where the background (auto-fluorescence) has decayed completely and the probe continues to fluoresce. Herein we report a strategy utilizing the long fluorescence lifetime of BSA Au cluster as a fluorescence lifetime and time gated intensity imaging probe.
Experimental Section
Materials
Chloroauric acid (HAuCl4), bovine serum albumin, fraction V (BSA) and acetone (reagent grade) were purchased from Sigma Aldrich Co. LLC. RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Pen-strep), and EDTA were purchased from Gibco, Life Technologies.
Synthesis of BSA Au clusters
The Au nanoclusters used in this study were synthesized using an approach developed by Xie et al.11. Typically, 5 mL of 10 mM HAuCl4 was mixed with 5 mL of 50 mg/mL BSA and incubated overnight at 37°C. The light brown cluster solution was further dialyzed (2000 MWCO membrane, Slide-A-lyzer, dialysis cassette, Thermo Scientific) against de-ionized water for at least 12 hr with periodic change of water to remove any small impurities. The dialyzed cluster solution was filtered using a 0.02 μm syringe filter and then used for subsequent measurements.
Cellular staining
4T1 murine mammary carcinoma cell line obtained from American Type Culture Collection (ATCC), Manassas, VA, USA was grown to seventy percent confluence in RPMI supplemented with 10% FBS and 1% Pen-Strep. Cells were trypsinized using 0.25 % Trypsin-EDTA and seeded on 20 mm round glass cover slips. After 24 hrs, cells were fixed using cold acetone (reagent grade-Sigma Aldrich) for 10 min at 4 °C followed by blocking with 1% BSA solution for 15–20 min at room temperature. Cells were washed with PBS and incubated with BSA Au clusters (5 μM) and fluorescein (0.2 μM) for 60 min and rinsed with PBS. The cluster loaded cells on the cover-slips were air dried and mounted on 24×24 mm cover glass using ProLong® Gold Antifade Reagent and stored at 4°C for fluorescence lifetime imaging experiments.
Lifetime and time gated imaging
Laser excitation was provided by a pulsed laser diode (PDL-470) emitting 470 nm light and driven by a PDL 828 “Sepia II” driver. This driver was operated at 315 KHz. Measurements were performed on a MicroTime 200 time-resolved, confocal microscope by PicoQuant. The excitation and emission light was focused by a 60× 1.2 NA Olympus objective in an Olympus IX71 microscope, and the emission light was filtered by a 488 long wave pass filter before passing through a 50 μm pinhole. Detection was made by a hybrid photomultiplier assembly. The resolution of the time correlated single photon counting (TCSPC) module was set to 512 ps/bin in order to facilitate the detection of the long-lived dye, producing a measurement window around 1.2 μs in length. All data analysis was performed using the SymPhoTime software, version 5.3.2. All experimental equipment and the SymPhoTime software were provided by PicoQuant, GmbH as part of the MicroTime 200 system.
Results and discussion
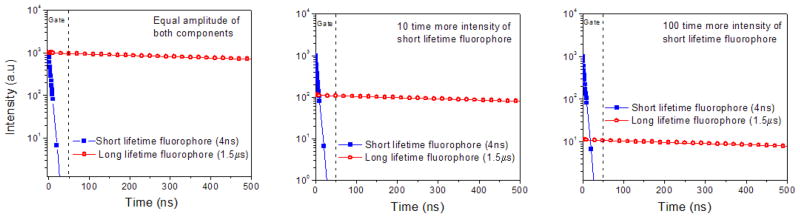
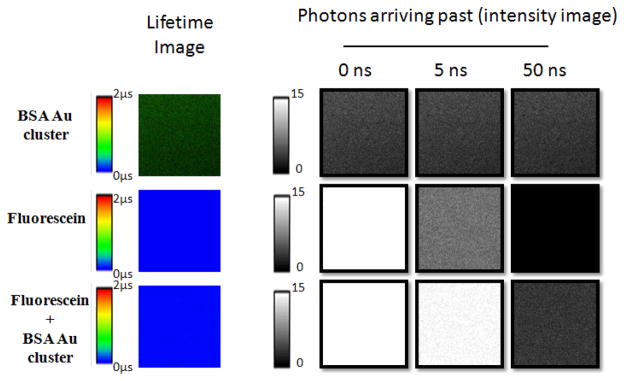
In cellular imaging using fluorescence intensity, the contribution from light scattering and sample auto-fluorescence imparts large background signal to all images. Fluorescence lifetime imaging microscopy (FLIM) offers two big advantages, namely the separation of fluorescence emission not only in the energy/color scale but also in time. FLIM can not only be used to image/measure lifetimes of the individual fluorophores due to environmental effects, but can also be used to differentiate between distinct fluorophores of the same color that stain different parts of the cell. The obstacle for FLIM has been the available dyes. Today, all common, bright dyes have lifetimes in the range of 0.2–5 ns making it difficult to distinguish two dyes from each other 31–33. One of the strengths of the BSA Au clusters is its very long fluorescence lifetime that enables us to suppress the background signal using gated detection. Figure 1 shows the simulated fluorescence intensity decays (at 1:1, 10:1 and 100:1 intensity ratio of a short to a long lifetime fluorophore) of typical fluorophores with lifetime 4 ns and BSA Au cluster (long lifetime fluorophore, 1.5 μs). It is visible that even with 10 times and 100 times brighter background one can collect the photons/signal coming from BSA Au clusters, if we collect the photons past 50 ns of the excitation pulse owing to the complete decay of short lifetime background. It is evident that the addition of long lived BSA Au clusters opens up the entire lifetime spectrum in FLIM and time gated imaging, allowing multiple probes to be detected in the same color channel. We tested our hypothesis by placing a drop of each of the three samples on a glass cover slip: BSA Au clusters, fluorescein and a mixture of fluorescein and BSA Au clusters. Lifetime images and intensity images at 5 and 50 ns post excitation pulse were acquired for all the three samples. Figure 2 shows the results of this measurement. The top panel depicting BSA Au clusters shows that even after 50 ns post excitation, we did not lose much of the emission signal. The fluorescein only sample (middle panel), on the other hand, shows reduced intensity after 5 ns gating and complete signal depletion after 50 ns gate. Furthermore, a mixture of fluorescein and BSA Au cluster in the lower panel shows strong signal from fluorescein even after gating for 5 ns. However, the 50 ns gate completely removed the fluorescein signal and BSA Au clusters’ emission dominated the signal intensity. This suggests that BSA Au clusters can be used to probe the systems with enormously high background samples.
Figure 1.
Simulated fluorescence intensity decays (assuming single exponential decay) of representative short lifetime fluorophore and long lifetime fluorophore at different situations. Equal intensity (left panel), 10 times (middle panel) and 100 times (right panel) more intensity of short lifetime fluorophore than long lifetime probe.
Figure 2.
Fluorescence lifetime and time gated intensity images of BSA Au cluster, Fluorescein and mixture of BSA Au cluster with fluorescein prepared on glass cover slips.
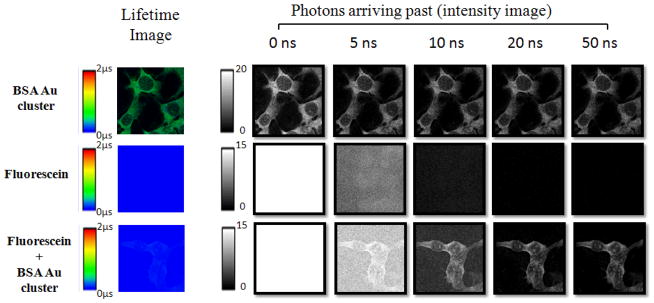
To demonstrate the applicability in a biological setting, a comparative study was initiated using fluorescein to mimic background fluorescence of enormous intensity, a situation far worse than is likely to be experienced with real life biological samples (worst case scenario). The long lived nanoclusters, in this case BSA Au clusters, could give a signal significant enough to be distinguished from the fluorescein background. It can be concluded that with gated imaging, suppressing all background is possible using this novel dye. The results of this experiment are shown in figure 3. First the fluorescence lifetime image is shown, followed by a series of images showing the intensity of emission collected after 5 different delay times. The important information gained from the images of individual dyes is that no fluorescein emission is observed after 20 ns; however the BSA Au clusters are still bright enough. More importantly/significantly, the difference between the dyes is very clear in the cells stained with both dyes. Here the intensity of BSA Au clusters dominates after 10 ns and it is the only type present after 20 ns. The important finding is that with just the addition of a short gating time, the background suppression is clearly visible using these long lifetime BSA Au clusters, making their use very attractive in the biological sample fluorescence imaging.
Figure 3.
Fluorescence lifetime and time gated intensity images of BSA Au cluster, Fluorescein and mixture of BSA Au cluster and fluorescein treated breast cancer cell line (4T1) mounted on glass slide.
Conclusions
We have shown that BSA Au clusters can be potentially used as novel fluorescent probe to stain cells. BSA Au clusters can be used as a cellular stain, advancing the bioimaging technology via their long fluorescence lifetime. BSA Au clusters allows for application in FLIM and time gated detection to visualize the stained parts of cells with close to zero background.
Acknowledgments
This work was supported by the NIH grant R01EB12003 (Z.G) and NSF grant CBET-1264608(I.G). We would like to thank Smrithi Rajendiran for proofreading the manuscript.
Notes and references
- 1.Rich RM, Mummert M, Gryczynski Z, Borejdo J, Sorensen TJ, Laursen BW, Foldes-Papp Z, Gryczynski I, Fudala R. Anal Bioanal Chem. 2013;405:4887–4894. doi: 10.1007/s00216-013-6879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee DK, Gnanasammandhan MK, Zhang Y. Small. 2010;6:2781–2795. doi: 10.1002/smll.201000418. [DOI] [PubMed] [Google Scholar]
- 3.Baker SN, Baker GA. Angewandte Chemie International Edition. 2010;49:6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- 4.Baker M. Nature Methods. 2010;7:957–962. doi: 10.1038/nmeth0710-494. [DOI] [PubMed] [Google Scholar]
- 5.Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WC. Small. 2010;6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 6.Derfus AM, Chan WC, Bhatia SN. Nano Letters. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu L, Han S, Parveen S, Yuan Y, Zhang L, Xu G. Biosens Bioelectron. 2012;32:297–299. doi: 10.1016/j.bios.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Le Guevel X, Daum N, Schneider M. Nanotechnology. 2011;22:275103. doi: 10.1088/0957-4484/22/27/275103. [DOI] [PubMed] [Google Scholar]
- 9.Zhou T, Huang Y, Li W, Cai Z, Luo F, Yang CJ, Chen X. Nanoscale. 2012;4:5312–5315. doi: 10.1039/c2nr31449e. [DOI] [PubMed] [Google Scholar]
- 10.Chen TH, Tseng WL. Small. 2012;8:1912–1919. doi: 10.1002/smll.201102741. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Zheng Y, Ying JY. J Am Chem Soc. 2009;131:888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- 12.Sakanaga I, Inada M, Saitoh T, Kawasaki H, Iwasaki Y, Yamada T, Umezu I, Sugimura A. Applied Physics Express. 2011;4:5001. [Google Scholar]
- 13.Wu Z, Jin R. Nano Lett. 2010;10:2568–2573. doi: 10.1021/nl101225f. [DOI] [PubMed] [Google Scholar]
- 14.Wen X, Yu P, Toh Y, Tang J. The Journal of Physical Chemistry C. 2012;116:11830–11836. [Google Scholar]
- 15.Wen X, Yu P, Toh Y, Hsu A, Lee Y, Tang J. The Journal of Physical Chemistry C. 2012;116:19032–19038. [Google Scholar]
- 16.Raut S, Chib R, Rich RM, Shumilov D, Gryczynski Z, Gryzcynski I. Nanoscale. 2013 doi: 10.1039/c3nr34152f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu M, Aikens CM, Hollander FJ, Schatz GC, Jin R. J Am Chem Soc. 2008;130:5883–5885. doi: 10.1021/ja801173r. [DOI] [PubMed] [Google Scholar]
- 18.Wang XX, Wu Q, Shan Z, Huang QM. Biosens Bioelectron. 2011;26:3614–3619. doi: 10.1016/j.bios.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Qian S, Chen J, Cai J, Wu S, Cai Z. Talanta. 2012;94:240–245. doi: 10.1016/j.talanta.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Qian S, Chen X, Gao W, Lin Y. Analyst. 2012;137:4356–4361. doi: 10.1039/c2an35786k. [DOI] [PubMed] [Google Scholar]
- 21.Durgadas CV, Sharma CP, Sreenivasan K. Analyst. 2011;136:933–940. doi: 10.1039/c0an00424c. [DOI] [PubMed] [Google Scholar]
- 22.Retnakumari A, Setua S, Menon D, Ravindran P, Muhammed H, Pradeep T, Nair S, Koyakutty M. Nanotechnology. 2010;21:055103. doi: 10.1088/0957-4484/21/5/055103. [DOI] [PubMed] [Google Scholar]
- 23.Retnakumari A, Jayasimhan J, Chandran P, Menon D, Nair S, Mony U, Koyakutty M. Nanotechnology. 2011;22:285102. doi: 10.1088/0957-4484/22/28/285102. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Li S, Li B, Ren X, Li S, Mahounga DM, Cui S, Gu Y, Achilefu S. Nanoscale. 2012;4:6050–6064. doi: 10.1039/c2nr31616a. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Li B, Ren X, Li S, Ma Y, Cui S, Gu Y. Biomaterials. 2012 doi: 10.1016/j.biomaterials.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Chen J, Irudayaraj J. ACS Nano. 2011;5:9718–9725. doi: 10.1021/nn2032177. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, He X, Wang K, Xie C, Zhou B, Qing Z. Nanoscale. 2010;2:2244–2249. doi: 10.1039/c0nr00359j. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Sutter JU, Birch D, Chen Y. 2012:1–4. [Google Scholar]
- 29.Becker W. J Microsc. 2012;247:119–136. doi: 10.1111/j.1365-2818.2012.03618.x. [DOI] [PubMed] [Google Scholar]
- 30.Schneckenburger H, Wagner M, Weber P, Strauss WS, Sailer R. J Fluoresc. 2004;14:649–654. doi: 10.1023/b:jofl.0000039351.09916.cc. [DOI] [PubMed] [Google Scholar]
- 31.Sauer M, Han K, Müller R, Nord S, Schulz A, Seeger S, Wolfrum J, Arden-Jacob J, Deltau G, Marx N. J Fluoresc. 1995;5:247–261. doi: 10.1007/BF00723896. [DOI] [PubMed] [Google Scholar]
- 32.Berlier JE, Rothe A, Buller G, Bradford J, Gray DR, Filanoski BJ, Telford WG, Yue S, Liu J, Cheung C. Journal of Histochemistry & Cytochemistry. 2003;51:1699–1712. doi: 10.1177/002215540305101214. [DOI] [PubMed] [Google Scholar]
- 33.Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung W, Haugland RP. Journal of Histochemistry & Cytochemistry. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]