ABSTRACT
We have previously shown that the carboxyl terminus (cT) of human follicle-stimulating hormone (FSH, follitropin) receptor (FSHR) is clipped before insertion into the plasma membrane. Surprisingly, several different constructs of FSHR fluorescent fusion proteins (FSHR-FPs) failed to traffic to the plasma membrane. Subsequently, we discovered that substituting the extreme cT of luteinizing hormone (LH) receptor (LHR) to create an FSHR-LHRcT chimera has no effect on FSHR functionality. Therefore, we used this approach to create an FSHR-LHRcT-FP fusion. We found this chimeric FSHR-LHRcT-FP was expressed in HEK293 cells at levels similar to reported values for FSHR in human granulosa cells, bound FSH with high affinity, and transduced FSH binding to produce cAMP. Quantitative fluorescence resonance energy transfer (FRET) analysis of FSHR-LHRcT-YFP/FSHR-LHRcT-mCherry pairs revealed an average FRET efficiency of 12.9 ± 5.7. Advanced methods in single-molecule analyses were applied in order to ascertain the oligomerization state of the FSHR-LHRcT. Fluorescence correlation spectroscopy coupled with photon-counting histogram analyses demonstrated that the FSHR-LHRcT-FP fusion protein exists as a freely diffusing homodimer in the plasma membrane. A central question is whether LHR could oligomerize with FSHR, because both receptors are coexpressed in differentiated granulosa cells. Indeed, FRET analysis revealed an average FRET efficiency of 14.4 ± 7.5 when the FSHR-LHR cT-mCherry was coexpressed with LHR-YFP. In contrast, coexpression of a 5-HT2cVSV-YFP with FSHR-LHR cT-mCherry showed only 5.6 ± 3.2 average FRET efficiency, a value indistinguishable from the detection limit using intensity-based FRET methods. These data demonstrate that coexpression of FSHR and LHR can lead to heterodimerization, and we hypothesize that it is possible for this to occur during granulosa cell differentiation.
Keywords: follicle-stimulating hormone (FSH/FSH receptor), gonadotropins, granulosa cells, mechanisms of hormone action, oocyte maturation
INTRODUCTION
The pituitary glycoprotein follicle-stimulating hormone (FSH) binds to and activates the FSH receptor (FSHR). This receptor is a G protein-coupled receptor (GPCR) that is expressed in the granulosa cells of the ovary and the Sertoli cells of the testis. Successful maturation of an ovarian antral follicle to a preovulatory follicle with a mature oocyte requires FSH-regulated granulosa cell proliferation and differentiation. Therefore, binding of FSH to FSHR initiates a series of events referred to as signal transduction pathways. The most canonical of these is the activation of adenylate cyclase and generation of cAMP. Nevertheless, the complexity of FSH actions has only been appreciated in recent years, and although the activation of protein kinase A by cAMP may be necessary for many downstream actions of the activated FSHR, the nuances of FSHR signaling after the rush of cAMP production has subsided are only now nuancing dogma. For example, following FSH binding, FSHR binds to the adaptor protein β-arrestin and signals via alternate pathways that are independent of G proteins. Thus, the canonical view that as a GPCR, FSHR response to FSH only involves the activation of G proteins and subsequent effectors, such as adenylate cyclase and phospholipase C, producing cAMP and extracellular calcium influx, respectively, in minutes, has been modified by new studies.
Recent structural studies have provided evidence that the large extracellular domain (ECD) of FSHR self-associates into a quaternary structure comprising three individual ECDs. Lacking crystal structures of either the full-length monomeric FSHR or oligomeric transmembrane domains (TMs), a greater understanding of in situ FSHR quaternary structure has been sought from live cell imaging. Fluorescent protein fusion with the luteinizing hormone (LH) receptor (LHR), a homologous receptor to the FSHR, has been reported. FSHR-fluorescent protein fusions that have been demonstrated to have full biological activity (i.e., traffic to the cell surface and bind hormone) have not been reported, quite possibly because the carboxyl terminal tail (cT) of FSHR is clipped before it is trafficked to the plasma membrane [1]. The lack of this scientific tool has hampered our ability to develop a complete understanding of the live cell dynamics of FSHR and its interactions with intracellular adapter proteins [2], to create in vivo models to study FSHR regulation, and to investigate whether FSHR interacts with the LHR, which is simultaneously expressed in the same cell type in the ovary, the granulosa cell.
Using a highly specific monoclonal antibody against human FSHR (hFSHR) ECD (FSHRECD) and using an immunofluorescence resonance energy transfer (immuno-FRET) acceptor photobleach protocol in living HEK293 cells, we have shown that hFSHR forms homodimers/oligomers on the plasma membrane [1]. To obviate a concern of bivalency of the antibodies, Fab fragments of this monoclonal antibody were labeled with the organic fluorophores Alexa 568 or Alexa 647, which served as FRET donor and FRET acceptor, respectively. This immuno-FRET approach was chosen to enable us to study unmodified native FSHRs that were expressed in HEK293 cells in culture. Using FRET methods, dimerization/oligomerization has been demonstrated for the other two members of the glycoprotein hormone receptor family: the LHR and the thyroid-stimulating hormone receptor (TSHR), which were directly labeled with a fluorescent protein as a fusion protein and expressed on the plasma membrane of cells in culture. The human LHR (hLHR)-YFP expressed in Chinese hamster ovary (CHO) cells was shown to self-associate in response to hormone treatment [3]. Similarly, TSHR tagged with YFP, a color variant of green fluorescent protein (GFP) from Aequorea victoria (and red fluorescent protein [RFP] from Discosoma sp. and coexpressed in CHO cells) exhibited FRET, suggesting the presence of homo-oligomers on the plasma membrane [4].
All GPCRs share a common structure consisting of seven α-helical TMs connected by alternating extracellular (e) and intracellular (i) loops (L), with an extracellular NH2-terminal domain and an intracellular cT. Taking advantage of these similarities, several groups have constructed chimeric receptors in which a specific domain of known function from one GPCR is substituted for the corresponding domain of a related/homologous GPCR, and the resultant chimera is assayed for specific functions ascribed to those domains. For example, construction of chimeric α2- and β2-adrenergic receptors to identify domains involved in effector coupling and ligand-binding specificity is an approach that has been used extensively to probe receptor/function relationships (reviewed in Rivero-Muller et al. [5]). Hirsch et al. [6] substituted the NH2 terminus of the FSHR for the NH2 terminus of the LHR and showed that the FSHR/LHR chimera, when bound by FSH, underwent activation and signaled similarly to the native LHR. Uribe et al. [7] constructed a chimeric receptor hFSHR/rat (r) LHR-cT (hFSHR/rLHR-cT) to determine the functional significance of the palmitoylation of cysteine residues present in the cT of the hFSHR. During those studies, the hFSHR/rLHR-cT was expressed on the plasma membrane of HEK293 cells and those receptors, when exposed to FSH, stimulated maximal production of cAMP at the same level as the wild-type (WT) FSHR. Because an LHR fusion protein has been shown to traffic to the plasma membrane and retain its signaling capabilities [3, 8], we constructed several hFSHR/rLHR-cT chimeras in which a fluorescent protein (GFP, YFP, RFP, and mCherry) had been incorporated at the carboxyl terminus. This report describes the preparation of FSHR-LHR chimeric fluorescent fusion proteins with full biological activity and their use in live cell imaging.
In particular, using fluorescence correlation spectroscopy (FCS) and photon-counting histogram (PCH) analysis, we demonstrate that the hFSHR/rLHR-cT-FP chimera is present on the plasma membrane of transfected HEK293 cells as a freely diffusing homodimer in live cells. Further, using an intensity-based quantitative FRET assay called Precision FRET Analysis (PFRET) [9, 10], we show that the hFSHR/rLHR-cT-FP chimera forms homodimers in the plasma membrane of transfected HEK293 cells, and when cotransfected with WT rLHR-FP, the hFSHR/rLHR-cT chimera forms heterodimers with the WT rLHR-FP.
MATERIALS AND METHODS
Construction of Plasmids for Fluorescent hFSHRs
The hFSHR WT-GFP was prepared by amplifying WT hFSHR cDNA (GenBank accession no. S59900) in pSG5 using the oligonucleotide primers 5′-gactcagatctcgaggccaccatggccctgctcctggtctctttgctg-3′ and 5′-cgactgcag aattcggttttgggctaaatgacttagagggacaag-3′, which included the XhoI and EcoRI restriction site sequences at the 5′ and 3′ ends but not the stop codon. The PCR product was cloned into the pGEM-T Easy vector (Promega) at XhoI and EcoRI restriction enzyme sites for initial sequencing. The cDNA was then digested with XhoI and EcoRI and ligated to complementary restriction sites in pEGFP-N1 vector, which encodes the F64L and S65T GFP variant (Clontech Laboratories Inc.). The construction of the hFSHR WT-RFP cDNA was performed by PCR using as template the hFSHR-GFP cDNA in pEGFP-N1, to which the DNA sequence coding for the GFP protein had been replaced with that of the DsRed Monomer (Clontech) at SmaI and NotI restriction sites.
Construction of hFSHR/LHR-cT
The chimeric hFSHR/rat LHR cT cDNA (hFSHR/rLHR-cT) was constructed as previously described [7]. This chimera contains amino acid residues 1–611 of the hFSHR and residues 604–674 of the rLHR (GenBank accession no. NM_012978), and it is highly expressed at the plasma membrane, efficiently binds agonist, and promotes cAMP accumulation upon exposure to agonist. The hFSHR/LHR-cT cDNA construct in pSG5 was amplified by PCR using the oligonucleotides 5′-ggtggtctcgaggatctagccaggatggccctgctc-3′ and 5′-gccgcccggtaccgtctggtgagttaacgctctcggtggtagg-3′ as primers, which included XhoI and KpnI restriction site sequences at the 5′ and 3′ ends but not the stop codon. The PCR cDNA product was ligated into the pGEM-T Easy vector at XhoI and KpnI restriction sites for initial sequencing, and then into the pEGFP-N1 vector, to which the cDNA sequence coding for the GFP protein had been replaced with that of the DsRed Monomer (see above).
Construction of hFSHR/rLHR-cT-EGFP
The hFSHR/LHR-cT cDNA, cloned into pcDNA3, was excised from this vector using EcoRI at the 5′ end and ApaI at the 3′ end; this 2219-bp fragment was then ligated into the EcoRI and ApaI sites of pEGFP-N1 (Clontech). The stop codon TAG was deleted by site-directed mutagenesis method. To bring the EGFP sequence in frame with the hFSHR/rLHR-cT sequence and to eliminate, at the same time, the Kozak consensus sequence surrounding the first ATG of EGFP, we performed site-directed mutagenesis using a forward and reverse primer to delete one C and change the other C to T, 5′ to ATG: 5′-ccaccggtcgccatatggtgagcaagggc-3′. Automated sequencing was performed to ensure the integrity of the hFSHR/rLHR-cT-EGFP sequence.
Construction of phFSHR/rLHR-cT-dsRed and of phFSHR/rLHR-cT-YFP
The hFSHR/rLHR-cT was excised from phFSHR/rLHR-cT-EGFP using EcoRI and ApaI and was cloned into pmCherry-n1 and pEYFP-n1 (Clontech). The original sequence surrounding the first ATG of dsRed cDNA of pmCherry and YFP cDNA of pEYFP (5′-CCACCGGTCGCCACCATGGTGAGCAAGGGC-3′) was altered using the mutagenesis primer (5′-CCACCGGTCGCCATATGGTGAGCAAGGGC-3′). This brought the hFSHR/rLHR-cT cDNA in reading frame with the dsRed and YFP cDNA. At the same time, this primer altered the strong Kozak consensus sequence at the 5′ of dsRed and YFP cDNA: one base pair, C/G, was removed, and another, C/G, was changed to T/A. Automated sequencing was performed to ensure the integrity of the hFSHR/rLHR-cT-dsRed and hFSHR/rLHR-cT-YPF sequence. The identity of the final constructs (hFSHR WT-GFP, hFSHR WT-RFP, and hFSHR/LHR-cT-RFP, hFSHR/LHR-cT-GFP) and the correctness of the PCR-derived sequences were finally verified by DNA automatic sequencing.
Plasmid DNA Constructs for FRET
The following cDNA constructs were prepared to serve as FRET donors: FSHR-LHR-cT-YPF, LHR-YFP, and 5-HT2cR-YFP (VSV isoform). FSHR-LHR-cT-mCherry served as the FRET acceptor for all donors. The pair YFP/mCherry was chosen for FRET detection because it had been shown that this pair exhibits an Ro of 5.66 nm, exceeding the largest value reported for any other combination of visible fluorescent proteins [11]. HEK293 cells were singly transfected with each cDNA plasmid to allow for correction of FRET signal contamination due to spectral bleed-through (SBT). FSHR-LHR-cT-YFP and FSHR-LHR-cT-mCherry plasmids were doubly transfected to verify FSHR homodimer formation using intensity-based FRET (see below). Wild-type LHR-YFP and FSHR-LHR-cT-mCherry were doubly transfected to detect heterodimer/oligomer formation between gonadotropin receptors. As a negative control for heterodimerization, we doubly transfected HEK293 cells with FSHR-LHR-cT-mCherry and an unrelated family A GPCR, the serotonin 5-HT2c receptor, which is almost exclusively expressed in the central nervous system [12].
Cell Culture and Transfection of WT and Mutant hFSHR cDNAs for Binding and Activation Studies
HEK293 cells were maintained in a humidified atmosphere of 5% CO2 at 37°C in low-glucose Dulbecco modified Eagle medium (DMEM; Life Technologies Inc.), supplemented with 5% fetal calf serum, 5 μg/ml geneticin, and antibiotic-antimycotic reagent (Life Technologies). Cells were grown to 70%–80% in 75 cm2 flasks (Costar), replated on 60-mm diameter plates or 15.6-mm wells (in 24-well cell culture plates; Corning Life Sciences), and cultured for 24 h at 37°C. Cells were then washed with unsupplemented DMEM and transfected with either 400 ng (for 15.6-mm culture wells) or 4 μg (for 60-mm plates) using plasmids encoding various receptor cDNA and fusion proteins (listed in Table 1) by liposome-mediated endocytosis in OPTIMEM (Life Technologies). Experiments were performed 48 h after transfection.
TABLE 1.
HEK293 cells were transfected with the following fluorescent protein cDNA constructs.

In Vitro Bioassay of cAMP Production
HEK293 cells were cultured in 24-well plates and transfected as described above. Forty-eight hours after the start of transfection the medium was removed, the cells were washed twice with DMEM-5% fetal calf serum, and the cells were then stimulated with increasing (0–50 ng/ml) doses of recombinant FSH (Merck Serono) in DMEM-5% fetal calf serum supplemented with 0.125 mM 3-isobutyl-methyl-xanthine (Sigma-Aldrich). At the end of the incubation period (18 h), the medium was removed and total (extracellular plus intracellular) cAMP accumulation was measured in acetylated samples by radioimmunoassay as described previously [13].
Receptor-Binding Assay
Human pituitary FSH (specific activity, 24 μCi per microgram of protein) was radiolabeled as previously described [14]. HEK293 cells cultured in 60-mm dishes were transfected with the hFSHR cDNAs, and 24 h after transfection the cells were replated in 24-well plates. Forty-eight hours after transfection, the medium was removed and replaced with fresh medium, and cells were allowed to continue incubation at 37°C for 1 h. After the preincubation period, the medium was removed and serum-free DMEM containing 20 ng/ml 125I-hFSH was added to each well in the presence or absence of 1 μg/ml recombinant FSH to assess for nonspecific binding [7]. Hormone was allowed to bind for 1 h at 37°C before the cultures were placed on ice and washed twice with ice-cold PBS. Cell surface [125I]-FSH was eluted with ice-cold 50 mM glycine/100 mM NaCl, pH 3.0, for 10 min on ice, and the eluate was removed to a glass tube and analyzed for radioactivity content.
Western Blot Analyses
Western blot analyses of FSHR were carried out essentially as described previously [1]. HEK293 cells cultured in 60-mm diameter culture wells were cotransfected as described above. After 48 h from the start of transfection, cells were lysed with Passive Lysis Buffer (Promega) containing 1% Igepal (Sigma-Aldrich); 0.4% desoxycholate (Sigma-Aldrich); 10 mM Tris, pH 7.5; and 6.6 mM ethylenediamine tetraacetic acid, and protein extracts (15 μg per lane) were resolved by 7.5% SDS-PAGE. Following electrophoreses, proteins were transferred to Immobilon-P membranes (EMD Millipore), probed with primary antibody mAb 106.105 (5 μg) [15], and then incubated with secondary anti-mouse immunoglobulin G (IgG) horseradish peroxidase conjugate (Biosource International). Signal was developed using the Pierce ECL Western Blotting detection kit. Equal protein loading was confirmed in a stripped, washed, and reprobed membrane with a 1:2000 anti-glyceraldehyde-3-phosphate dehydrogenase antibody (Sigma) and 1:10 000 goat-anti-mouse IgG conjugated with horseradish peroxidase (Biosource).
Cell Culture and Transfection of WT and Mutant hFSHR cDNAs for Fluorescence Microscopy
HEK293 cells from the American Type Culture Collection were cultured in Dulbecco modified Eagle medium (Corning cellgro, Mediatech) with 10% fetal bovine serum at 37°C, 5% CO2. HEK293 cells (7 × 105 cells per well of a six-well plate) were transfected with 0.5 μg of hFSHR/rLHR-cT-RFP plasmid DNA using Lipofectamine Reagent (Life Technologies) for 5 h and were cultured after transfection in phenol red-free DMEM containing 10% fetal bovine serum. Twenty-four hours later the cells were imaged.
Confocal Microscopy
The hFSHR-fluorescent proteins hFSHR-GFP, hFSHR-rLHR-cT-GFP, hFSHR-rLHR-cT-YFP, hFSHR-rLHR-cT-RFP, and hFSHR-rLHR-cT-mCherry were singly transfected into HEK293 cells, and images of live cells or fixed cells were acquired on a Zeiss LSM510 META-NLO laser scanning microscope system equipped with a Plan-Apochromat 63×/1.4 NA oil objective lens, an Argon laser (at 488 nm) for excitation of GFP and YFP, a green HeNe diode laser (543 nm) for excitation of mCherry, and emission filters BP 500–530 for GFP and YFP, and LP590 for RFP and mCherry.
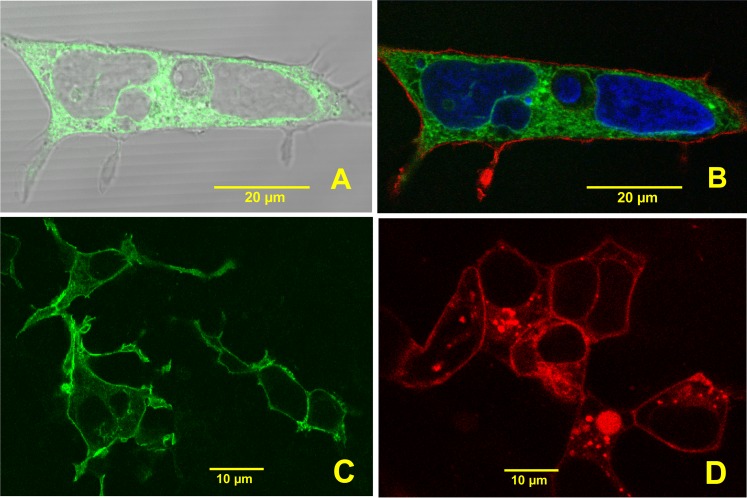
To assess trafficking of hFSHR-GFP to the plasma membrane, live cells were imaged with fluorescence and differential interference contrast (DIC), and the channels merged (Fig. 1). The hFSHR-GFP chimeric protein appeared to be retained in the endoplasmic reticulum and not present on the cell surface. To confirm this observation, cells were fixed with 4% paraformaldehyde in PBS and were incubated in a solution containing a DiI derivative, SP-DiIC183, a lipophilic sulfophenylcarbocyanine fluorescent dye (Invitrogen, Molecular Probes Inc.) at 2 μg/ml in PBS for 3 min at room temperature, followed by washing in PBS. The cell nucleus was labeled with DAPI, 2-(4-amidinophenyl)-1H-indole-6-carboxamidine. The hFSHR-GFP was imaged as above, the DiI derivative was excited with 543-nm light, and emission was collected through a 565–615 BP filter. The DAPI was imaged by two-photon excitation at 720-nm light from a Chameleon Ti:Sa laser (Coherent Inc.), and fluorescence emission was collected with a 435- to 485-nm BP filter. To confirm that the hFSHR-rLHR-cT-RFP chimera trafficked to the plasma membrane, cells expressing this chimeric protein were incubated with an anti-hFSHR mAb 106.105 that had been labeled with Alexa 657 (Invitrogen, Molecular Probes Inc.) as previously described [1]. The RFP was imaged as above, Alexa 647 was excited with a red HeNe diode laser (633 nm), and fluorescence emission was collected with a 650- to 710-nm BP filter. In confocal images, the fluorescence emission from Alexa 647 was pseudocolored green.
FIG. 1.
Expression of hFSHR-GFP and hFSHR-rLHR-cT-FP in HEK293 cells. A) Image of a live cell expressing hFSHR-GFP merged with DIC image. The hFSHR-GFP is not present on the cell surface and is retained in the endoplasmic reticulum. B) The same cell as in A was fixed and incubated with DiI to stain the plasma membrane and DAPI to label the nucleus. The absence of trafficking of the hFSHR-GFP is clearly demonstrated in the triple-labeled image. C and D) Trafficking of the hFSHR-rLHR-FPs to the plasma membrane of HEK293 cells is clearly evident. C) hFSHR-rLHR-cT-GFP. D) hFSHR-rLHR-cT-RFP.
Confocal FRET Microscopy
Images were acquired on a Zeiss LSM510 META-NLO laser scanning microscope system as described above. Optimal acquisition settings were determined using a double-labeled sample to avoid saturation. In the AIM 4.2 software (Zeiss USA), the image display was configured for three channels: a donor excitation/donor emission (donor channel), an acceptor excitation/acceptor emission (acceptor channel), and a donor excitation/acceptor emission (FRET channel). Images were collected sequentially at 512 × 512 pixels, 8-bit depth, mean of two images, and zoom 1×. Photomultiplier gain and black-level settings, laser power, and pinhole were set at identical levels and remained unchanged for all subsequent collections. Double-labeled, single-label acceptor and single-label donor images were collected with identical settings. Single-labeled images were used to correct for FRET signal contamination (i.e., SBT) in double-labeled images. Donor SBT results from donor emission that crosses over into the acceptor emission, and acceptor SBT results from acceptor absorption that is excited by the donor excitation.
Precision FRET Analysis
To calculate the FRET energy transfer efficiency (E%), we used a quantitative analysis algorithm called PFRET [16] that removes the donor and acceptor SBT and corrects the variation in fluorophore expression level associated with FRET imaging. Nine images were collected for background correction, SBT correction, and FRET analysis using the donor, acceptor, and FRET channels described above: three single-label donor reference images, three single-label acceptor reference images, and three double-label images [16]. The three double-labeled images were named as follows: quenched donor (qD; i.e., donor excitation/donor emission [donor channel]); acceptor (A; i.e., acceptor excitation/acceptor emission [acceptor channel]); and uncorrected FRET (uFRET; i.e., donor excitation/acceptor emission [FRET channel]). Then, images were background-subtracted and processed by the PFRET software, which removed donor and acceptor SBT pixel by pixel on the basis of matched fluorescence levels between the double-label specimen and the single-label reference specimens in order to generate the corrected FRET images (PFRET images) that represent the actual energy-transfer levels (PFRET levels) and are used for quantitative analyses.
 |
A custom-written analysis program was used to select above threshold regions of interest (ROIs; 10 × 10 pixels) of the eight-bit gray scale fluorescence intensities of uFRET, A, and qD images, excluding zero and saturated pixels [16]. Under our imaging conditions, there was less than 5% saturated pixels. Appropriate ROIs were automatically selected from the uFRET image. These ROIs were subsequently applied to the other images to extract the different gray scale fluorescence intensity values for the different parameters tested, including PFRET (actual energy transfer levels as per the PFRET SBT correction algorithm), uFRET, qD, and A levels. These values were transferred to an Excel spreadsheet (Microsoft) for calculation of the additional parameters E%, unquenched D (D = PFRET + qD), and D:A ratios. These values were averaged over ROIs containing 10 × 10 pixels and were used for further FRET clustering analysis. The E% is calculated as a percentage of energy transfer in relation to the unquenched donor, that is, D = qD + γ·PFRET as described in the equation E% = 100 × (γ·PFRET/D) or E% = 100 × (1 – [qD/D]). Gamma value (γ) is a function of the quantum yield of the fluorophores and of the detection setup. Because all of our imaging conditions remain constant, the γ value does not affect the interpretation of the relative E% data when E% is calculated assuming
 |
Discrimination Between a Clustered and a Random Receptor Distribution
When acceptor and donor pairs are confined to a planar membrane and expressed in high concentrations, it is important to determine that the FRET results from specific protein-protein interactions, such as dimer/oligomer formation (receptor clustering). It is important to rule out if protein overexpression resulted in high levels of donor and acceptor sufficiently close to produce FRET because they are tightly packed in a small region of the membrane (random proximity/molecular crowding effect). Mathematical models have been used to discriminate a clustered from a random membrane protein organization based on the relationship between E% and A levels at specific ranges of D:A ratios, which are experimentally determined using quantitative FRET analysis [17–23]. In a random situation, the likelihood of an acceptor colocalizing with a given donor is positively correlated with acceptor levels and leads to an increase in E%. Conversely, in a clustered situation, where molecules by definition are in proximity either in dimer or higher-order oligomeric complexes, E% is largely independent of A levels, and it does not decrease to zero when A levels approach zero [17, 18, 23, 24].
Fluorescence Correlation Spectroscopy
Fluorescence correlation spectroscopy measurements were performed essentially as described in Herrick-Davis and Mazurkiewicz [25]. Briefly, fluorescence fluctuations were detected using a Zeiss LSM-780 confocal microscope equipped with a Plan-Apochromat 40×/1.2 NA water objective lens, an Argon laser (at 488 nm) for excitation of GFP, and a gallium arsenide phosphide linear array spectral detector for fluorescence emission in the range of 520–625 nm (Carl Zeiss). The FCS measurements were recorded at 23°C in HEPES-buffered MEM (without phenol red). For each recording, the confocal volume was first positioned on the cell nucleus in x and y, and then, while monitoring fluorescence count rate, it was scanned vertically in z until it was placed on the upper plasma membrane. As the fluorescence-tagged receptors pass through the laser-illuminated observation volume, the fluctuations in fluorescence intensity are recorded in real time by the photon-counting detector, and a fluorescence intensity trace for the observation period is generated. Fluorescence fluctuations were recorded for 100 sec, as 10 consecutive 10-sec intervals. Autocorrelation analyses were performed and fitted using Zeiss Aim 4.2 software (Zeiss). The rate at which the fluorescence-tagged receptor diffuses within the plasma membrane is reported as the average dwell time (τD) within the observation volume and is calculated from the midpoint of the autocorrelation decay curve. The autocorrelation curve depicts the fluorescence intensity fluctuations as a function of particle number and diffusion time. A free two-dimensional (2D) diffusion model with two components and an added pre-exponential term to account for fluorescent fluctuations due to photophysical events (triplet, blinking, stretched exponentials) within GFP was used to fit the data [26, 27]. The fit provided apparent dwell times of τD1 and τD2 for each component. We interpreted the faster component, τD1, measured in microseconds, as being related to the photophysical properties of the fluorescent probe, and the slower component, τD2, measured in milliseconds, as representing the translational diffusion of the hFSHR/rLHR-cT-EGFP chimera in the plasma membrane. The number of fluorescent molecules in the observation volume, N, was derived from its inverse relationship to the amplitude of the τD2 component and is derived from the autocorrelation curve. The diffusion coefficient (D) for lateral diffusion of the hFSHR/rLHR-cT-EGFP within the plasma membrane was calculated using the equation:
 |
where ω0 is the radial waist of the observation volume.
Photon-Counting Histogram
Fluorescence fluctuation data recorded during the FCS experiment were used to generate PCHs, which provide quantitative information about the number of fluorescent molecules and the number of photon counts per molecule, reported as molecular brightness [28, 29]. The molecular brightness is proportional to the number of fluorescent molecules present within a protein complex; thus, a GPCR monomer with a single fluorescent tag would have a molecular brightness of x, a dimer carrying two fluorescent tags would be 2x, a tetramer would be 4x, and so forth. Controls of known monomeric (CD-86) and dimeric (CD-28) plasma membrane receptors with C-terminal GFP (CD-86/GFP and CD-86/GFP-GFP) were used to determine the molecular brightness of GFP monomers and dimers and to decode the molecular brightness determined for the hFSHR/rLHR-cT-EGFP chimera [25].
RESULTS
Confocal Microscopy Imaging of Cells Expressing hFSHR-GFP
When singly transfected cells were imaged live, the hFSHR-GFP fusion protein was strongly expressed in the cytoplasm. However, there was no clear localization at the plasma membrane when viewing a DIC image merged with GFP fluorescence from hFSHR-GFP expression (Fig. 1A). Following fixation with 4% paraformaldehyde in PBS, cells were incubated with SP-DiIC18-3, a sulfonated derivative of the lipophilic dye DiI, to label the plasma membrane. Indeed, the three-color image in Figure 1B clearly shows that the hFSHR-GFP is retained in the endoplasmic reticulum. Further, when cells expressing this fusion protein were treated with human FSH there was no evidence of cAMP production, and 125I-hFSH binding was not detectable in cells expressing the hFSHR-GFP fusion protein (data not shown).
Cells Expressing hFSHR-rLHR-Fluorescent Proteins Trafficked to the Plasma Membrane
When cells were singly transfected with each of the hFSHR-rLHR-FP constructs and imaged, there was variable but consistent expression of the chimeric fluorescent proteins; however, they all trafficked to the plasma membrane. Representative images of cells that were transfected with hFSHR-rLHR-cT-GFP or hFSHR-rLHR-cT-RFP are shown in Figure 1, C and D.
To conclusively demonstrate that hFSHR-rLHR-cT-RFP chimeric proteins traffic to the plasma membrane, live cell cultures were incubated with mAb106.105, a monoclonal antibody directed against hFSHR ECD that had been labeled with Alexa 647 and imaged [1]. Figure 2A shows a single cell expressing hFSHR-rLHR-cT-RFP on its plasma membrane, and Figure 2B shows the same cell labeled with mAB106.105-Alexa 647 (pseudocolored green) on the surface of the cell. The colocalization of the two fluorescent proteins is illustrated in the merged image, Figure 2C. The localization of hFSHR-rLHR-cT-RFP on the cell surface was further confirmed using standard biochemical techniques (see the section on biochemical characterization below).
FIG. 2.
HEK293 cell expressing hFSHR-rLHR-cT-RFP and incubated with mAb directed against hFSHREDC. A) hFSHR-rLHR-cT-RFP (red channel). B) Monoclonal antibody 105.106-Alexa 647 (pseudocolored green). C) Merged image demonstrating colocalization of the two fluorescent proteins on the plasma membrane of the cell.
FRET Analysis Suggests That the hFSHR-rLHR-cT Chimera Is Self-Associated on the Cell Surface
A quantitative FRET protocol was used to detect the presence of homodimers of FSHR-rLHR-cT chimeras on the cell surface. FSHR-rLHR-cT-YFP and FSHR-rLHR-cT-mCherry were cotransfected into HEK293 cells. The chimeric proteins hFSHR-rLHR-cT-YFP and hFSHR-rLHR-cT-mCherry served as donor and acceptor of a FRET pair, respectively. FRET was detected between these receptors on the plasma membrane with an average E% of 12.82 ± 1.7 (Table 2), demonstrating that intrinsically labeled fluorescent FSHR-chimeric proteins form homodimers on the cell surface. As shown in Figure 3, both hFSHR-rLHR-cT-YFP (qD) and hFSHR-rLHR-cT-mCherry (A) show accumulation at the plasma membrane. Furthermore, ROIs automatically selected from the PFRET image (Fig. 3, D and E) show predominant plasma membrane localization. This confirms previous work from our laboratory in which we had shown that hFSHR forms homodimers on the plasma membrane of a stable HEK293 cell line expressing hFSHR, using an immuno-FRET acceptor photobleach protocol using mAb106.105 labeled with Alexa 568 as donor and mAb106.105 labeled with Alexa 647 as acceptor [1].
TABLE 2.
Summary of FRET data used to assay the formation of FSHR homodimers and FSHR-LHRcT/LHR heterodimers on the plasma membrane of HEK293 cells.
n = 88 ROIs selected from 23 cells collected from 8 images.
n = 320 ROIs selected from 33 cells collected from 11 images.
n = 15 ROIs selected from 9 cells collected from 5 images.
FIG. 3.
FSHR chimeric receptors form homodimers on the cell surface. Representative images are shown of HEK293 cells that were cotransfected with FSHR-rLHR-cT-YFP and FSHR-rLHR-cT-mCherry. A) Acceptor excitation/acceptor channel shows acceptor fluorescence intensities. B) Donor excitation/donor channel shows the qD fluorescence intensities. C) Uncorrected FRET represents donor excitation/acceptor channel, which includes energy transfer levels plus the two contaminants in the FRET signal: donor cross talk and acceptor bleed-through. D) PFRET image. This image represents the actual energy transfer levels and was processed by the correction algorithm, which removes donor cross talk and acceptor bleed-through. E) The PFRET image overlaid with the automatically selected ROIs produced by the software. The images were modified with ImageJ using the same settings to enhance contrast for better visualization.
FCS Analysis Combined with PCH Directly Demonstrates That the hFSHR-rLHR-cT Chimera Is Present as a Freely Diffusing Homodimer on the Surface of Live Cells
The FCS and PCH analyses were applied to determine the diffusion dynamics and oligomer status of the hFSHR/rLHR-cT-EGFP receptor. Autocorrelation analysis assessed the time dependence of the fluctuations and provided 1) information about the number of mobile fluorescent particles in the volume (N), inversely proportional to the autocorrelation curve amplitude, and 2) the average fluorescent molecule dwell time (τD), measured at the midpoint of the curve decay, from which the diffusion coefficient D was calculated (shown in Fig. 4). Diffusion time, reported in milliseconds, represents the average dwell time of the receptor in the observation volume. Diffusion coefficients (μm2/sec) were calculated using a 2D model for the lateral diffusion of receptors within the plasma membrane. Autocorrelation analysis revealed a dwell time of 74.7 ± 11.3 msec and a lateral diffusion coefficient of 0.3 ± 0.05 μm2/sec for the hFSHR/rLHR-cT-EGFP receptor in HEK293 cells (Table 3). The number of molecules, N, measured in the observation volume ranged from 3 to 12, with a mean of 9.2 ± 1.2. Receptor expression levels can be estimated using N, the area of the plasma membrane in the observation volume (determined experimentally to be 0.28 μm2), and the average total surface area of an HEK cell (determined to be 2591 μm2 [30, 31]). Thus, in the present study expression levels ranged from 28 000 to 110 000 receptors per cell, equivalent to 10–40 receptors per square micrometer of plasma membrane. This number is physiologically relevant because it is within the range reported for native GPCR [32]. Further, granulosa cells are much smaller than HEK cells, with a reported mean volume of 1140 μm3 [33]. If the granulosa cell is considered a sphere, the surface area can be calculated to be 528 μm2, and with a similar surface density for hFSHR/rLHR-cT-EGFP of 3–12 molecules per 0.28 μm2 of plasma membrane, then the calculated number of receptors per granulosa cell would range from 5000 to 23 000. The reported number of FSHRs on granulosa cells in cell culture immediately after extraction was 5000 per cell. This number increased to 7000 when cells were transduced with adenoviral-mediated FSHR gene [34]. Very early studies suggested ∼5000 receptors per cell in undifferentiated granulosa cells derived from estradiol-treated immature animals [35].
FIG. 4.
Fluorescence correlation spectroscopy recording from the plasma membrane of an HEK293 cell expressing FSHR-rLHR-cT-GFP. A) Fluorescence intensity trace for one 10-sec observation period. B) Autocorrelation analysis of the fluorescence intensity trace. The red line represents the autocorrelation of the observed fluorescence signal, and the green line represents the fit to a two-component model: a fast component (240 μsec) related to the photophysical properties of the fluorescent probe, and a slower component (70 msec) representing the translational diffusion of FSH receptors in the plasma membrane. Dividing the average photon count rate (kHz), determined from the fluorescence intensity trace (A), by the number of fluorescent molecules, determined from the amplitude of the autocorrelation curve, predicts the average molecular brightness of the sample. C) Photon-counting histogram of the corresponding FCS recording. To generate a histogram, the 10-sec fluorescence intensity trace (A) was broken down into 1 million 10-μsec intervals or bins (PCH bin time = 10 μsec). The number of bins is plotted on the y-axis and photon counts on the x-axis. The resulting histogram depicts the number of bins that registered 1, 2, 3…n photon counts during one 10-sec observation period. D) The residuals of the PCH curve fit plot the number of bins on the y-axis and photon counts on the x-axis. The data were fit to a one-component model for a single, homogenous population of homodimers. The residuals of the curve fit are less than two standard deviations and are randomly distributed about zero, indicating that the data are a good fit for the selected model, with reduced χ2 equal to unity.
TABLE 3.
Diffusion (FCS) and molecular brightness (PCH) analyses of HEK293 cells expressing GFP-tagged FSH-LHRcT chimeric receptors.a
Data represent the mean ± SEM for the number of cells (N) as indicated.
Diffusion time, reported in milliseconds (msec), represents the average dwell time of the receptor in the observation volume.
Diffusion coefficients (μm2/sec) were calculated using a 2D model for the lateral diffusion of receptors within the plasma membrane.
PCH molecular brightness analysis was performed by fitting the data to a one-component model for a single, homogeneous population of homodimers. Molecular brightness values are reported as counts per second per molecule (CPSM).
Fluorescence fluctuation data recorded during an FCS experiment were used to generate PCHs, which provided quantitative information about the number of fluorescent receptors and the number of photon counts produced by a single fluorescent receptor, from which the molecular brightness of a single fluorescent receptor was estimated. The molecular brightness of the hFSHR/rLHR-cT-EGFP receptor was estimated to be 18 085 ± 696 counts per second per molecule, a value that reports the molecular species (hFSHR/rLHR-cT-EGFP receptor) diffusing through the observation volume as a dimer. This conclusion was arrived at by comparing this value with the molecular brightness of a known monomeric receptor (CD-86) labeled with a single GFP (CD-86-GFP) and with tandem GFPs (CD-86/GFP-GFP) that were measured as 9549 ± 348 (monomer) and 18 175 ± 469 (dimer), respectively.
The hFSHR-rLHR-cT Chimera Forms Heterodimers/Hetero-oligomers with LHR on the Cell Surface
The intensity-based PFRET protocol was used to determine whether the hFSHR-rLHR-cT chimera can form a heterodimer with LHR. LHR-YFP and FSHR-rLHR-cT-mCherry were cotransfected into HEK293 cells, and images were collected and processed as for the cotransfected FSHR-rLHR-cT-FP chimeras as described in Figure 4. FRET was detected on the cell surface between these two gonadotropin receptors with an average E% of 14.41 ± 0.82 (Table 2). As a negative control for heterodimerization, an unrelated family A GPCR, the serotonin 5-HT2cVSV-YFP receptor, was cotransfected with FSHR-rLHR-cT-mCherry and assayed for FRET. An E% of 5.5 ± 1.46 was calculated for this heterologous GPCR pair, a value indistinguishable from the detection limit using intensity-based FRET methods [36]. This value is also consistent with observation of lower levels for the so-called stochastic FRET, a phenomenon that can occur between the donor and acceptor proteins diffusing at random in the plane of a biological membrane and colliding briefly in the absence of stable physical interaction [37]. The 5-HT2cVSV receptor was chosen because it is exclusively expressed in brain, is capable of forming homodimers on the cell surface when expressed in HEK293 cells, and is present as a homodimer on primary hippocampal neurons in culture [38].
To specifically address the concern that FRET could still result from molecular crowding because the GPCRs are restricted in the plane of the lipid bilayer and, if overexpressed, are forced close enough to give FRET as a consequence of simply being tightly packed in a small region of the membrane (random proximity effect), we applied mathematical models that discriminate a clustered from a random membrane protein organization based on the relationship between E% and acceptor (A) levels at specific ranges of D:A ratios [17, 18, 39]. In a random distribution model, E% is positively correlated with acceptor levels and increases with increasing acceptor density. In a clustered arrangement either as a dimer or higher-order oligomers, E% is independent of acceptor levels, and it does not decrease to zero when acceptor trends to zero. The FRET data suggest a clustering for FSHR-chimera homodimers and for FSHR-chimera/LHR heterodimers, the dimer being the minimum configuration of a cluster (Fig. 5).
FIG. 5.
FSHR-rLHR-cT chimeric receptors form homodimers (A) and form heterodimers with rLHR (B). For both pairs, E% is independent of acceptor levels at a D:A ≈ 1. In addition, the E% values do not trend to zero with decreasing A levels, and correlation coefficients show R2 = 0.0096 and slope s-value = 0.0027 for the FSHR-rLHR-cT homodimer, and R2 = 0.0566 and s-value = 0.089 for the FSHR-rLHR-cT/rLHR heterodimer. This behavior is indicative of the case where molecules, here hFSHR-rLHR-cT/hFSHR-rLHR-cT or FSHR-rLHR-cT/LHR, are present as clusters either as a dimer or as a higher-order oligomer.
Biochemical Characterization of hFSHR-rLHR-cT Chimera-RFP
Western blot analysis demonstrated that hFSHR-rLHR-cT (Fig. 6A, lane 1) was expressed comparably to the hFSHR-rLHR-cT-RFP (Fig. 6A, lane 2), the latter of which evidenced an appropriate shift in higher molecular weight due to the fusion. Notably there was no evidence of clipped hFSHR-rLHR-cT-RFP (Fig. 6A, lane 2), which could give rise to fully functional hFSHR-rLHR-cT at the cell surface and falsely attribute an FSH response to the hFSHR-rLHR-cT-RFP chimera. In comparison, hFSHR-RFP (Fig. 6A, lane 5) also evidenced a high-molecular weight shift compared with hFSHR (Fig. 6A, lane 4), validating its expression.
FIG. 6.

Biochemical characterization of FSHR-LHR C-tail chimera-fluorescent fusion proteins. A) Western blot of hFSHR fusion proteins. 1, hFSHR-rLHR-cT chimera; 2, hFSHR-rLHR-cT chimera-RFP; 3, mock transfection; only nonspecific second antibody staining; 4, hFSHR; and 5, hFSHR-RFP. B) Signal transduction by hFSHR fusion proteins. C) FSH-binding activity of 125I-FSHR with fusion proteins. Open circles, hFSHR; black boxes, hFSHR-RFP; gray triangles, hFSHR/rLHR-cT; and red diamonds, hFSHR/rLHR cT-RFP.
The hFSHR-rLHR-cT-RFP was able to signal normally and responded to an FSH challenge with a robust cAMP response that was indistinguishable from the response by hFSHR and hFSHR-rLHR-cT (Fig. 6B). In contrast, hFSHR-RFP did not respond to FSH challenge with an increase in cAMP production (Fig. 6B). In addition, 125I-hFSH binding was similar for hFSHR, hFSHR-rLHR-cT and the hFSHR-rLHR-cT-RFP chimera (Fig. 6C).
DISCUSSION
In the present study we report on the construction of a chimeric GPCR, hFSHR-rLHR-cT-FP, consisting of the exofacial and TM of the hFSHR coupled to the cytoplasmic domain of the rLHR and to a number of different fluorescent proteins. These fluorescent-chimeric proteins were expressed in HEK293 cells in cell culture, a classic cell line used for the expression of recombinant proteins and the study of their functions that include, in our case, biosynthesis and trafficking of FSHR to the plasma membrane, and signal responses subsequent to binding of the agonist, FSH. Heretofore, FSHR proved recalcitrant to standard approaches for the preparation of a FSH-responsive, plasma membrane-localized fluorescent fusion FSHR protein, in contrast with the other members of the glycoprotein hormone GPCR family, LHR and TSHR. This was likely due to the fact the cT of the FSHR is clipped prior to trafficking to the plasma membrane [1]. Because intracellular forms of the FSHR that are not clipped form detergent-resistant associations with cellular proteins, likely chaperones, it was reasoned that a molecular recognition site had to be removed to allow appropriate trafficking of FP-labeled FSHR. When the cT of hFSHR (which does not traffic to the cell surface when coupled with fluorescent proteins) was substituted with the cT of the rLHR (which does traffic to the plasma membrane), we observed that the resulting chimeric protein, hFSHR-rLHR-cT-FP, exhibited a robust endoplasmic reticulum-associated expression as well as cell surface plasma membrane expression. Further, the chimera was able to evoke intracellular signaling in response to agonist that was indistinguishable from that seen with cell surface-expressed nonfluorescent WT hFSHR and an hFSHR-rLHR-cT chimera.
In the present study we used the HEK cell as a tool to assess the intrinsic properties of hFSHR-rLHR-cT-FP that were expressed in live cells, such as oligomerization state, proper trafficking to the plasma membrane, and signaling activity subsequent to agonist stimulation. We were aware of the consequences that can result from overexpression of transfected proteins and thus analyzed cells that had low expression. Fluorescence correlation spectroscopy allowed us to quantify the expression of our receptors in live cells because the number of fluorescent receptors in the region of the plasma membrane observation volume can be directly determined from the autocorrelation curve. Knowing this number provides the receptor density per square micrometer, and it is a straightforward process to calculate the total number of receptors in the plasma membrane of an HEK cell once the surface area of a cell is known. It is estimated that there are 5000 FSHRs per granulosa cell [35], and the approximate surface area of a granulosa cell is reported to be 528 μm2 [33]. Therefore, the estimated expression level of native FSHRs in granulosa cells is 10 receptors per square micrometer of plasma membrane. This is similar to the FSHR expression levels in our transiently transfected HEK293 cells (10–40 receptors per square micrometer) used in the FCS studies. Because the biological activity of the hFSHR-rLHR-cT-FP was retained in the HEK cells, it can be logically extended that the molecular interaction between receptors in the plasma membrane and with molecules involved in signaling would be the same in a granulosa cell.
Whether GPCRs are expressed on the cell surface as dimers or higher-order oligomers, either constitutively or transiently subsequent to ligand interaction [40–42], is the subject of active debate [43–46]. In some GPCRs, homomerization and/or heteromerization has proven to modulate intracellular trafficking of the receptor complex to and from the plasma membrane, these have also been proven to modulate some pharmacological properties, including ligand affinity, functional co-operativity, and biased signaling [47]. For example, it has been shown that coexpression of two distinct nonfunctional TSHRs, FSHRs, or LHRs can rescue the functional activity of that receptor through binding of agonist to a signaling-deficient protomer within the putative dimer, which communicates with a neighboring agonist binding-deficient protomer to propagate signal [5, 48, 49]. Similarly, coexpression of thyrotropin-releasing hormone receptor pairs deficient in either signaling or phosphorylation led to G protein-coupled kinase-mediated phosphorylation of the complex in response to thyrotropin-releasing hormone [50]. In a different scenario, it has been shown that a variety of mutant GPCRs, including the hFSHR [51], the hLHR [52], the gonadotropin-releasing hormone receptor [53, 54], the TSHR [55], and the V2 vasopressin receptor [56], among some, may exhibit intracellular association with their WT counterparts and evoke dominant negative effects on WT receptor expression, which eventually may lead to disease [55]. In previous studies, we demonstrated the constitutive association of hFSHR homodimers on the cell surface of HEK293 cells using an immuno-FRET acceptor photobleach protocol [1]. In the present study, the hFSHR-rLHR-cT-FP chimera, in which the fluorescent tags are directly coupled to the receptor, displayed robust FRET (Table 2), indicating that these chimeras can form homodimers/oligomers when trafficked to the cell surface. The quantitative FRET assay we used reports on average FRET efficiency (E%) and cannot directly report on the number of monomers in the cluster (e.g., two, three, four, etc.). However, using the simplest model, it can be stated that hFSHR can exist as homodimers on the plasma membrane. This is consistent with several recent reports that conclude that the dimer is the basic signaling unit for the serotonin 5-HT2C receptor [38], that the minimal in situ configuration of the muscarinic M3 acetylcholine receptor is a dimer [57], and that biogenic amine receptors freely diffusing within the plasma membrane are predominantly homodimers [58].
Here we report that the hFSHR-rLHR-cT-FP chimera was present on the cell surface of live cells only as a dimer, without monomers or tetramers. The molecular composition of the hFSHR-rLHR-cT-FP chimeric receptor present on the cell surface was determined using the single-particle fluorescence fluctuation analysis method of FCS combined with PCH, which allows the determination of the molecular brightness of the hFSHR-rLHR-cT-FP chimeric receptor. The results showed that the molecular brightness value for the hFSHR-rLHR-cT chimeric receptor was similar to that of the dimeric control (CD-86-GFP-GFP) and twice that of the monomeric control (CD-86-GFP), consistent with a homodimer structure for the hFSHR-rLHR-cT-FP chimera.
Interestingly, Jiang et al. [59] have reported on the crystal structure of FSH in complex with the ectodomain of the FSHR. In addition to describing the interaction of FSH with the FSHRECD on its concave surface, they showed that the hinge region of the ECD is an integral part of the ectodomain, lending rigidity to this region where the ECD is in close proximity to the first TM. They further described a potential exosite on the convex surface of the ECD. This exosite could provide for additional FSH-FSHRECD interactions that suggest the formation of a trimeric association of FSHR following interaction with FSH. The biological significance of such an FSHR trimer remains to be examined, and it may require the generation of an active-state FSHR-G protein-FSH complex
The gonadotropin hormones, LH and FSH, play essential roles in folliculogenesis and the maturation of the developing oocyte. Key in the process of ovarian follicle maturation is FSH-induced granulosa cell proliferation and differentiation, as well as LH-promoted maturation of follicular cells and, in concert with FSH, enhanced steroidogenesis in these cells. In addition, a surge of LH triggers ovulation by promoting rupture of the preovulatory follicle and release of the ovum. As follicular maturation progresses, the receptors for these hormones are concurrently expressed in granulosa cells. As with the concept that GPCRs can form homodimers/oligomers, there is growing literature suggesting that GPCRs might also form heterodimers or hetero-oligomers [60].
We offer the following hypothesis for future testing: Because the regulation of folliculogenesis is the result of a complex interaction between the trophic activities of these hormones, formation of FSHR and LHR heterodimers/hetero-oligomers in granulosa cells represents an additional level of signal control and modulation not attained by either receptor alone. Of course, such studies will be challenging. Although we have immunochemical probes for human and rat FSHR, it will be necessary to develop and validate immunochemical probes for human and rat LHR. Additionally, the sensitivity of detection may limit the ability to detect FRET in native cells with a low receptor number. Still, this is a laudable goal and one which it would seem is well worth the effort.
Mindful of this, we do find that in congruence with our structural findings presented here are biochemical studies that suggest that the hLHR and hFSHR can specifically associate into heterodimers and that these interactions result in an attenuation of LH-stimulated signaling through the hLHR and an attenuation of FSH signaling through the FSHR [61]. In this scenario, attenuation of the LH response by hFSHR/hLHR heterodimers in early stages of follicular maturation might prevent premature luteinization of granulosa cells, whereas formation of hLHR/hFSHR heterodimers in the mature follicle would prevent further FSH-promoted cell proliferation, excessive estradiol production, and interference with LH effects on oocyte maturation. Here again, however, it will be necessary to demonstrate in vivo and with low levels of receptor that heterodimerization can occur. In this regard, we do not hypothesize that newly synthesized LHR would compete for FSHR at the plasma membrane. We envision that newly synthesized monomeric LHR could associate with ongoing FSHR monomers synthesized in granulosa cell endoplasmic reticulum. In fact, recent studies in experimental animals have shown that persistent FSHR signaling may lead to aberrant outcomes, such as luteinized unruptured follicles and the development of multiple hemorrhagic cysts [62].
In summary, the evidence provided herein of FSHR/LHR heterodimerization might have significant implications for both the physiology and pharmacology of gonadotropin receptors. For the latter, in particular, FSHR/LHR association may convey advantages for the management of fertility and infertility with gonadotropins and small molecule gonadotropin agonists and antagonists. A question is whether the heterodimeric receptors are fully active regarding ligand binding and activation. Although it has been shown that a binding-defective but signaling-functional receptor can rescue a signaling-defective but binding-functional protomer as a partner in the pas de deux, it is less clear that this is so for heterodimeric partners. If heteromerization results in mutual neutralization, then one might predict that FSH- induced expression of LHR will lead to quenching of further FSHR effects, and eventually an LH-dominant effect. Alternatively, one receptor of the heteromer could dominate, leading to preferential activation by one ligand. The advantage of this scenario is that one need only invoke an excess of the molar concentration of the dominant receptor in order to form sufficient heteromers to effectively neutralize all of the subordinate partners. In this regard it is worth noting that the LHR seems far more susceptible to activating mutations than the FSHR. This has led to the hypothesis that there is an inherent difference in the flexibility of the two receptors. It is tempting to speculate that the coevolution of these two receptors necessitated one rigid and one malleable receptor, so that in the pas de deux, one can lead and the other will follow.
ACKNOWLEDGMENT
The FCS experiments were performed at the Center for Cell Analysis and Modeling Microscopy User Facility at the University of Connecticut Health Center. Confocal microscopy was performed in the Imaging Core Facility at Albany Medical College.
Footnotes
Supported in part by National Institutes of Health grant HD18407 to J.A.D., by CONACyT, Mexico grant 240619, and by R21MH086796 to K.H.D.
REFERENCES
- Thomas RM, Nechamen CA, Mazurkiewicz JE, Muda M, Palmer S, Dias JA. Follicle-stimulating hormone receptor forms oligomers and shows evidence of carboxyl-terminal proteolytic processing. Endocrinology. 2007;148:1987–1995. doi: 10.1210/en.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias JA, Nechamen CA, Atari R. Identifying protein interactors in gonadotropin action. Endocrine. 2005;26:241–247. doi: 10.1385/ENDO:26:3:241. [DOI] [PubMed] [Google Scholar]
- Wolf-Ringwall AL, Winter PW, Liu J, Van Orden AK, Roess DA, Barisas BG. Restricted lateral diffusion of luteinizing hormone receptors in membrane microdomains. J Biol Chem. 2011;286:29818–29827. doi: 10.1074/jbc.M111.250969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J Biol Chem. 2001;276:45217–45224. doi: 10.1074/jbc.M103727200. [DOI] [PubMed] [Google Scholar]
- Rivero-Muller A, Chou YY, Ji I, Lajic S, Hanyaloglu AC, Jonas K, Rahman N, Ji TH, Huhtaniemi I. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci U S A. 2010;107:2319–2324. doi: 10.1073/pnas.0906695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch B, Kudo M, Naro F, Conti M, Hsueh AJ. The C-terminal third of the human luteinizing hormone (LH) receptor is important for inositol phosphate release: analysis using chimeric human LH/follicle-stimulating hormone receptors. Mol Endocrinol. 1996;10:1127–1137. doi: 10.1210/mend.10.9.8885247. [DOI] [PubMed] [Google Scholar]
- Uribe A, Zarinan T, Perez-Solis MA, Gutierrez-Sagal R, Jardon-Valadez E, Pineiro A, Dias JA, Ulloa-Aguirre A. Functional and structural roles of conserved cysteine residues in the carboxyl-terminal domain of the follicle-stimulating hormone receptor in human embryonic kidney 293 cells. Biol Reprod. 2008;78:869–882. doi: 10.1095/biolreprod.107.063925. [DOI] [PubMed] [Google Scholar]
- Horvat RD, Barisas BG, Roess DA. Luteinizing hormone receptors are self-associated in slowly diffusing complexes during receptor desensitization. Mol Endocrinol. 2001;15:534–542. doi: 10.1210/mend.15.4.0622. [DOI] [PubMed] [Google Scholar]
- Elangovan M, Wallrabe H, Chen Y, Day RN, Barroso M, Periasamy A. Characterization of one- and two-photon excitation fluorescence resonance energy transfer microscopy. Methods. 2003;29:58–73. doi: 10.1016/s1046-2023(02)00283-9. [DOI] [PubMed] [Google Scholar]
- Wallrabe H, Bonamy G, Periasamy A, Barroso M. Receptor complexes cotransported via polarized endocytic pathways form clusters with distinct organizations. Mol Biol Cell. 2007;18:2226–2243. doi: 10.1091/mbc.E06-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrap N, Seidel T, Barisas BG. Forster distances for fluorescence resonant energy transfer between mCherry and other visible fluorescent proteins. Anal Biochem. 2010;402:105–106. doi: 10.1016/j.ab.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, MacDermott AB, Axel R, Jessell TM. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988;241:558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Barrios-De-Tomasi J, Cardenas M, Ulloa-Aguirre A. Studies on the relative in-vitro biological potency of the naturally-occurring isoforms of intrapituitary follicle stimulating hormone. Mol Hum Reprod. 1996;2:563–571. doi: 10.1093/molehr/2.8.563. [DOI] [PubMed] [Google Scholar]
- Weiner RS, Dias JA. Identification of assembled epitopes on the alpha-subunit of human follicle stimulating hormone. Mol Cell Endocrinol. 1992;85:41–52. doi: 10.1016/0303-7207(92)90123-n. [DOI] [PubMed] [Google Scholar]
- Lindau-Shepard BA, Brumberg HA, Peterson AJ, Dias JA. Reversible immunoneutralization of human follitropin receptor. J Reprod Immun. 2001;49:1–19. doi: 10.1016/s0165-0378(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Talati R, Vanderpoel A, Eladdadi A, Anderson K, Abe K, Barroso M. Automated selection of regions of interest for intensity-based FRET analysis of transferrin endocytic trafficking in normal vs. cancer cells. Methods. 2014;66:139–152. doi: 10.1016/j.ymeth.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI- anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrabe H, Elangovan M, Burchard A, Periasamy A, Barroso M. Confocal FRET microscopy to measure clustering of ligand-receptor complexes in endocytic membranes. Biophys J. 2003;85:559–571. doi: 10.1016/S0006-3495(03)74500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrabe H, Chen Y, Periasamy A, Barroso M. Issues in confocal microscopy for quantitative FRET analysis. Microsc Res Tech. 2006;69:196–206. doi: 10.1002/jemt.20281. [DOI] [PubMed] [Google Scholar]
- Wallrabe H, Stanley M, Periasamy A, Barroso M. One- and two-photon fluorescence resonance energy transfer microscopy to establish a clustered distribution of receptor-ligand complexes in endocytic membranes. J Biomed Opt. 2003;8:339–346. doi: 10.1117/1.1584444. [DOI] [PubMed] [Google Scholar]
- Zimet DB, Thevenin BJ, Verkman AS, Shohet SB, Abney JR. Calculation of resonance energy transfer in crowded biological membranes. Biophys J. 1995;68:1592–1603. doi: 10.1016/S0006-3495(95)80332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentcheva T, Edidin M. Clustering of peptide-loaded MHC class I molecules for endoplasmic reticulum export imaged by fluorescence resonance energy transfer. J Immunol. 2001;166:6625–6632. doi: 10.4049/jimmunol.166.11.6625. [DOI] [PubMed] [Google Scholar]
- Spiliotis ET, Pentcheva T, Edidin M. Probing for membrane domains in the endoplasmic reticulum: retention and degradation of unassembled MHC class I molecules. Mol Biol Cell. 2002;13:1566–1581. doi: 10.1091/mbc.01-07-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Mazurkiewicz JE. Fluorescence correlation spectroscopy and photon-counting histogram analysis of receptor-receptor interactions. Methods Cell Biol. 2013;117:181–196. doi: 10.1016/B978-0-12-408143-7.00010-4. [DOI] [PubMed] [Google Scholar]
- Briddon SJ, Kellam B, Hill SJ. Design and use of fluorescent ligands to study ligand-receptor interactions in single living cells. Methods Mol Biol. 2011;746:211–236. doi: 10.1007/978-1-61779-126-0_11. [DOI] [PubMed] [Google Scholar]
- Briddon SJ, Middleton RJ, Cordeaux Y, Flavin FM, Weinstein JA, George MW, Kellam B, Hill SJ. Quantitative analysis of the formation and diffusion of A1-adenosine receptor-antagonist complexes in single living cells. Proc Natl Acad Sci U S A. 2004;101:4673–4678. doi: 10.1073/pnas.0400420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Muller JD, Berland KM, Gratton E. Fluorescence fluctuation spectroscopy. Methods. 1999;19:234–252. doi: 10.1006/meth.1999.0854. [DOI] [PubMed] [Google Scholar]
- Chen Y, Muller JD, So PT, Gratton E. The photon counting histogram in fluorescence fluctuation spectroscopy. Biophys J. 1999;77:553–567. doi: 10.1016/S0006-3495(99)76912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerhage F, Helpenstein R, Rauf A, Wrobel G, Offenhausser A, Ingebrandt S. Membrane allocation profiling: a method to characterize three-dimensional cell shape and attachment based on surface reconstruction. Biomaterials. 2008;29:3927–3935. doi: 10.1016/j.biomaterials.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Lindsley T, Cowan A, Mazurkiewicz JE. Oligomer size of the serotonin 5-hydroxytryptamine 2C (5-HT2C) receptor revealed by fluorescence correlation spectroscopy with photon counting histogram analysis: evidence for homodimers without monomers or tetramers. J Biol Chem. 2012;287:23604–23614. doi: 10.1074/jbc.M112.350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Lindsley T, Teitler M, Mancia F, Cowan A, Mazurkiewicz JE. Native serotonin 5-HT2C receptors are expressed as homodimers on the apical surface of choroid plexus epithelial cells. Mol Pharmacol. 2015;87:660–673. doi: 10.1124/mol.114.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A, Dockery P. WS O, Turner K, Lenton EA, Cooke ID. The human ovarian granulosa cell: a stereological approach J Anat 1996. 188 (pt 3): 671 676 [PMC free article] [PubMed] [Google Scholar]
- Serikawa T, Fujita K, Nagata H, Oite T, Tanaka K. Maintenance of in vitro granulosa cell function by adenoviral mediated follicle stimulating hormone receptor gene transduction. J Assist Reprod Genet. 2006;23:199–206. doi: 10.1007/s10815-005-9000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht M, Ranta T, Catt KJ. Granulosa cell differentiation in vitro: induction and maintenance of follicle-stimulating hormone receptors by adenosine 3′,5′-monophosphate. Endocrinology. 1983;113:949–956. doi: 10.1210/endo-113-3-949. [DOI] [PubMed] [Google Scholar]
- Raicu V, Stoneman MR, Fung R, Melnichuk M, Jansma DB, Pisterzi LF, Rath S, Fox M, Wells JW, Saldin DK. Determination of supramolecular structure and spatial distribution of protein complexes in living cells. Nat Photon. 2009;3:107–113. [Google Scholar]
- Singh DR, Raicu V. Comparison between whole distribution- and average-based approaches to the determination of fluorescence resonance energy transfer efficiency in ensembles of proteins in living cells. Biophys J. 2010;98:2127–2135. doi: 10.1016/j.bpj.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Mazurkiewicz JE. Biochemical and biophysical characterization of serotonin 5-HT2C receptor homodimers on the plasma membrane of living cells. Biochemistry. 2004;43:13963–13971. doi: 10.1021/bi048398p. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M. Imaging fluorescence resonance energy transfer as probe of membrane organization and molecular associations of GPI-anchored proteins. Methods Mol Biol. 1999;116:37–49. doi: 10.1385/1-59259-264-3:37. [DOI] [PubMed] [Google Scholar]
- Szidonya L, Cserzo M, Hunyady L. Dimerization and oligomerization of G-protein-coupled receptors: debated structures with established and emerging functions. J Endocrinol. 2008;196:435–453. doi: 10.1677/JOE-07-0573. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- Cottet M, Faklaris O, Maurel D, Scholler P, Doumazane E, Trinquet E, Pin JP, Durroux T. BRET. and time-resolved FRET strategy to study GPCR oligomerization: from cell lines toward native tissues. Front Endocrinol (Lausanne) 2012;3:92. doi: 10.3389/fendo.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M, Hebert TE. Bouvier Michel, Hebert Terence E., editors. J Physiol. 2014;592:2447. doi: 10.1113/jphysiol.2014.274233. Rebuttal from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M, Hebert TE. CrossTalk proposal: weighing the evidence for Class A GPCR dimers, the evidence favours dimers. J Physiol. 2014;592:2439–2441. doi: 10.1113/jphysiol.2014.272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA, Javitch JA. Lambert Nevin A., Javitch Jonathan A., editors. J Physiol. 2014;592:2449. doi: 10.1113/jphysiol.2014.274241. Rebuttal from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA, Javitch JA. CrossTalk opposing view: weighing the evidence for class A GPCR dimers, the jury is still out. J Physiol. 2014;592:2443–2445. doi: 10.1113/jphysiol.2014.272997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66:413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji I, Lee C, Jeoung M, Koo Y, Sievert GA, Ji TH. Trans-activation of mutant follicle-stimulating hormone receptors selectively generates only one of two hormone signals. Mol Endocrinol. 2004;18:968–978. doi: 10.1210/me.2003-0443. [DOI] [PubMed] [Google Scholar]
- Lee C, Ji I, Ryu K, Song Y, Conn PM, Ji TH. Two defective heterozygous luteinizing hormone receptors can rescue hormone action. J Biol Chem. 2002;277:15795–15800. doi: 10.1074/jbc.M111818200. [DOI] [PubMed] [Google Scholar]
- Song GJ, Jones BW, Hinkle PM. Dimerization of the thyrotropin-releasing hormone receptor potentiates hormone-dependent receptor phosphorylation. Proc Natl Acad Sci U S A. 2007;104:18303–18308. doi: 10.1073/pnas.0702857104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarinan T, Perez-Solis MA, Maya-Nunez G, Casas-Gonzalez P, Conn PM, Dias JA, Ulloa-Aguirre A. Dominant negative effects of human follicle-stimulating hormone receptor expression-deficient mutants on wild-type receptor cell surface expression: rescue of oligomerization-dependent defective receptor expression by using cognate decoys. Mol Cell Endocrinol. 2010;321:112–122. doi: 10.1016/j.mce.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Feng X, Wu X, Zhang M, Zhang X, Hebert TE, Segaloff DL. Bioluminescence resonance energy transfer studies reveal constitutive dimerization of the human lutropin receptor and a lack of correlation between receptor activation and the propensity for dimerization. J Biol Chem. 2009;284:7483–7494. doi: 10.1074/jbc.M809150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Calebiro D, de Filippis T, Lucchi S, Covino C, Panigone S, Beck-Peccoz P, Dunlap D, Persani L. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Wess J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry. 1998;37:15773–15784. doi: 10.1021/bi981162z. [DOI] [PubMed] [Google Scholar]
- Patowary S, Alvarez-Curto E, Xu TR, Holz JD, Oliver JA, Milligan G, Raicu V. The muscarinic M3 acetylcholine receptor exists as two differently sized complexes at the plasma membrane. Biochem J. 2013;452:303–312. doi: 10.1042/BJ20121902. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Cowan A, Mazurkiewicz JE. Fluorescence correlation spectroscopy analysis of serotonin, adrenergic, muscarinic, and dopamine receptor dimerization: the oligomer number puzzle. Mol Pharmacol. 2013;84:630–642. doi: 10.1124/mol.113.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Liu H, Chen X, Chen PH, Fischer D, Sriraman V, Yu HN, Arkinstall S, He X. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci U S A. 2012;109:12491–12496. doi: 10.1073/pnas.1206643109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zhang M, Guan R, Segaloff DL. Heterodimerization between the lutropin and follitropin receptors is associated with an attenuation of hormone-dependent signaling. Endocrinology. 2013;154:3925–3930. doi: 10.1210/en.2013-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoketo H, Strauss L, Karjalainen R, Zhang M, Stamp GW, Segaloff DL, Poutanen M, Huhtaniemi IT. Female mice expressing constitutively active mutants of FSH receptor present with a phenotype of premature follicle depletion and estrogen excess. Endocrinology. 2010;151:1872–1883. doi: 10.1210/en.2009-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]