ABSTRACT
Maternal nutritional restriction has been shown to induce impairments in a number of organ systems including the ovary. We have previously shown that maternal undernutrition induces fetal growth restriction and low birth weight, and results in an offspring ovarian phenotype characteristic of premature ovarian aging with reduced ovarian reserve. In the present study, we set out to investigate the underlying mechanisms that lead offspring of undernourished mothers to premature ovarian aging. Pregnant dams were randomized to 1) a standard diet throughout pregnancy and lactation (control), 2) a calorie-restricted (50% of control) diet during pregnancy, 3) a calorie-restricted (50% of control) diet during pregnancy and lactation, or 4) a calorie-restricted (50% of control) diet during lactation alone. The present study shows that early life undernutrition-induced reduction of adult ovarian follicles may be mediated by increased ovarian endoplasmic reticulum stress in a manner that increased follicular apoptosis but not autophagy. These changes were associated with a loss of ovarian vessel density and are consistent with an accelerated ovarian aging phenotype. Whether these changes are mediated specifically by a reduction in the local antioxidant environment is unclear, although our data suggest the possibility that ovarian melatonin may play a part in early life nutritional undernutrition and impaired offspring folliculogenesis.
Keywords: apoptosis, ER stress, follicles, maternal nutrition, ovary, reproduction
INTRODUCTION
Early life events, including changes in nutritional status, trigger processes that prepare the individual for particular circumstances that are anticipated in the postnatal environment. However, where the intrauterine and postnatal environments differ markedly, such changes to the developmental trajectory may prove maladaptive in later life [1–5]. Research into the developmental programming of disease risk has integrated aspects of metabolic, cardiovascular, and reproductive physiology, and evidence indicates that reproductive maturation and function is similarly influenced by early life events, which has been established in human populations [6–12]. In experimental models, the impact of early life nutritional adversity on reproductive function has been documented by our group [13–15] and others [16–20]. We have previously reported that rat offspring born to undernourished mothers have reduced ovarian follicle numbers and increased ovarian oxidative stress as adults [13]. Others have shown consistent effects where maternal malnutrition during lactation impairs folliculogenesis in rat offspring at puberty [21] and in sheep results in an increase in apoptosis-regulating genes [22]. Although evidence for early life nutritional influences on reproductive function is mounting, the signaling pathways that elicit ovarian impairment after early life undernutrition are still poorly understood.
We have shown previously that fetal growth restriction in the rat following maternal undernutrition results in advancements in pubertal onset [14] in a manner similar to that reported in low birth weight girls [6, 7, 23]. Offspring of undernourished mothers also demonstrated reductions in primordial, secondary, and antral follicles as adults [13] in a manner that was dependent upon the timing of undernutrition. Importantly we have shown that pregnancy is the critical window of fetal ovarian vulnerability, where offspring of mothers undernourished during pregnancy and lactation or pregnancy alone, but not during lactation alone, demonstrated a loss of oocyte-derived follicular growth factors as well as increased oxidative stress levels [13]. Previous studies have shown in vitro that ovarian oxidative stress induces granulosa cell apoptosis and subsequently follicle atresia in preovulatory rat follicles [24]. These data are characteristic of premature ovarian aging [25] and are similar to our observations in offspring born to undernourished mothers [13].
Thus, in the present study, we set out to investigate the underlying mechanisms that lead offspring of undernourished mothers to premature ovarian aging. We determined potential signaling molecules responsible for our observed increase in oxidative stress and loss of ovarian reserve [13]. In doing so, we concentrated on signaling pathways known to be stimulated by the accumulation of reactive oxygen species, including endoplasmic reticulum (ER) stress [26], phosphoinositide 3-kinase (PI3K) [27], and melatonin [28]. We hypothesized that increased ovarian ER stress could be associated with follicular loss through apoptosis and/or autophagy [29] and that this could be PI3K-inflammation mediated. Ovarian aging is associated with decreased vascularization and ovarian stromal blood flow [30]. Compromised perifollicular vascularization has been proposed to contribute to an accumulation of oxidative stress in aging follicles [31], and altered vascular support may have long-term effects on follicle depletion [32]. Thus, we investigated whether early life undernutrition altered ovarian levels of a known angiogenic agent vascular endothelial growth factor (VEGF), its receptor (VEGFR2), and blood vessel density. Because VEGF and VEGFR2 binding has been suggested to protect cells from apoptosis [33], we anticipated a decrease in VEGF in offspring ovaries accompanied by a loss in ovarian vascularization.
Melatonin induces ovarian cell survival [34] and is a powerful antioxidant and free radical scavenger [35], reducing atresia [34] and improving in vitro fertilization rates [36] and oocyte quality [37]. In brain tissue, melatonin induces protein kinase B (Akt) phosphorylation [38] because its receptors induce PI3K signaling [39]. We were interested in investigating whether the capacity to produce ovarian melatonin was diminished in undernourished offspring, implicating this important hormone in adult ovarian oxidative stress and potential follicle loss. Because melatonin and circadian locomotor output cycles kaput (Clock) genes share a close relationship [40] and recent work has suggested that clock genes are altered in offspring ovaries after early life nutritional manipulation [41], we also determined whether early life undernutrition altered adult ovarian clock gene levels.
MATERIALS AND METHODS
Animal Model
We used an established model of global maternal nutrient manipulation [13, 14, 42] to induce fetal growth restriction. Briefly, female Wistar rats (aged 120 days) were time mated, and after confirmation of mating, rats were housed individually in standard rat cages with free access to water. All rats were kept in the same room with a constant temperature maintained at 25°C and a 12L:12D cycle. Pregnant dams were randomized into one of four groups: 1) dams fed a control diet of 18% protein, 5% fat, and 3.4 kcal/gm digestible energy (Diet 2018; Harlan Teklad) ad libitum throughout pregnancy and lactation (Cont; n = 8); 2) undernourished dams fed 50% of Cont intake throughout pregnancy and lactation (UNPL, n = 7); 3) undernourished dams fed 50% of Cont intake throughout pregnancy only (UNP, n = 6); and 4) undernourished dams fed 50% of Cont intake throughout lactation only (UNL, n = 6). After birth, the pups were sexed, weighed, and litter size was standardized to eight pups that best represented (were closest to) the mean litter weight. Each litter contained four females and four males. At weaning (Day 22), the offspring were weighed, and female offspring were weight-matched within maternal dietary groups, housed two per cage, and fed the control diet ad libitum for the remainder of the study. At 150 days of age, offspring (from different litters) were fasted overnight and killed by decapitation following pentobarbitone anesthesia (60 mg/kg, subcutaneously). All the ovarian tissue was collected between 0900 and 1200. While under anesthesia, vaginal smears were performed for determination of estrous stage [13–15]. Both ovaries were collected; one was fixed in Bouin solution (Sigma-Aldrich) and processed for the determination of follicular counts [13] or for immunohistochemistry and the other snap-frozen and stored at −80°C for molecular analyses. In all cases, all biological replicates are from different litters. All the animal experiments were approved by the Animal Ethics Committee, University of Auckland (Approval R402).
Molecular Analyses
RNA extraction and reverse transcription.
Ovarian tissues were collected, snap-frozen, and stored at −80°C in preparation for RNA extraction and reverse transcribed as previously described [13, 43, 44]. Total RNA was extracted using AllPrep DNA/RNA Mini kit (80204; Qiagen). Genomic DNA was removed with RNase-free DNase (Invitrogen Life Technologies). RNA quantity and purity were analyzed using a NanoDrop spectrophotometer (ND-1000; BioLab Ltd.) and NanoDrop software (version 3.1.2). RNA samples were stored at −80°C.
Five micrograms of total RNA was used for first-strand cDNA synthesis using the Moloney Murine Leukemia Virus Reverse Transcriptase enzyme (M-MLV-RT) (Promega Corp.) and a standard thermocycler (GeneAmpH PCR System 9700; Applied Biosystems). A master mix was prepared containing 5 ml M-MLV 56 buffer (M531A; In Vitro Technologies), 0.5 ml M-MLV-RT (M170B; In Vitro Technologies), and 1.25 ml 10 mM deoxyribonucleotides (R0181; Global Science) under the following cycling conditions: denaturation stage of 5 min at 96°C, followed by 30 cycles of 30 sec each of 96°C (denaturation), 60°C (annealing stage), and 72°C (extension stage). Complementary DNA was stored at −20°C.
Quantitative polymerase chain reaction assays.
A quantitative PCR assay was performed as previously described [13] using the Roche LightCycler 480 System (Roche Diagnostics) and LightCycler 480 SYBR Green I Master (04707516001; Roche Diagnostics). Primers for all genes for ER stress, autophagy, inflammation, gonadotropin receptor, and clock genes—except Period and hydroxyindole O-methyltransferase (HIOMT)—were designed using Primer BLAST software available at the National Center for Biotechnology Information Web site (blast.ncbi.nlm.nih.gov; Table 1). Primers were manufactured by Invitrogen (Invitrogen Life Technologies). Ready-made primers for Period (per1, per2) and HIOMT genes were purchased from Qiagen (Table 2).
TABLE 1.
Primer sequences.
BMAL1, brain and muscle Arnt-like protein 1; CLOCK, circadian locomotor output cycles kaput; P13Kca (p110), phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha; CRY1 and CRY2, cryptochrome 1 and 2, respectively; P1K3r1 (p85), phosphatidylinositol 3-kinase regulatory subunit 1 (alpha); p21, cyclin-dependent kinase inhibitor 1 (also known as CDK-interacting protein 1); XBP1s and XBP1t, X-box binding protein 1 spliced and total, respectively.
TABLE 2.
Qiagen QuantiTect primer assays.
PER1 and PER2, Period 1 and 2 genes, respectively; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL-6, interleukin 6; IL-1β, interleukin 1-beta; MAP1LC3a, microtubule-associated protein 1 light chain 3 alpha; HIOMT, hydroxyindole O-methyltransferase.
Optimal primer conditions were adjusted to the following cycling conditions: length = 20 bp (range 17–23 bp), Tm = 63°C (range 60°C–65°C), and amplicon length = 100–350 bp. Dissociation analyses were performed to ensure specificity, and samples producing a single peak in dissociation curves were used. A subset of amplified products was visualized on an agarose gel using the E-GelH CloneWell 0.8% SYBR Safe gel (G6618-08; Invitrogen) run on the E-GelH iBaseTM Power System (G6400; Invitrogen).
All the quantitative PCRs were carried out with an initial denaturation at 95°C for 5 min followed by amplification of the gene product through 45 successive cycles of 95°C for 10 sec, 60°C for 10 sec, and 72°C for 10 sec. A standard curve was generated from the mean cycle threshold of eight standards (1:5 serial dilution) of a known concentration in triplicate, while amplification and dissociation curves were generated for all standards and samples (Roche Lightcycler 480 System; Roche Diagnostics). Each sample was run in triplicate. All the mRNA data is expressed relative to the geometric mean of two different reference genes (cyclophilin and beta-actin), the levels of which did not differ between nutritional groups.
Immunohistochemistry and Immunofluorescence
Fixed ovaries were processed for microscopy, embedded in paraffin, and the entire ovary was serially sectioned at 8 μm as described previously [13]. Sections that were not used for follicle counts were then used for immunohistochemistry/immunofluorescence.
Determination of Follicular Apoptosis
Detection of apoptotic cells in follicles was performed as previously described [33]. A terminal deoxynucleotidytransferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) kit (Roche Applied Science) was used according the manufacturer's protocol. Briefly, ovarian tissue sections (8 μm) were permeabilized in 0.1% Triton X-100 (Sigma), washed in PBS, and incubated for 60 min with the fluorescein isothiocyanate-conjugated TUNEL enzyme to detect DNA fragmentation. Nuclei were counterstained with propidium iodide. Apoptotic cells showing TUNEL-positive staining were counted in six fields of view per section at 250× magnification (n = 5 per nutritional group). Image analysis was performed using an Olympus BX-61 microscope and integrated morphometry software (MetaMorph). Data are expressed as apoptotic-positive follicular cells as a proportion of the total.
Determination of Ovarian Blood Vessel Density and Immunolocalization of VEGF and VEGFR2
Evaluation of blood vessel density was performed as described previously using the endothelial cell marker, CD31 [45]. Briefly, ovarian sections (8 μm) were immersed in 30% hydrogen peroxide to inhibit endogenous peroxidase activity for 10 min. Antigen retrieval was performed by incubating tissues in 10 mM sodium citrate buffer with Tween, pH 6.0, at 90°C for 12 min. Nonspecific binding was blocked with 5% bovine serum albumin for 10 min at room temperature (RT) and incubated overnight at 4°C with anti-CD31 primary antibody (1:100 dilution; Abcam). The following day, tissue sections were incubated at RT for 2 h with anti-mouse biotinylated secondary antibody (Sigma-Aldrich Canada Ltd.) then incubated with ExtrAvidin (Sigma-Aldrich Canada Ltd.) for 1 h. CD31 was visualized with 3,3′-diaminobenzidine (DAB) (D4293; Sigma-Aldrich), and all sections were counterstained with Gills 2 hematoxylin (GHS216; Sigma-Aldrich). For blood vessel density determination, CD31-immunopositive (endothelial cells) blood vessels were measured in six fields of view per section at 250× magnification (n = 5 per nutritional group). Image analysis was performed using an Olympus BX-61 microscope and integrated morphometry software (MetaMorph). Data are expressed as vessel area as a proportion of total ovarian area analyzed.
Protein localization of vascular endothelial growth factor (VEGF), its receptor type 2 (VEGFR2), and their colocalization was performed using immunofluorescence on ovarian sections (8 μm). Sections were incubated for 1 h with rabbit polyclonal anti-human VEGF (1:600 dilution; Santa Cruz Biotechnology), washed with PBS, and incubated for 1 h with an Alexa Fluor 488-conjugated anti-rabbit immunoglobulin G (IgG; heavy and light chains), F(ab′)2 fragment (1:250 dilution, 8889; Cell Signaling). Sections were then washed twice for 2 min with 1× PBS and incubated with rabbit polyclonal anti-human VEGFR2 (1:500 dilution; Santa Cruz Biotechnology). After a 1-h incubation, tissue sections were washed with PBS and incubated for another hour with Alexa Fluor 594-conjugated anti-rabbit IgG (heavy and light chains), F(ab') fragment. Nuclei were counterstained with propidium iodide or 4′,6-diamino-2-phenylindole, and specimens were imaged with a BX-61 fluorescent microscope (Olympus). Stained slides were stored at 4°C. Total VEGF and VEGFR2 immunostaining was quantified by determining the proportion of immunopositive cells in six fields of view per section at 250× magnification (n = 5 per nutritional group). Image analysis was performed using an Olympus BX-61 microscope and integrated morphometry software (MetaMorph). Data are expressed as VEGF/VEGFR2-positive cells as a proportion of total ovarian area analyzed. The amount of area where VEGF and VEGFR2 colocalized was also calculated and expressed as a proportion of total area analyzed. All the image analyses were performed by an investigator blind to the study groups.
Immunolocalization of the Autophagy Regulator, Beclin1
Immunolocalization of Beclin1 in ovarian tissue sections was performed as described above with the following modifications. Ovarian sections were incubated with Beclin1 antibody (1:300 dilution, ab55878; Cedarlane) overnight at 4°C. The following day, sections were incubated with biotinylated secondary antibody (1:10 dilution, Vectastain Elite ABC kit peroxidase rabbit IgG; Vector Labs) for 2 h at RT then incubated with avidin-biotin peroxidase complex solution (4:10 dilution using 1% bovine serum albumin in 1× PBS) for 2 h at RT. The antibody complex was visualized using DAB, and the sections were counterstained with Gills II hematoxylin. Cover slips were mounted using Permount (SP15-500; Fisher Scientific), and immunopositive cells were imaged using the NIS-Elements AR imaging program on a Nikon 90i microscope at 200× magnification. Total Beclin1 immunostaining was quantified by determining average density of brown (DAB substrate) immunopositive cells in six fields of view per section at 200× magnification (n = 5 per nutritional group) using the NIS-Elements AR imaging program (Nikon). In all the immunohistochemical assays, negative controls were incubated in the absence of the primary antibody and were included in each assay run. All the image analyses were performed by an investigator blinded to the study groups.
Statistical Analyses
All the data were analyzed by one-way ANOVA. Data that were not normally distributed were log-transformed to achieve normality. Post-ANOVA multiple comparisons among means were performed using Bonferroni post hoc analysis where appropriate, and a P < 0.05 was considered statistically significant. All the data are presented as means ± SEM. Statistical analysis was performed using SigmaStat for Windows version 2.03 (Jandel Corp.) and GraphPad Prism version 6.00 for Mac (GraphPad Software).
RESULTS
Body Weights
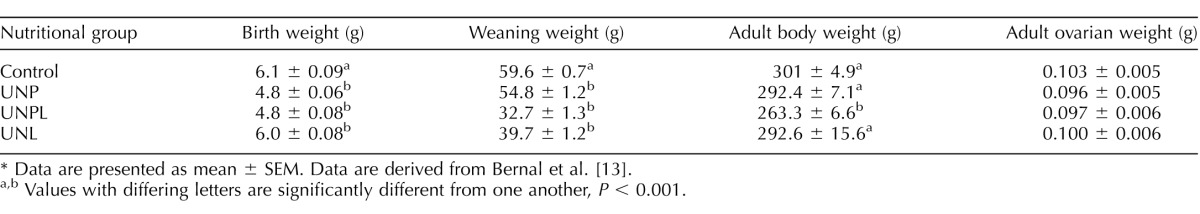
At birth, sex ratios were the same across groups (data not shown). Early life undernutrition significantly reduced birth weight in UNP and UNPL offspring compared to Cont; this reduction in body weight persisted to weaning, and in UNPL, it persisted to adulthood [13] (Table 3). We found no effect of early life undernutrition exposure on adult ovarian weight [13].
TABLE 3.
Offspring body and ovarian weights.*
Data are presented as mean ± SEM. Data are derived from Bernal et al. [13].
Values with differing letters are significantly different from one another, P < 0.001.
Early Life Undernutrition Exposure Increased Ovarian ER Stress and Follicular Apoptosis but Decreased Ovarian Autophagy in Adult Offspring
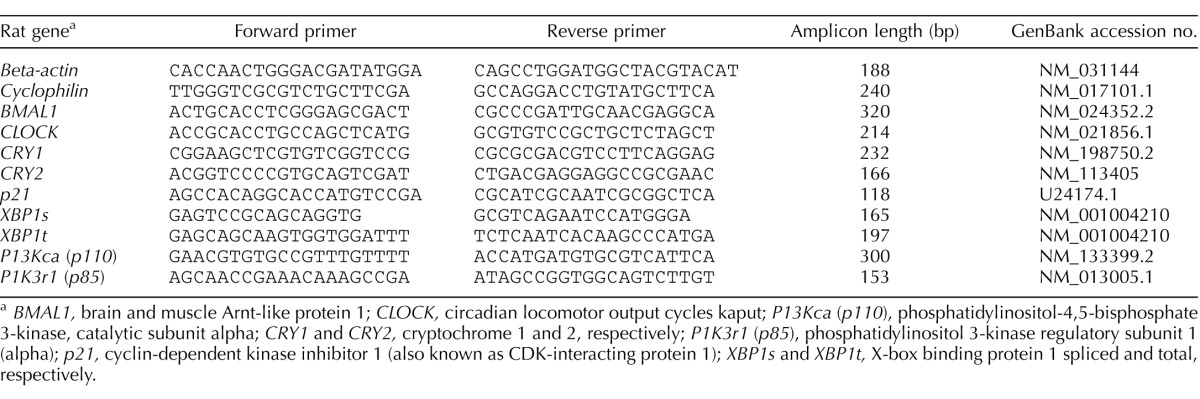
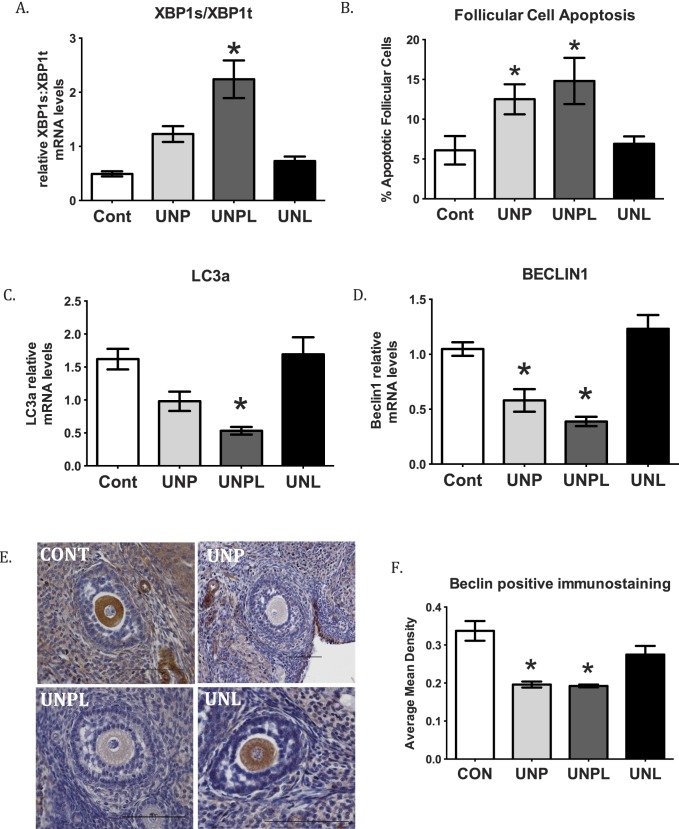
Ovarian X-box binding protein 1 spliced (XBP1s), total (XBP1t), and their ratio were determined as a marker of ER stress in offspring ovaries. Ovarian XBPT1s:XBP1t mRNA ratios were significantly increased in ovaries from offspring born to undernourished mothers (P < 0.001) (Fig. 1A). Post hoc analysis demonstrated that although UNP and UNPL offspring both showed an increased XBP1s:XBP1t ratio compared to Cont offspring, but only UNPL reached statistical significance (UNPL; P < 0.001) (Fig. 1A). Follicular apoptosis was significantly increased in ovaries from offspring born to undernourished mothers (P < 0.001) (Fig. 1B). Post hoc analysis demonstrated a statistically significant increase in the proportion of apoptotic follicular cells in ovaries of UNP (P < 0.05), and UNPL (P < 0.05) groups compared to Cont offspring (Fig. 1B).
FIG. 1.
Early life exposure to undernutrition increases ovarian ER stress and follicular apoptosis but decreases ovarian autophagy in adult offspring. A) Maternal undernutrition during pregnancy and lactation (UNPL) resulted in an increase mRNA levels of ER stress-related genes expressed as a ratio of XBP1s to XBP1t (n = 6–7 per group). B) Maternal undernutrition during pregnancy and during pregnancy and lactation resulted in an increase in follicular cell apoptosis (measured as TUNEL-positive staining) as a proportion of total area analyzed. C) Maternal undernutrition decreased key components of the autophagy process, including microtubule-associated protein 1A/1B-light chain 3 (LC3a) in UNP ovaries. D) Beclin 1 in ovaries of UNP and UNPL offspring. E) Photographs represent ovarian sections stained for immunopositive Beclin1 protein using DAB substrate and counterstained with hematoxylin. Immunopositive Beclin1 protein is identified by brown staining. Bars = 100 μm. Negative controls show no positive staining (data not shown); n = 5 per group. F) Gene expression data are presented as means ± SEM. One-way ANOVA main effects: maternal diet P < 0.05 for all genes. Post hoc analyses (Bonferroni): *P < 0.05 for undernourished compared to control and UNL offspring. Cont, offspring of mothers fed a control diet; UNP, offspring of mothers undernourished during pregnancy alone; UNPL, offspring of mothers undernourished during pregnancy and lactation; UNL, offspring of mothers undernourished during lactation alone; n = 6–7 per group.
Early life undernutrition exposure resulted in a significant decrease in adult ovarian mRNA levels of Beclin1 (P < 0.001), an established component of the autophagic machinery, and of the autophagasome marker LC3 (P < 0.001; Fig. 1C). Post hoc analyses showed a statistically significant decrease in Beclin1 mRNA levels in UNP (P = 0.005) and UNPL (P < 0.001) offspring compared with Cont offspring (Fig. 1D). Although both UNP and UNPL groups showed lower levels of LC3 mRNA, post hoc analyses showed a statistically significant decrease in LC3 mRNA levels only in UNPL offspring (P < 0.001; UNP: P = 0.06) (Fig. 1C). Ovarian sections were immunostained for Beclin1 protein to determine follicular localization. We observed that Beclin1 protein is localized in ovarian blood vessels, oocytes, and ovarian stroma (Fig. 1E). Consistent with mRNA levels, we observed a decrease in the mean density of immunostaining of Beclin1 protein in ovarian sections in UNP (P < 0.001), and UNPL (P < 0.05) groups compared to the Cont group (Fig. 1, E and F). UNL offspring showed similar staining to controls (Fig. 1, E and F). Immunopositive Beclin 1 was highly localized to oocytes and blood vessels and less so in stromal, granulosa, and thecal cells. All the negative controls in immunohistochemical staining assays showed no positive staining (data not shown).
Early Life Undernutrition Exposure Reduced Ovarian Blood Vessel Density and VEGF and VEGFR2 Immunostaining in Offspring Ovaries
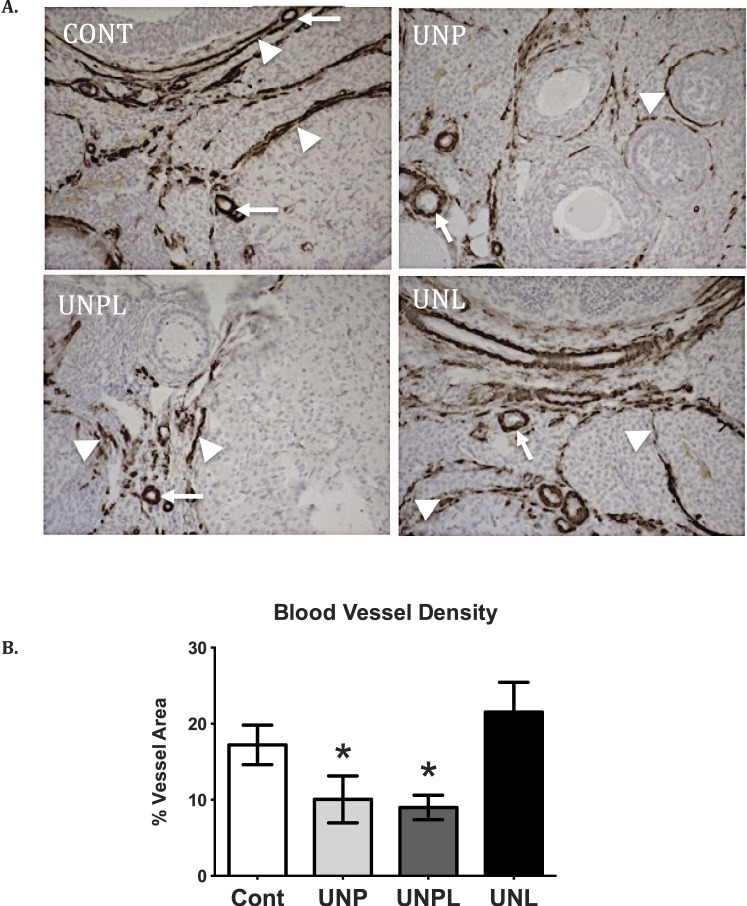
Early life events have been shown to modulate vascularization of organs in offspring [46, 47]; thus, we set out to investigate whether blood vessel density was similarly altered in the ovary. Early life undernutrition exposure significantly decreased ovarian vascularization in a manner that was dependent upon timing of the undernutrition. Blood vessel density, including perifollicular capillaries, as defined by the presence of endothelial cell marker CD31 immunostaining as a proportion of total area, was significantly decreased in UNP (P < 0.05) and UNPL (P < 0.05) offspring compared to Cont (Fig. 2). Vascularization in UNL offspring was not different to that of the Cont group.
FIG. 2.
Early life undernutrition reduces ovarian blood vessel density. Photographs represent ovarian sections immunostained for endothelial cell marker CD31 using DAB substrate and counterstained with hematoxylin. A) CD31 immunopositive protein is identified by brown staining indicative of DAB substrate. CD31-positive endothelial cell staining within ovarian blood vessel structures are indicated by white arrows. Perifollicular capillaries, that provide vascular supply to the developing follicle and/or corpus luteum, are indicated by white arrowheads. Negative controls show no positive staining (data not shown). Original magnification ×200. B) Maternal undernutrition resulted in a significant decrease in the proportion of CD31 immunostaining (expressed as percent of vessel area of total ovarian area analyzed) in offspring ovaries. Data are presented as means ± SEM. One-way ANOVA main effects: maternal diet P < 0.05 for undernourished compared to Cont offspring. Post hoc analyses (Bonferroni): *P < 0.05 compared to Cont offspring; n = 5 per group. Cont, offspring of mothers fed a control diet; UNP, offspring of mothers undernourished during pregnancy alone; UNPL, offspring of mothers undernourished during pregnancy and lactation; UNL, offspring of mothers undernourished during lactation alone.
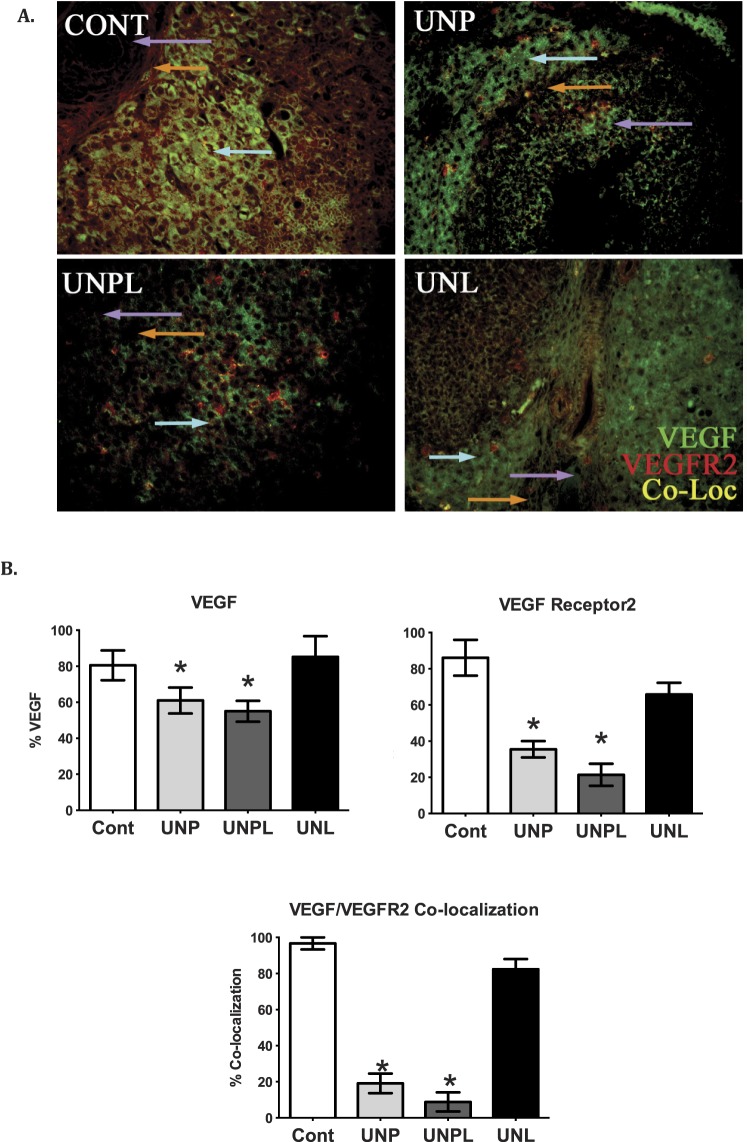
VEGFR2 has been found to be highly expressed in stromal, granulosa, and theca cells in the rat ovary [48], and critical changes in sensitivity of granulosa cells to VEGF appear to be mediated through VEGFR2 [49]. VEGFR2 has also been shown not only to mediate vascularization, but also to function as an antiapoptotic agent in rat granulosa cells and is necessary for dominant follicle selection. Consistent with our observed increase in apoptosis and loss of vessel density, early life nutrient restriction resulted in a significant reduction in VEGF and VEGFR2 immunopresence in adult ovaries (Fig. 3). Immunohistochemical staining showed a significant decrease in VEGF and VEGFR2 protein in stromal and follicular cells as a proportion of total area analyzed in UNP (P < 0.05) and UNPL (P < 0.05) offspring compared to Cont offspring. Similarly, offspring born to undernourished mothers demonstrated decreased VEGF and VEGFR2 colocalization in UNP (P < 0.05) and UNPL (P < 0.05) offspring compared to Cont offspring (Fig. 3). All negative controls showed no positive staining (data not shown).
FIG. 3.
Early life undernutrition reduces VEGF, VEGFR2, and their colocalization in offspring ovaries. A) Photographs represent immunopositive staining of VEGF (green), VEGFR2 (red), and the colocalization of VEGF/VEGFR2 (yellow). Negative controls show no positive staining (data not shown). Original magnification ×200. B) Early life undernutrition resulted in a significant decrease in the proportion of VEGF, VEGFR2, and VEGF/VEGFR2 immunostaining (expressed as percent of positive stained area of total ovarian area analyzed) in offspring ovaries. One-way ANOVA main effects: maternal diet P < 0.05. Post hoc analyses (Bonferroni): *P < 0.05 for undernourished compared to control offspring (n = 5 per group). Cont, offspring of mothers fed a control diet; UNP, offspring of mothers undernourished during pregnancy alone; UNPL, offspring of mothers undernourished during pregnancy and lactation; UNL, offspring of mothers undernourished during lactation alone. Cell types are indicated by colored arrows: granulosa cells (purple arrow), theca cells (orange arrow), and ovarian stroma (light blue arrow).
Early Life Undernutrition Exposure Altered Proinflammatory Cytokines and Upstream Signaling Molecules in Offspring Ovaries
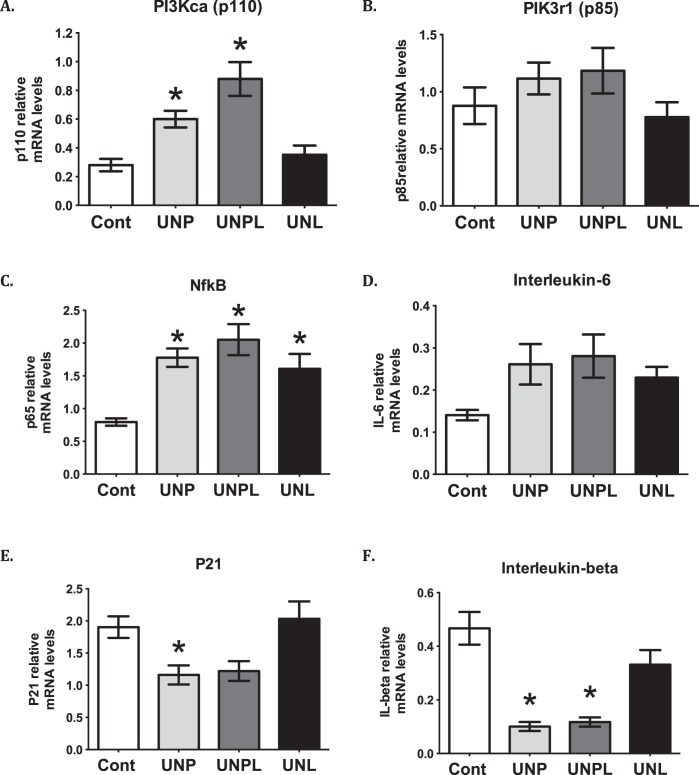
We have previously reported that offspring exposed to early life undernutrition demonstrate increased levels of ovarian oxidative stress [13], and because we now demonstrated an increased in a marker of ER stress, we therefore investigated a central signaling pathway that could contribute to these observed changes. Offspring born to undernourished mothers demonstrated elevated mRNA levels of the genes that encode both the catalytic subunit (p110α) and the regulatory subunit (p85α) of PI3K, although only p110α reached statistical significance (P < 0.001) (Fig. 4). Post hoc analysis demonstrated that mRNA levels of PIK3CA (p110α) were significantly elevated in UNP (P = 0.023) and UNPL (P < 0.001), but not UNL offspring, compared to Cont offspring (Fig. 4). Consistent with this, ovarian nuclear factor kappa light chain enhancer of B cells (NFκB or RelA/p65) mRNA levels were significantly increased in ovaries from offspring born to undernourished mothers (P < 0.001) (Fig. 4). Post hoc analysis demonstrated an increase in NFκB mRNA levels in UNP (P = 0.002), UNPL (P < 0.001), and UNL (P = 0.017) compared to Cont offspring (Fig. 4). Although offspring born to undernourished mothers tended to demonstrate increased mRNA levels of the proinflammatory cytokine IL-6, this difference was not statistically significant (P = 0.063) compared to Cont offspring (Fig. 4). Ovarian mRNA levels of IL-1β, a proinflammatory factor shown to suppress apoptosis in the rodent ovary [50], was decreased in offspring born to undernourished mothers (P < 0.001). Post hoc analysis demonstrated that mRNA levels of IL-1β were significantly decreased in UNP (P < 0.001) and UNPL (P < 0.001) but not UNL offspring compared to Cont (Fig. 4).
FIG. 4.
Early life undernutrition alters proinflammatory cytokines and upstream signaling molecules in offspring ovaries. Early life undernutrition resulted in a significant increase in the catalytic subunit of PI3 kinase PI3KCA (A), p85 (B), and NFκB (C) mRNA levels, but a decrease in p21 (E) and proinflammatory IL-1β (F). D) No effect on IL-6 was observed. Data are presented as means ± SEM. One-way ANOVA main effects: maternal diet P < 0.05 for all genes except for IL-6 undernourished compared to Cont offspring. Post hoc analyses (Bonferroni): *P < 0.05 compared to Cont offspring for PI3KCA and p21 and for IL-1β *P < 0.05 compared to Cont and as well when compared to UNL (n = 6–7 per group). Cont, offspring of mothers fed a control diet; UNP, offspring of mothers undernourished during pregnancy alone; UNPL, offspring of mothers undernourished during pregnancy and lactation; UNL, offspring of mothers undernourished during lactation alone.
Maternal nutrient restriction also decreased mRNA levels of the gene encoding cyclin-dependent kinase inhibitor 1 (p21) (gene CDKN1A) (P = 0.005) (Fig. 4). Post hoc analysis demonstrated that this is largely attributable to a significant decrease in UNP offspring (P = 0.049) and not UNPL or UNL groups compared to Cont offspring (Fig. 4). This decrease is consistent with our previously observed loss of ovarian follicles [13] and p21's role in inhibiting cell proliferation.
Early Life Undernutrition Exposure Reduced Ovarian Levels of the Melatonin Synthesizing Enzyme HIOMT
Melatonin, in addition to its role in circadian rhythmicity, is also a known antioxidant in the ovary [39]. Because we have previously shown that early life undernutrition resulted in increased ovarian oxidative stress levels [13], we investigated whether this may be associated with changes in the capacity of the ovary to produce melatonin. Early life undernutrition resulted in a significant decrease in HIOMT mRNA levels (P = 0.002) (Fig. 5). Post hoc analysis demonstrated that although UNP offspring tended to have lower HIOMT mRNA levels, differences were significant only in UNPL (P = 0.038) offspring compared to Cont and UNL offspring (Fig. 5). We were unable to find detectable mRNA levels of the two known melatonin receptors in the ovary [51].
FIG. 5.
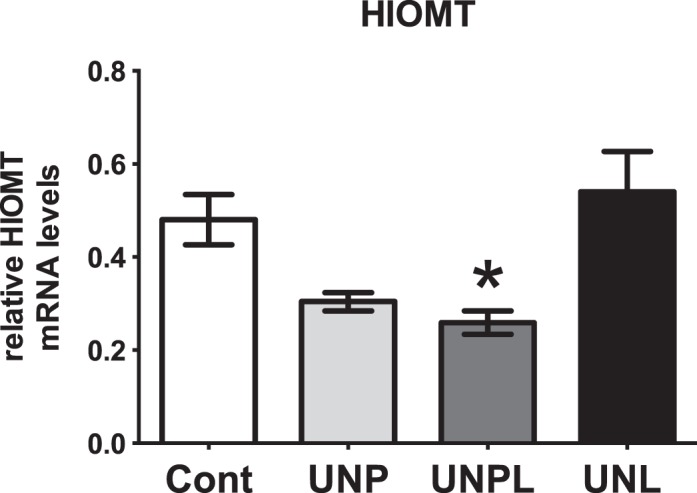
Early life undernutrition alters ovarian levels of HIOMT. Early life undernutrition resulted in a significant decrease in ovarian mRNA expression of hydroxyindole O-methyltransferase (HIOMT) in a manner that is dependent upon the timing of nutrient restriction. Data are presented as means ± SEM. One-way ANOVA main effect: maternal diet P < 0.05 for undernourished compared to Cont offspring. Post hoc analyses (Bonferroni): *P < 0.05 for UNPL (P = 0.1 UNP) compared to Cont and UNL offspring (n = 6–7 per group). Cont, offspring of mothers fed a control diet; UNP, offspring of mothers undernourished during pregnancy alone; UNPL, offspring of mothers undernourished during pregnancy and lactation; UNL, offspring of mothers undernourished during lactation alone.
Early Life Undernutrition Exposure Altered Core Circadian Clock Genes in Offspring Ovaries
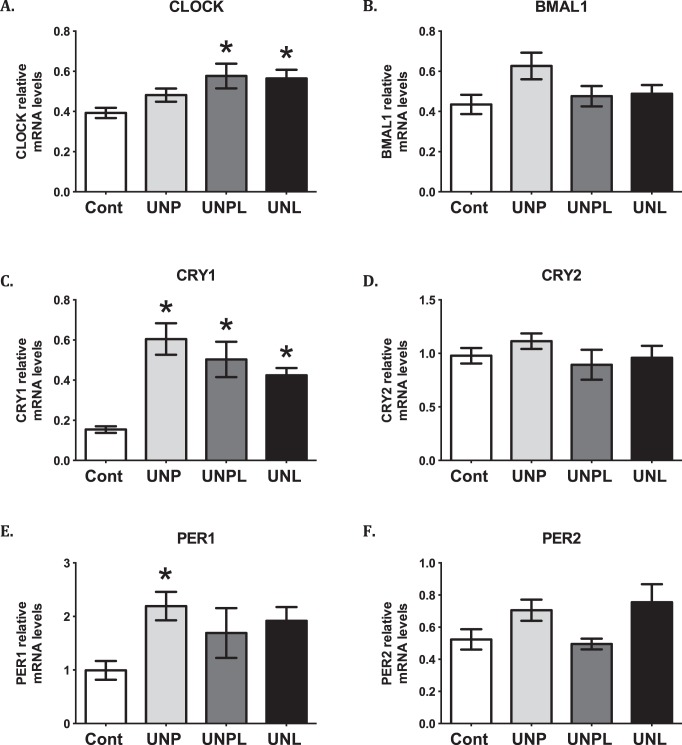
A close relationship exists between melatonin and clock genes [40, 52], and alterations in clock-controlled gene expression due to prenatal malnutrition have been linked to metabolic dysfunction in offspring later in life [53]. Clock gene knockout mice have illustrated the importance of clock genes in the regulation of reproductive function [54], and recently, maternal obesity has been associated with altered clock gene expression in offspring ovaries [41]. Thus, we investigated the effect of early life undernutrition exposure on offspring ovarian mRNA levels of core circadian clock genes. Early life undernutrition resulted in a significant increase in CLOCK mRNA levels (P = 0.015). Post hoc analysis demonstrated an increase in ovarian Clock mRNA levels in UNPL (P = 0.028) and UNL (P = 0.046), but not UNP offspring compared to Cont offspring (Fig. 6). Although early life undernutrition tended to increase BMAL1 (also known as Arntl) mRNA levels, this difference was not statistically significant (P = 0.081). Ovarian cryptochrome 1 (CRY1) mRNA levels were significantly higher in undernourished offspring (main effect P < 0.001; Fig. 6). Post hoc analysis demonstrated that undernourished offspring showed higher ovarian CRY1 mRNA levels compared to Cont offspring, regardless of the timing of maternal undernutrition (UNP, P < 0.001; UNPL, P = 0.004; UNL, P = 0.031) (Fig. 6). There was no effect of early life undernutrition exposure on ovarian CRY2 mRNA levels (P = 0.464). Early life undernutrition exposure resulted in a significant increase in ovarian Period 1 (PER1) mRNA levels (P = 0.033) in offspring. Post hoc analysis demonstrated that this increase was largely due to an increase in ovarian PER1 mRNA levels in UNP (P = 0.02) compared to Cont offspring (Fig. 6); although UNPL and UNL levels tended to be higher, these differences were not statistically significant. There was a significant main effect of early life undernutrition on ovarian PER2 mRNA levels in offspring (P = 0.025), but post hoc analysis demonstrated no differences between groups. The mRNA levels of the transcriptional repressor Rev-erbα, involved in circadian rhythmicity, were not affected by early life undernutrition (P = 0.1) (data not shown).
FIG. 6.
Early life undernutrition alters core circadian clock genes in offspring ovaries. A, B, C, E) Early life undernutrition resulted in a significant increase in ovarian mRNA expression of core clock genes in the ovary in a manner that is dependent upon the timing of nutrient restriction: CLOCK (A), BMAL 1 (B), CRY1 (C), PER1 (E). Data are presented as means ± SEM. One-way ANOVA main effects: maternal diet P < 0.05 for all genes except for PER2 (F) and CRY2 (D) levels in undernourished compared to Cont offspring. Post hoc analyses (Bonferroni): *P < 0.05 compared to Cont offspring (n = 6–7 per group). Cont, offspring of mothers fed a control diet; UNP, offspring of mothers undernourished during pregnancy alone; UNPL, offspring of mothers undernourished during pregnancy and lactation; UNL, offspring of mothers undernourished during lactation alone.
DISCUSSION
Our data highlight the possible mechanisms that may be responsible for the postnatal ovarian phenotype characterized by premature ovarian aging after early life exposure to caloric restriction. We demonstrate that outcomes are dependent on the timing of nutrient restriction, where in most outcomes measured, pregnancy is a critical time window of vulnerability as evidenced by the fact that UNP and UNPL offspring demonstrate the most significant changes. We show that offspring have increased follicular apoptosis and reduced blood vessel density that may be facilitated by a loss of VEGF and its receptor. We hypothesize that this ovarian phenotype may be mediated by increased ER stress and a loss of the capacity to produce the antioxidant melatonin. These observations are consistent with our previous observations whereby offspring that are exposed to early life undernutrition have a loss of follicles and demonstrate increased oxidative stress [13].
Our previously reported loss of follicles in undernourished offspring [13] could be mediated by ER stress-induced follicular apoptosis. Although we report only XBP1 as a marker of ER stress, it has previously been shown that many upstream unfolded protein response (UPR)-sensitive genes may converge on XBP1 transcription and mRNA splicing [55], and indeed XBP1 has been directly associated with disease states [56] and with autophagy [57]. Recent work has highlighted the role of ER stress-mediated UPR in granulosa cells [58]. Consistent with our observations, both ER stress and autophagy have been shown to be present in the goat ovary and regulate granulosa cell apoptosis [58]. Recent in vitro work shows that UPR signaling pathways that are activated by ER stress appears to play a role in luteal stage progression and ER stress-mediated apoptosis in induced corpus luteal regression [59]. It is possible that our observed increase in XBP1 ratio is due to corpus luteum regression, although it is likely that follicular UPR also contributes to our observation because these ovaries are within the follicular stage of the rat estrous cycle. [60, 61]
Antral follicle loss may occur through other possible molecular targets, including XIAP (X-linked inhibitor apoptosis protein). XIAP is localized within granulosa, theca, and luteal cells where it serves to regulate follicle loss through atresia [62, 63] and associates with survival signaling pathways, including PI3K, Akt, and NFκB [64]. Importantly, ER stress induces transcription of IAPs [65] and interacts with the PI3K/Akt pathway where transcription of XIAP was up-regulated by PI3K/Akt, at least in vitro [66], to play a critical role in controlling cell survival by resisting ER stress-induced cell death signaling [66]. It is tempting to speculate that the survival-to-death balance within the ovary, particularly in growing (antral) follicle subtypes, has been tipped in undernourished offspring, whereby ER stress-triggered PI3K signaling is insufficient to restrain ER stress-induced apoptosis in undernourished offspring. Our previous observations that undernourished offspring have increased oxidative stress and reduced ability to cope with oxidative stress [13] is consistent with this notion.
It is important to recognize that PI3K signaling is a master regulator of diverse outcomes. In the ovary, PI3K signaling of Akt induces downstream effects that are not only dependent upon the cell type, (granulosa versus oocyte) but also the follicle subtype (primordial versus antral). Importantly, stimulation of PI3K/Akt leads to accelerated primordial recruitment in normal ovaries [67]. It is possible that early life exposure to undernutrition results in PI3K-induced accelerated primordial follicle recruitment; this may explain our previously reported loss of primordial follicle in UNPL offspring in adulthood [13]. Indeed early life exposure to high levels of testosterone (mimic of polycystic ovary syndrome) has been suggested to result in accelerated primordial follicle recruitment [68]. We would hypothesize that these recruited primordial follicles undergo atresia and contribute to a decrease in ovarian reserve [13]. We are unable dissect out specific PI3K-Akt roles in whole ovarian tissue, and as such, we recommend future studies engage in more culture-based queries or laser microdissection of specific follicles [69] to not only specifically pinpoint the exact downstream effect of an increase in PI3KCA (the catalytic subunit of PI3K) in UNP, UNL, and UNPL offspring but to investigate the role of PI3K in different follicle subtypes. Nevertheless, our data strongly implicate the PI3K pathway in producing offspring ovarian impairment after early life undernutrition and in mediating our previously reported premature loss of ovarian reserve in adulthood [13].
Although our results suggest autophagy is not involved in the observed follicle loss in the UNP and UNPL adult offspring ovaries, previous studies have indicated that Beclin-independent pathways may also contribute toward the activation of autophagy. However to date, this work has been shown in cortical neuronal cells, and ovarian carcinoma cells and the mechanisms by which Beclin-independent autophagy occurs varies according to cell type and stimuli [70, 71].
Proinflammatory cytokines are crucial in ovarian physiology; including ovulation, the maintenance of ovarian follicles, and regulating follicle atresia [50]. Proinflammatory IL-6 and its soluble receptor promote granulosa cell survival, regulating follicle growth [72]. IL-1β has been shown to be a survival factor, influencing growth and preventing follicles from undergoing atresia by increasing nitric oxide production within granulosa cell and macrophages, inhibiting apoptosis [73]. Our observed reduction in IL-1β mRNA levels suggests that UNP and UNPL offspring ovaries may be in a state of ER stress-induced apoptosis, aided by a decrease in IL-1β. Interestingly, VEGF is induced by IL-1β, which contributes toward perifollicular blood flow, ultimately promoting macrophage recruitment [74]. This is consistent with our observed loss of immunopositive VEGF and VEGFR2 in UNP and UNPL offspring, suggesting that local growth factors such as VEGF likely participate in impairments in folliculogenesis after early life undernutrition. Indeed, it has been previously shown that VEGF protects granulosa cells from atresia [33], promotes early folliculogenesis [75], and more recently is implicated in granulosa cell-oocyte cross talk [69]. Our observed loss of VEGF/VEGFR2 may be indicative of a loss of this protection from atresia.
Our study is the first to demonstrate that early life exposure to nutrient restriction alters adult offspring ovarian mRNA levels of core circadian genes and has associated changes in clock-controlled genes. Circadian clocks have been shown previously to exist in the ovary [76–78], and clock gene-null mice have delayed pubertal onset, altered estrus cyclicity, reduced ovarian weight [54, 79], reduced lifespan, and premature aging [80]. At the molecular level, clock genes, Clock and Bmal1, heterodimerize and result in the direct rhythmic transcription of Per and Cry gene families [81]. Our observation of increased Clock, Per1, and Cry1 gene levels in undernourished offspring suggests that early life exposure to nutrient restriction may result in the resetting of the ovarian clock in undernourished offspring. Recently, a maternal high fat diet has also been shown to modulate core clock genes in offspring ovaries [41]. The heterodimer CLOCK/Bmal1 controls the transcription of ∼10% of the genes in a given cell; it binds to E-boxes that are located in the promoters of clock-controlled genes, including those that regulate steroidogenesis [82], apoptosis [83], cell cycle [84], inflammation [85], and angiogenesis [86], all putative regulators of ovarian folliculogenesis. How clock gene resetting modifies ovarian function is presently unknown, although a strong relationship exists between ovarian clock gene activation and gonadotropin regulation of follicle development [87]. It has been suggested that immature granulosa cells do not exhibit a strong circadian gene periodicity and that during the process of follicular differentiation, FSH provides a cue for the development of a functional clock [88]. We have previously reported that undernourished offspring have similar circulating FSH and FSH receptor levels compared to controls [13]. Thus, it is unclear how an increase in the core clock genes in UNPL (and modestly in UNP) offspring specifically affects follicle dynamics. We have, however, observed changes in ovarian steroidogenic capacity [13] and now show changes in apoptosis, cell cycle regulation, inflammation, and angiogenic pathways. It has been shown that follicular cells express cell autonomous circadian rhythmicity, which can be affected by gonadotropins FSH and LH [87], an effect that is dependent on the cell's stage of differentiation. It is possible that clock gene signaling in FSH-dependent follicles may be compromised in these offspring, consistent with our previously observed loss of antral follicle number in undernourished offspring [13].
Melatonin has known effects on clock gene expression [40] and is a well-characterized antioxidant within the ovary [34, 35]. It induces ovarian cell survival [34] via reducing follicular atresia [34], improving in vitro fertilization rates [36] and oocyte quality [37]. We have shown that early life undernutrition decreases mRNA levels of the rate-limiting enzyme (HIOMT) involved in the production of melatonin. A decreased capacity to produce melatonin in UNP and UNPL offspring ovaries would be consistent with our observation that undernourished ovaries have increased oxidative stress [13] and follicular apoptosis. Moreover, it is possible that melatonin's regulation of oxidative stress could be through its known immune-modulatory actions via the NFkB pathway [89]. Melatonin suppresses NFkB signaling in many disease states [90]. We hypothesize that UNP and UNPL offspring have lower ovarian melatonin levels, which would be consistent with our observed increase in NFkB mRNA levels, and would be consistent with a loss of antral follicles due to oxidative stress and apoptosis.
Although there has been some dispute about whether the ovary actually produces melatonin [28], our data together those of others [91] suggest that the ovary is capable of producing melatonin. Serum melatonin levels show seasonal and circadian variation and are related to reproduction [92], and there are many reports linking melatonin with reproductive outcome and ovarian function [93–98]. Melatonin has been localized in the human ovary [34]. Although we were unable to detect melatonin receptor mRNA levels in offspring ovaries, others have localized melatonin, its receptors, and synthesizing enzyme (HIOMT) to granulosa and theca cells in the rodent ovary using immunostaining [51, 91, 99], although their role in these cell types is not well established. It is likely that they are expressed at very low levels and thus could explain why we were unable to detect receptor presence in whole ovaries.
We recognize that our study is not without limitations. Our mRNA data represent whole ovarian homogenates and do not represent specific follicle subtypes. However, we consistently demonstrate that, even at the whole ovarian level, robust changes in mRNA levels and immunolocalization of key proteins and growth factors in offspring that were exposed to early life undernutrition. Further, although our tissue collection occurred within a strict 3-h time frame beginning at 0900, the clock gene data reflect only one time point within the 24-h circadian cycle. Given that we demonstrate such strong changes in both core clock genes and other key factors involved in folliculogenesis suggests that these changes are robust and reflective of real changes in gene expression within undernourished ovaries compared to controls. These changes are consistent with others that have collected ovarian tissue at one time point during the day and show changes in clock gene levels [41].
In conclusion, we present novel data that maternal undernutrition during pregnancy and/or lactation significantly increased ovarian ER stress in adult offspring that was accompanied by decreased ovarian VEGF, vascularization, and autophagy. It appears that a local ovarian antioxidant, melatonin, may be reduced because its synthesizing enzyme, HIOMT, is suppressed, a result that is consistent with our previous observations of increased ovarian oxidative stress in offspring of undernourished mothers [13]. We hypothesize that these changes may be downstream of clock gene expression patterns in the ovary. Critically, these results are dependent upon the timing of maternal undernutrition, and the offspring whose undernutrition exposure included fetal life are particularly vulnerable.
Footnotes
D.M.S. is supported by Canada Research Chair in Perinatal Programming, J.J.P. is supported by Canadian Institutes for Health Research, and M.H.V. is supported by Gravida. This study was in part funded by the Health Research Council of New Zealand, and Gravida. Presented in part at the 47th Annual Meeting of the Society for the Study of Reproduction, 19–23 July 2014, Grand Rapids, Michigan.
These authors contributed equally to the preparation of this manuscript.
REFERENCES
- Gluckman PD, Beedle AS. Migrating ovaries: early life influences on later gonadal function. PLoS Med. 2007;4:e190. doi: 10.1371/journal.pmed.0040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS, Spencer HG. Predictive adaptive responses in perspective. Trends Endocrinol Metab. 2008;19:109–110. doi: 10.1016/j.tem.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental origins of health and disease: moving from biological concepts to interventions and policy Int J Gynecol Obstet 2011. 115 (Supplement 1): S3 S5 [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: influences of prenatal and postnatal growth. J Clin Endocrinol Metab. 2007;92:46–50. doi: 10.1210/jc.2006-1378. [DOI] [PubMed] [Google Scholar]
- Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. Br J Obstet Gynaecol. 1996;103:814–817. doi: 10.1111/j.1471-0528.1996.tb09879.x. [DOI] [PubMed] [Google Scholar]
- Nunez-de la Mora A, Chatterton RT, Choudhury OA, Napolitano DA, Bentley GR. Childhood conditions influence adult progesterone levels. PLoS Med. 2007;4:e167. doi: 10.1371/journal.pmed.0040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV. Endocrinology and gynecology of girls and women with low birth weight. Fetal Diagn Ther. 2011;30:243–249. doi: 10.1159/000330366. [DOI] [PubMed] [Google Scholar]
- Hart R, Sloboda DM, Doherty DA, Norman RJ, Atkinson HC, Newnham JP, Dickinson JE, Hickey M. Circulating maternal testosterone concentrations at 18 weeks of gestation predict circulating levels of antimüllerian hormone in adolescence: a prospective cohort study. Fertil Steril. 2010;94:1544–1547. doi: 10.1016/j.fertnstert.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Coall D, Chisholm J. Reproductive development and parental investment during pregnancy: moderating influence of mother's early environment. Am J Hum Biol. 2010;22:143–153. doi: 10.1002/ajhb.20965. [DOI] [PubMed] [Google Scholar]
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;5:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One. 2009;4:e6744. doi: 10.1371/journal.pone.0006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KL, Vickers MH, Beltrand J, Meaney MJ, Sloboda DM. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J Physiol. 2012;590:2167–2180. doi: 10.1113/jphysiol.2011.223305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, Lippman M. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci U S A. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae MT, Palassio S, Kyle CE, Brooks AN, Lea RG, Miller DW, Rhind SM. Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction. 2001;122:915–922. [PubMed] [Google Scholar]
- da Silva Faria T. de Bittencourt Brasil F, Sampaio FJB, da Fonte Ramos C. Effects of maternal undernutrition during lactation on estrogen and androgen receptor expressions in rat ovary at puberty. Nutrition. 2010;26:993–999. doi: 10.1016/j.nut.2009.09.027. [DOI] [PubMed] [Google Scholar]
- da Silva Faria T. de Bittencourt Brasil F, Sampaio FJB, da Fonte Ramos C. Maternal malnutrition during lactation affects folliculogenesis, gonadotropins, and leptin receptors in adult rats. Nutrition. 2010;26:1000–1007. doi: 10.1016/j.nut.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Faria Tda S, Brasil Fde B, Sampaio FJ, da Fonte Ramos C. Maternal malnutrition during lactation alters the folliculogenesis and gonadotropins and estrogen isoforms ovarian receptors in the offspring at puberty. J Endocrinol. 2008;198:625–634. doi: 10.1677/JOE-08-0121. [DOI] [PubMed] [Google Scholar]
- Lea RG, Andrade LP, Rae MT, Hannah LT, Kyle CE, Murray JF, Rhind SM, Miller DW. Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary. Reproduction. 2006;131:113–124. doi: 10.1530/rep.1.00844. [DOI] [PubMed] [Google Scholar]
- Ibanez L, de Zegher F. Puberty after prenatal growth restraint Horm Res 2006. 65 (Suppl 3): 112 115 [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology. 2006;147:1224–1236. doi: 10.1210/en.2005-1281. [DOI] [PubMed] [Google Scholar]
- Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13. doi: 10.1507/endocrj.ej12-0263. [DOI] [PubMed] [Google Scholar]
- Gannon AM, Stampfli MR, Foster WG. Cigarette smoke exposure elicits increased autophagy and dysregulation of mitochondrial dynamics in murine granulosa cells. Biol Reprod. 2013;88:63. doi: 10.1095/biolreprod.112.106617. [DOI] [PubMed] [Google Scholar]
- Pan H-A, Cheng Y-C, Li C-H, Wu M-H, Chang F-M. Ovarian stroma flow intensity decreases by age: a three-dimensional power doppler ultrasonographic study. Ultrasound Med Biol. 2002;28:425–430. doi: 10.1016/s0301-5629(02)00486-6. [DOI] [PubMed] [Google Scholar]
- Li Q, Geng X, Zheng W, Tang J, Xu B, Shi Q. Current understanding of ovarian aging. Sci China Life Sci. 2012;55:659–669. doi: 10.1007/s11427-012-4352-5. [DOI] [PubMed] [Google Scholar]
- Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- Greenaway J, Connor K, Pedersen HG, Coomber BL, LaMarre J, Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145:2896–2905. doi: 10.1210/en.2003-1620. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y, Sugino N. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:5. doi: 10.1186/1757-2215-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan D-X, Sugino N, Reiter RJ. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92:328–343. doi: 10.1016/j.fertnstert.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Eryilmaz O, Devran A, Sarikaya E, Aksakal F, Mollamahmutoğlu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. 2011;28:815–820. doi: 10.1007/s10815-011-9604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Chun W, Kong P-J, Han JA, Cho BP, Kwon OY, Lee HJ, Kim S-S. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res. 2006;40:79–85. doi: 10.1111/j.1600-079X.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- Kong PJ, Byun JS, Lim SY, Lee JJ, Hong SJ, Kwon KJ, Kim SS. Melatonin induces Akt phosphorylation through melatonin receptor- and PI3K-dependent pathways in primary astrocytes. Korean J Physiol Pharmacol. 2008;12:37–41. doi: 10.4196/kjpp.2008.12.2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf H-W. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann N Y Acad Sci. 2005;1040:508–511. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- Cheong Y, Sadek KH, Bruce KD, Macklon N, Cagampang FR. Diet-induced maternal obesity alters ovarian morphology and gene expression in the adult mouse offspring. Fertil Steril. 2014;102:899–907. doi: 10.1016/j.fertnstert.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Howie GJ, Sloboda DM, Vickers MH. Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br J Nutr. 2012;108:298–307. doi: 10.1017/S000711451100554X. [DOI] [PubMed] [Google Scholar]
- Connor MHV, Cupido KL. C, Sirimanne E, Sloboda DM. Maternal high fat diet during critical windows of development alters adrenal cortical and medullary enzyme expression in adult male rat offspring. J Dev Origins Health Dis. 2010;1:245–254. doi: 10.1017/S2040174410000346. [DOI] [PubMed] [Google Scholar]
- Howie GJ, Sloboda DM, Reynolds CM, Vickers MH. Timing of maternal exposure to a high fat diet and development of obesity and hyperinsulinemia in male rat offspring: same metabolic phenotype, different developmental pathways? J Nutr Metab. 2013;2013:517384. doi: 10.1155/2013/517384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NE, Greenaway J, Henkin J, Moorehead RA, Petrik J. The thrombospondin-1 mimetic ABT-510 increases the uptake and effectiveness of cisplatin and paclitaxel in a mouse model of epithelial ovarian cancer. Neoplasia. 2010;12:275–283. doi: 10.1593/neo.91880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche AF, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small for dates infants. Br J Obstet Gynecol. 1977;84:751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia. 2002;45:856–866. doi: 10.1007/s00125-002-0833-6. [DOI] [PubMed] [Google Scholar]
- Abramovich D, Parborell F, Tesone M. Effect of a vascular endothelial growth factor (VEGF) inhibitory treatment on the folliculogenesis and ovarian apoptosis in gonadotropin-treated prepubertal rats. Biol Reprod. 2006;75:434–441. doi: 10.1095/biolreprod.106.051052. [DOI] [PubMed] [Google Scholar]
- Babitha V, Panda RP, Yadav VP, Chouhan VS, Dangi SS, Khan FA, Singh G, Bag S, Sharma GT, Silvia WJ, Sarkar M. Amount of mRNA and localization of vascular endothelial growth factor and its receptors in the ovarian follicle during estrous cycle of water buffalo (Bubalus bubalis) Anim Reprod Sci. 2013;137:163–176. doi: 10.1016/j.anireprosci.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Chun SY, Eisenhauer KM, Kubo M, Hsueh AJ. Interleukin-1 beta suppresses apoptosis in rat ovarian follicles by increasing nitric oxide production. Endocrinology. 1995;136:3120–3127. doi: 10.1210/endo.136.7.7540548. [DOI] [PubMed] [Google Scholar]
- Soares JM, Jr, , Masana MI, Ersahin C, Dubocovich ML. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther. 2003;306:694–702. doi: 10.1124/jpet.103.049916. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Nelson RJ. Acute melatonin treatment alters dendritic morphology and circadian clock gene expression in the hippocampus of Siberian hamsters. Hippocampus. 2015;25:142–148. doi: 10.1002/hipo.22358. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Centanni AV, Butler AA. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology. 2010;151:1570–1580. doi: 10.1210/en.2009-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;139:1077–1090. doi: 10.1530/REP-09-0523. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Lee A-H, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EES, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang Z, Wang W, Jiang Z, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 2013;288:859–872. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Yang Y, Li X, Chen F, Cui C, Hu L, Li Q, Liu W, Jin Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol Reprod Dev. 2012;79:423–432. doi: 10.1002/mrd.22045. [DOI] [PubMed] [Google Scholar]
- Park H-J, Park S-J, Koo D-B, Lee S-R, Kong I-K, Ryoo J-W, Park Y-I, Chang K-T, Lee D-S. Progesterone production is affected by unfolded protein response (UPR) signaling during the luteal phase in mice. Life Sci. 2014;113:60–67. doi: 10.1016/j.lfs.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Wu LL-Y, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Mol Endocrinol. 2012;26:562–573. doi: 10.1210/me.2011-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rippstein PU, Tsang BK. Role and gonadotrophic regulation of X-linked inhibitor of apoptosis protein expression during rat ovarian follicular development in vitro. Biol Reprod. 2003;68:610–619. doi: 10.1095/biolreprod.102.007807. [DOI] [PubMed] [Google Scholar]
- Li J, Kim J-M, Liston P, Li M, Miyazaki T, Mackenzie AE, Korneluk RG, Tsang BK. Expression of inhibitor of apoptosis proteins (IAPs) in rat granulosa cells during ovarian follicular development and atresia. Endocrinology. 1998;139:1321–1328. doi: 10.1210/endo.139.3.5850. [DOI] [PubMed] [Google Scholar]
- Phillipps HR, Hurst PR. XIAP: a potential determinant of ovarian follicular fate. Reproduction. 2012;144:165–176. doi: 10.1530/REP-12-0142. [DOI] [PubMed] [Google Scholar]
- Hamanaka RB, Bobrovnikova-Marjon E, Ji X, Liebhaber SA, Diehl JA. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2008;28:910–920. doi: 10.1038/onc.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hämäläinen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Bonnet A, Cabau C, Bouchez O, Sarry J, Marsaud N, Foissac S, Woloszyn F, Mulsant P, Mandon-Pepin B. An overview of gene expression dynamics during early ovarian folliculogenesis: specificity of follicular compartments and bi-directional dialog. BMC Genomics. 2013;14:904. doi: 10.1186/1471-2164-14-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy. 2011;7:1115–1131. doi: 10.4161/auto.7.10.16608. [DOI] [PubMed] [Google Scholar]
- Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–1881. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Kawano Y. Kosay Hasan Z, Narahara H, Miyakawa I. The clinical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin Exp Med. 2003;3:27–31. doi: 10.1007/s102380300012. [DOI] [PubMed] [Google Scholar]
- Matsumi H, Yano T, Osuga Y, Kugu K, Tang X, Xu JP, Yano N, Kurashima Y, Ogura T, Tsutsumi O, Koji T, Esumi H, et al. Regulation of nitric oxide synthase to promote cytostasis in ovarian follicular development. Biol Reprod. 2000;63:141–146. doi: 10.1095/biolreprod63.1.141. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol Reprod Dev. 2007;74:1095–1104. doi: 10.1002/mrd.20633. [DOI] [PubMed] [Google Scholar]
- Dolatshad H, Davis FC, Johnson MH. Circadian clock genes in reproductive tissues and the developing conceptus. Reprod Fertil Dev. 2008;21:1–9. doi: 10.1071/rd08223. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum Reprod Update. 2005;11:91–101. doi: 10.1093/humupd/dmh054. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol Metab. 2010;21:628–636. doi: 10.1016/j.tem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26:7916–7920. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- Lee JH, Sancar A. Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proc Natl Acad Sci U S A. 2011;108:12036–12041. doi: 10.1073/pnas.1108125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khapre RV, Samsa WE, Kondratov RV. Circadian regulation of cell cycle: molecular connections between aging and the circadian clock. Ann Med. 2010;42:404–415. doi: 10.3109/07853890.2010.499134. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi S, Kuramoto Y, Nakagawa H, Aramaki H, Ohdo S, Soeda S, Shimeno HA. Molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003;63:7277–7283. [PubMed] [Google Scholar]
- Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotropins. Endocrinology. 2009;150:4338–4347. doi: 10.1210/en.2008-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G, Misawa I, Chen H, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori M-A. Contribution of FSH and triiodothyronine to the development of circadian clocks during granulosa cell maturation. Am J Physiol Endocrinol Metab. 2012;302:E645–E653. doi: 10.1152/ajpendo.00470.2011. [DOI] [PubMed] [Google Scholar]
- Markus RP, Ferreira ZS. The immune-pineal axis: the role of pineal and extra-pineal melatonin in modulating inflammation. Adv Neuroimmune Biol. 2011;1:95–104. [Google Scholar]
- Li Z, Nickkholgh A, Yi X, Bruns H, Gross M-L, Hoffmann K, Mohr E, Zorn M, Büchler MW, Schemmer P. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res. 2009;46:365–372. doi: 10.1111/j.1600-079X.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- Itoh MT, Ishizuka B, Kudo Y, Fusama S, Amemiya A, Sumi Y. Detection of melatonin and serotonin N-acetyltransferase and hydroxyindole-O-methyltransferase activities in rat ovary. Mol Cell Endocrinol. 1997;136:7–13. doi: 10.1016/s0303-7207(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Boden MJ, Varcoe TJ. Circadian rhythms and fertility. Mol Cell Endocrinol. 2012;349:56–61. doi: 10.1016/j.mce.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Kauppila A, Kivela A, Pakarinen A, Vakkuri O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J Clin Endocrinol Metab. 1987;65:823–828. doi: 10.1210/jcem-65-5-823. [DOI] [PubMed] [Google Scholar]
- Huber S, Didham R, Fieder M. Month of birth and offspring count of women: data from the Southern hemisphere. Hum Reprod. 2008;23:1187–1192. doi: 10.1093/humrep/den079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DR. Human birth seasonality and sunshine. Am J Hum Biol. 2010;22:316–324. doi: 10.1002/ajhb.20987. [DOI] [PubMed] [Google Scholar]
- Lummaa V. Early developmental conditions and reproductive success in humans: downstream effects of prenatal famine, birthweight, and timing of birth. Am J Hum Biol. 2003;15:370–379. doi: 10.1002/ajhb.10155. [DOI] [PubMed] [Google Scholar]
- Paes De Almeida Ferreira Braga D, Setti A, Figueira RdCS, Iaconelli A, Borges E. Seasonal variability in the fertilization rate of women undergoing assisted reproduction treatments. Gynecol Endocrinol. 2012;28:549–552. doi: 10.3109/09513590.2011.649812. [DOI] [PubMed] [Google Scholar]
- Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. Shift work and subfecundity: a European multicenter study. J Occup Environ Med. 1996;38:352–358. doi: 10.1097/00043764-199604000-00012. [DOI] [PubMed] [Google Scholar]
- Itoh MT, Ishizuka B, Kuribayashi Y, Amemiya A, Sumi Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol Hum Reprod. 1999;5:402–408. doi: 10.1093/molehr/5.5.402. [DOI] [PubMed] [Google Scholar]