Abstract
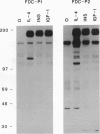
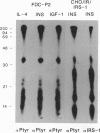
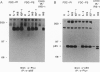
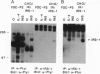
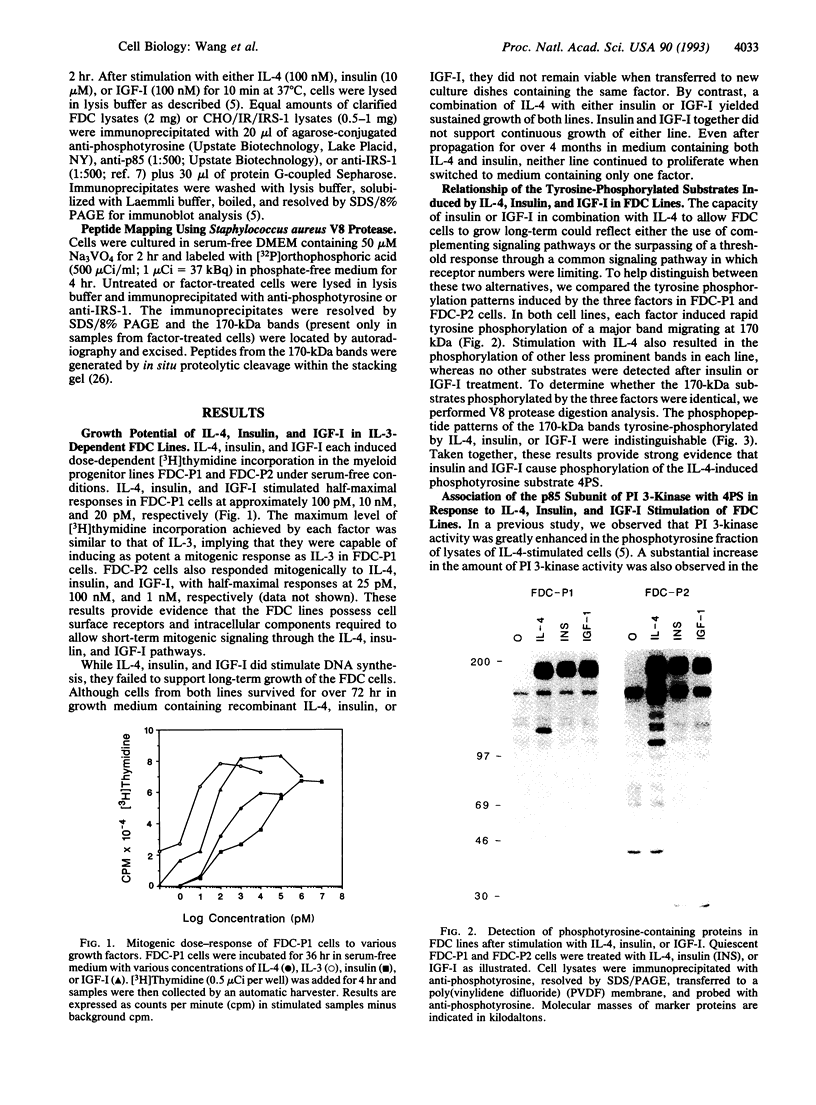
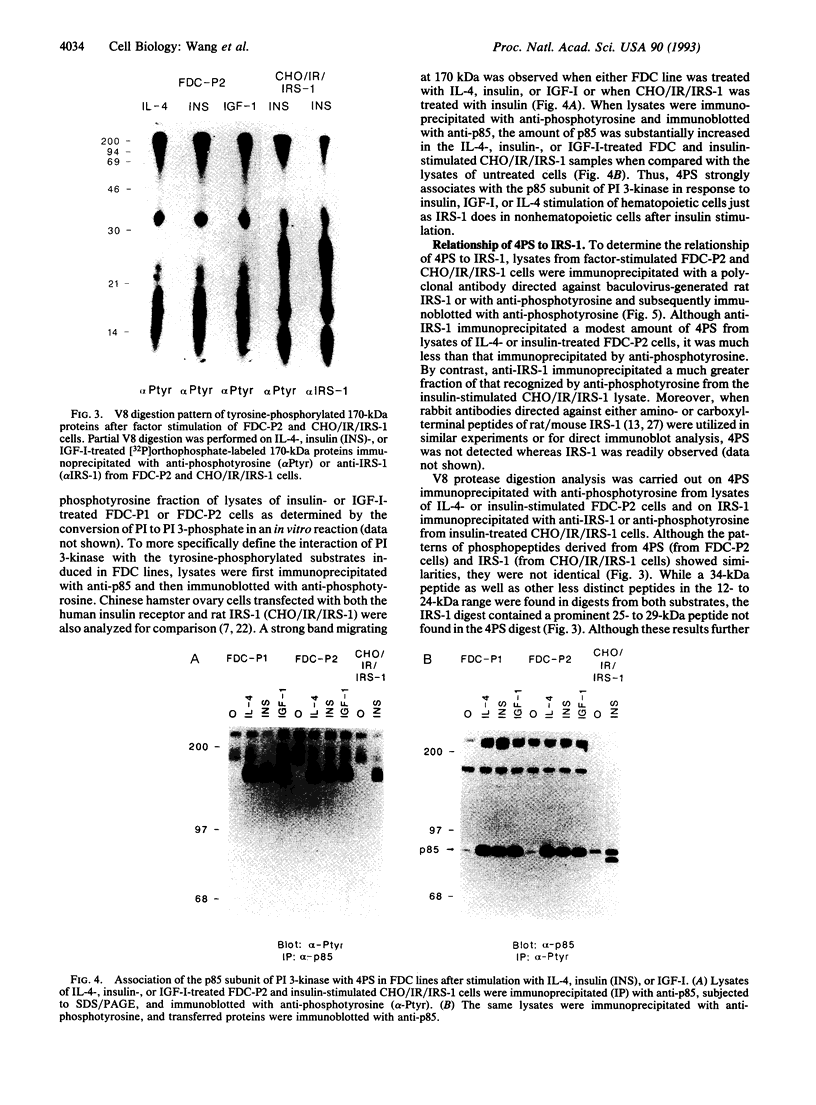
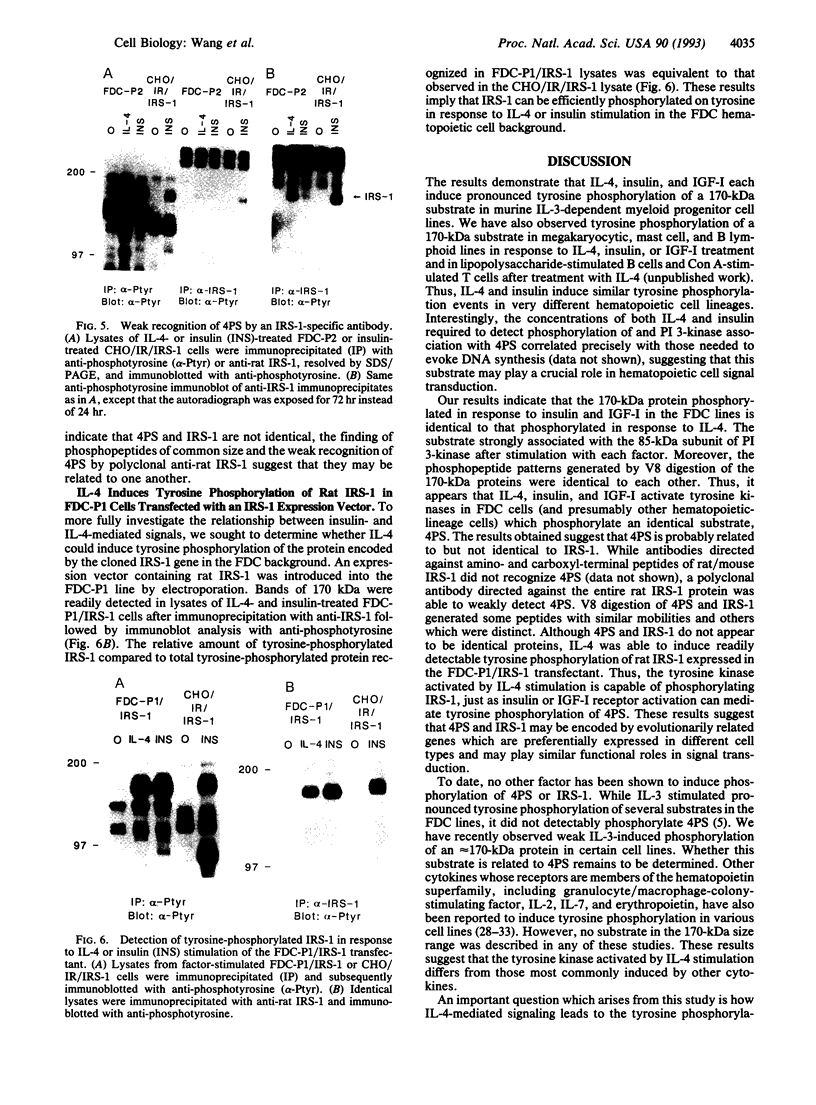
Interleukin 4 (IL-4), insulin, and insulin-like growth factor I (IGF-I) efficiently induced DNA synthesis in the IL-3-dependent murine myeloid cell lines FDC-P1 and FDC-P2. Although these factors could not individually sustain long-term growth of these lines, a combination of IL-4 with either insulin or IGF-I did support continuous growth. The principal tyrosine-phosphorylated substrate observed in FDC cells stimulated with IL-4, previously designated 4PS, was of the same size (170 kDa) as the major substrate phosphorylated in response to insulin or IGF-I. These substrates had phosphopeptides of the same size when analyzed by digestion with Staphylococcus aureus V8 protease, and each tightly associated with the 85-kDa component of phosphatidylinositol 3-kinase after factor stimulation. IRS-1, the principal substrate phosphorylated in response to insulin or IGF-I stimulation in nonhematopoietic cells, is similar in size to 4PS. However, anti-IRS-1 antibodies failed to efficiently precipitate 4PS, and some phosphopeptides generated by V8 protease digestion of IRS-1 were distinct in size from the phosphopeptides of 4PS. Nevertheless, IL-4, insulin, and IGF-I were capable of stimulating tyrosine phosphorylation of IRS-1 in FDC cells that expressed this substrate as a result of transfection. These findings indicate that (i) IL-4, insulin, and IGF-I use signal transduction pathways in FDC lines that have at least one major feature in common, the rapid tyrosine phosphorylation of 4PS, and (ii) insulin and IGF-I stimulation of hematopoietic cell lines leads to the phosphorylation of a substrate that may be related to but is not identical to IRS-1.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine J. A., Schlager J. W., Abraham R. T. Differential effects of interleukin-2 and interleukin-4 on protein tyrosine phosphorylation in factor-dependent murine T cells. Biochim Biophys Acta. 1990 May 2;1052(2):313–322. doi: 10.1016/0167-4889(90)90227-5. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., Cahill D. A., Ullrich A., White M. F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J Biol Chem. 1990 Sep 25;265(27):16450–16454. [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Schroeder G. G., Cahill D. A., Ullrich A., Siddle K., White M. F. Cytoplasmic juxtamembrane region of the insulin receptor: a critical role in ATP binding, endogenous substrate phosphorylation, and insulin-stimulated bioeffects in CHO cells. Biochemistry. 1991 Jul 2;30(26):6366–6372. doi: 10.1021/bi00240a003. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Schroeder G. G., Kahn C. R., Myers M. G., Jr, Wilden P. A., Cahill D. A., White M. F. Insulin stimulation of phosphatidylinositol 3-kinase activity maps to insulin receptor regions required for endogenous substrate phosphorylation. J Biol Chem. 1992 Jan 15;267(2):1367–1374. [PubMed] [Google Scholar]
- Blackshear P. J., Haupt D. M., App H., Rapp U. R. Insulin activates the Raf-1 protein kinase. J Biol Chem. 1990 Jul 25;265(21):12131–12134. [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Snijders A. J., Maassen J. A., van der Eb A. J., Bos J. L. Possible involvement of normal p21 H-ras in the insulin/insulinlike growth factor 1 signal transduction pathway. Mol Cell Biol. 1989 Oct;9(10):4312–4322. doi: 10.1128/mcb.9.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V., Welham M. J., Abraham S., Dryden P., Schrader J. W. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1587–1591. doi: 10.1073/pnas.89.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Linnekin D., Farrar W. L. Comparative analysis of IL-2 and IL-3 induced tyrosine phosphorylation. Lymphokine Res. 1989 Fall;8(3):215–224. [PubMed] [Google Scholar]
- Galizzi J. P., Zuber C. E., Harada N., Gorman D. M., Djossou O., Kastelein R., Banchereau J., Howard M., Miyajima A. Molecular cloning of a cDNA encoding the human interleukin 4 receptor. Int Immunol. 1990;2(7):669–675. doi: 10.1093/intimm/2.7.669. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. SH2 domains: elements that control protein interactions during signal transduction. Trends Biochem Sci. 1991 Dec;16(12):450–452. doi: 10.1016/0968-0004(91)90175-u. [DOI] [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Tamemoto H., Nagao M., Kadowaki T., Takaku F., Kasuga M. Insulin and platelet-derived growth factor stimulate phosphorylation of the c-raf product at serine and threonine residues in intact cells. J Biol Chem. 1991 Apr 25;266(12):7933–7939. [PubMed] [Google Scholar]
- Izumi T., White M. F., Kadowaki T., Takaku F., Akanuma Y., Kasuga M. Insulin-like growth factor I rapidly stimulates tyrosine phosphorylation of a Mr 185,000 protein in intact cells. J Biol Chem. 1987 Jan 25;262(3):1282–1287. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Keller S. R., Kitagawa K., Aebersold R., Lienhard G. E., Garner C. W. Isolation and characterization of the 160,000-Da phosphotyrosyl protein, a putative participant in insulin signaling. J Biol Chem. 1991 Jul 15;266(20):12817–12820. [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kovacina K. S., Yonezawa K., Brautigan D. L., Tonks N. K., Rapp U. R., Roth R. A. Insulin activates the kinase activity of the Raf-1 proto-oncogene by increasing its serine phosphorylation. J Biol Chem. 1990 Jul 25;265(21):12115–12118. [PubMed] [Google Scholar]
- Lamphere L., Lienhard G. E. Components of signaling pathways for insulin and insulin-like growth factor-I in muscle myoblasts and myotubes. Endocrinology. 1992 Nov;131(5):2196–2202. doi: 10.1210/endo.131.5.1385098. [DOI] [PubMed] [Google Scholar]
- Lavan B. E., Kuhné M. R., Garner C. W., Anderson D., Reedijk M., Pawson T., Lienhard G. E. The association of insulin-elicited phosphotyrosine proteins with src homology 2 domains. J Biol Chem. 1992 Jun 5;267(16):11631–11636. [PubMed] [Google Scholar]
- Le Gros G. S., Gillis S., Watson J. D. Induction of IL 2 responsiveness in a murine IL 3-dependent cell line. J Immunol. 1985 Dec;135(6):4009–4014. [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991 May 1;77(9):1859–1870. [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Quelle F. W., Wojchowski D. M. Proliferative action of erythropoietin is associated with rapid protein tyrosine phosphorylation in responsive B6SUt.EP cells. J Biol Chem. 1991 Jan 5;266(1):609–614. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., Chatterjee S., Chaudhuri M., White M. F. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Turner B., Rapp U., App H., Greene M., Dobashi K., Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Dibirdik I., Smith R., Tuel-Ahlgren L., Chandan-Langlie M., Schieven G. L., Waddick K. G., Hanson M., Ledbetter J. A. Interleukin 7 receptor ligation stimulates tyrosine phosphorylation, inositol phospholipid turnover, and clonal proliferation of human B-cell precursors. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3589–3593. doi: 10.1073/pnas.88.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. M., Keegan A. D., Paul W. E., Heidaran M. A., Gutkind J. S., Pierce J. H. IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO J. 1992 Dec;11(13):4899–4908. doi: 10.1002/j.1460-2075.1992.tb05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham M. J., Duronio V., Sanghera J. S., Pelech S. L., Schrader J. W. Multiple hemopoietic growth factors stimulate activation of mitogen-activated protein kinase family members. J Immunol. 1992 Sep 1;149(5):1683–1693. [PubMed] [Google Scholar]
- White M. F., Livingston J. N., Backer J. M., Lauris V., Dull T. J., Ullrich A., Kahn C. R. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell. 1988 Aug 26;54(5):641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Stegmann E. W., Dull T. J., Ullrich A., Kahn C. R. Characterization of an endogenous substrate of the insulin receptor in cultured cells. J Biol Chem. 1987 Jul 15;262(20):9769–9777. [PubMed] [Google Scholar]