Abstract
Patient: Female, 40
Final Diagnosis: Intravenous leiomyomatosis
Symptoms: Chest pain • syncope
Medication: —
Clinical Procedure: Thoracotomy
Specialty: Radiology • Cardiology
Objective:
Rare disease
Background:
Intravenous leiomyomatosis (IVL) is a rare tumor, which is usually of uterine origin, characterized by intravascular nodular masses of histologically benign smooth muscle that may extend variable distances, including into the inferior vena cava, right atrium and pulmonary arteries. Tumors may arise from uterine leiomyoma, walls of the uterine vessel, or myometrium. It usually occurs at between 20–70 years of age with a median age of 45 years. The most commonly affected women are pre-menopausal and multiparous. Intra-cardiac extension may represent a diagnostic challenge as it is usually misdiagnosed as a right atrial myxoma and may cause multiple symptoms, such as shortness of breath, tachycardia, chest pain, syncope, and even death.
Case Report:
We present the case of a 40-year-old female patient with past medical history of arterial hypertension, who was referred to a cardiovascular center due to an intra-cardiac mass found on 2D echocardiogram. The patient was given the rare diagnosis of intravenous leiomyomatosis of the uterus with extension into the gonadal veins, inferior vena cava, right atrium, right ventricle, and main pulmonary arteries. Imaging workup including trans-esophageal echocardiogram, cardiac catheterization, contrast-enhanced abdomen and pelvic CT scans, and cardiac MRI was performed for evaluation.
Conclusions:
Intravenous leiomyomatosis is a rare diagnosis that merits consideration in a young pre-menopausal female patient with cardiac symptoms associated with a right atrial mass. Radiologists play a vital role in the diagnosis and follow-up of patients with the diagnosis of intravenous leiomyomatosis. Differential diagnosis includes vascular thrombus as well as primary and metastatic tumors. Early detection is imperative for appropriate treatment and surgical planning.
MeSH Keywords: Angiomyoma; Leiomyoma; Leiomyomatosis; Magnetic Resonance Imaging, Cine
Background
Intravenous leiomyomatosis is a rare tumor, which is usually of uterine origin, characterized by intravascular nodular masses of histologically benign smooth muscle that may extend variable distances, including into the inferior vena cava right atrium and pulmonary arteries. The tumor may arise from uterine leiomyoma, walls of uterine vessels, or myometrium. Age of presentation is usually between 20–70 years with a median age of 45 years. The most commonly affected women are pre-menopausal and multiparous. Intra-cardiac extension may represent a diagnostic challenge as it is usually misdiagnosed as a right atrial myxoma and may cause multiple symptoms, such as shortness of breath, tachycardia, chest pain, syncope, and even death.
Case Report
A 40-year-old female patient (G3P3A0) with past medical history of arterial hypertension was first seen in January 2012 for evaluation of at least 2 episodes of syncope and loss of consciousness while dancing and during coitus. The patient referred a 2-year history of multiple episodes of progressive shortness of breath, palpitations, bilateral leg edema, and dyspnea on exertion. Her symptoms were initially attributed to arterial hypertension, for which her primary physician initially prescribed anti-hypertensive medications. The symptoms persisted and there was concomitant development of irregular menstrual cycles with menorrhagia. The patient was referred to a cardiologist for evaluation with 2D echocardiogram, which revealed an intra-cardiac mass.
At physical examination, the patient was awake and oriented in time, person, and place without evidence of distress at that moment. Physical examination showed bilateral mild (+1) pitting edema of the lower legs. Upon auscultation, normal S1 and S2 heart sounds were present with an additional third heart sound present (tumor plop) which was described as a systolic murmur 3/6 on intensity at the left parasternal border. No other abnormalities were found upon physical examination.
Complete blood cell count (CBC) and comprehensive metabolic panel (CMP) were normal. Electrocardiogram showed normal sinus rhythm with bi-atrial enlargement and prolonged QT interval. The patient was admitted to our cardiology service for evaluation and possible surgical intervention.
Imaging workups were subsequently done, including transesophageal echocardiogram (TEE) and cardiac catheterization, as well as contrast-enhanced abdomen and pelvic CT scans. TEE showed a large right heart tumor extending into the inferior vena cava protruding through the tricuspid valve and a moderate pericardial effusion (Figures 1, 2 and Video 1). Cardiac catheterization showed a large tubular mass extending from the IVC to the right heart chambers, minimal cardiomegaly, inverted systole, and obstructed right ureter (not shown).
Figure 1.
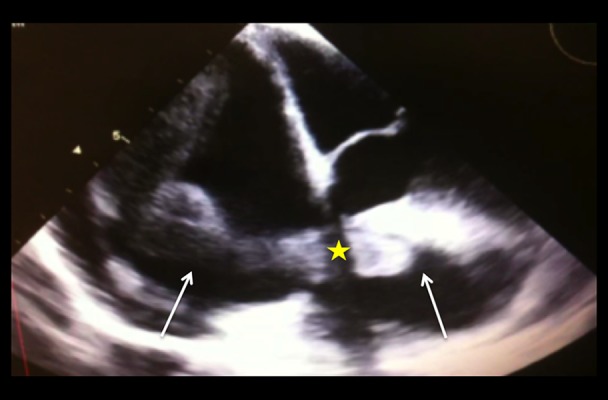
Transesophageal echocardiogram at the level of the right heart chambers. A hyperechoic, tubular, worm-like mass (white arrows) is visualized extending from the right atrium to the right ventricle through the tricuspid valve (star).
Figure 2.
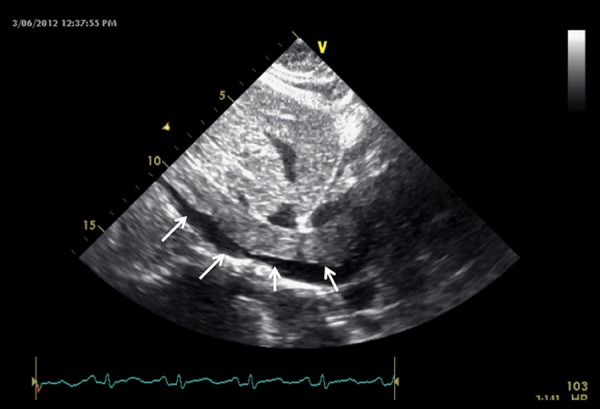
Transesophageal echocardiogram at the level of the IVC showing an elongated, tubular, worm-like echogenic mass within the lumen of the inferior vena cava (arrows).
Video 1.
Trans-esophageal echocardiogram shows an echogenic tubular mass extending from the inferior vena cava to the right atrium and through the tricuspid valve into the right ventricle.
Abdomen and pelvis contrast-enhanced CT demonstrated a large myomatous uterus with a complex pelvic mass extending through the gonadal veins to the IVC and into the right heart chambers (Figures 3–6). No pulmonary nodules or masses were identified.
Figure 3.
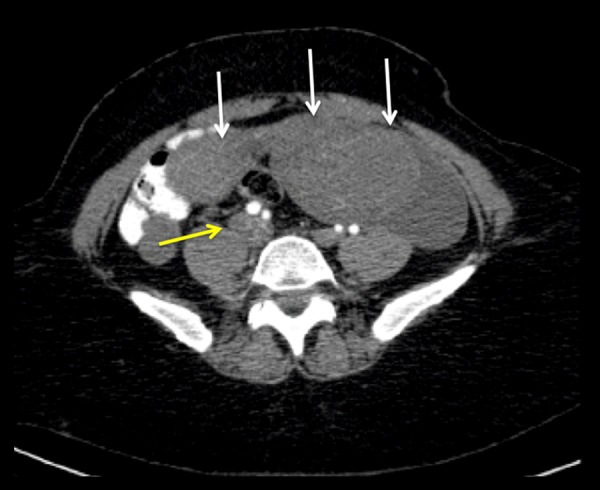
Axial image of a contrast-enhanced abdomen and pelvis CT scan at the level of the lower abdomen. The enlarged uterus is visualized with multiple heterogeneous enhancing masses (likely leiomyomas) (white arrows). The right common iliac vein is enlarged, with intraluminal material of intermediate attenuation related to tumoral involvement (yellow arrow).
Figure 4.
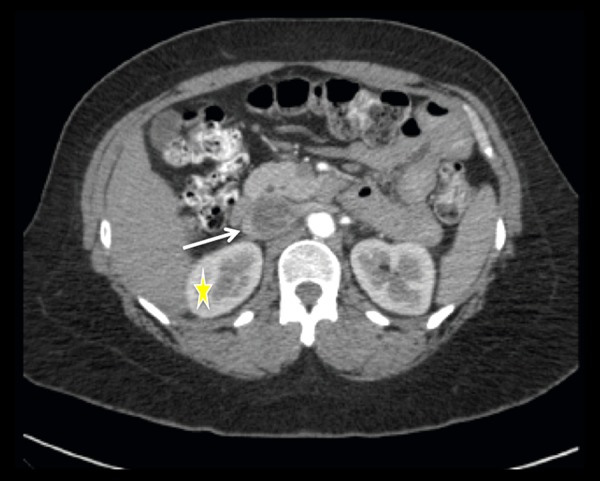
Axial image of a contrast-enhanced Abdomen and Pelvis CT scan at the level of the IVC and left renal vein. Hypoattenuating intraluminal material visualized within the IVC consistent with intravascular tumor (white arrow). Additional images (not shown) revealed symmetric nephrograms without evidence of masses or abnormalities (yellow star), making the diagnosis of invasive RCC unlikely.
Figure 5.
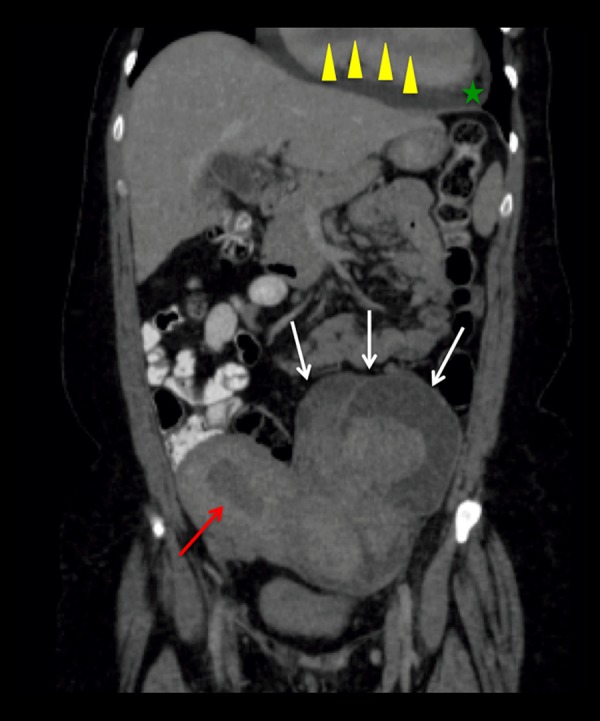
Coronal reformat image of a contrast-enhanced Abdomen and Pelvis CT Scan. Enlarged myomatous uterus with multiple large masses (white arrows) showing heterogeneous enhancement in this contrast enhanced image, probably secondary to cystic degeneration of myomas. There is small amount of fluid within the endometrial cavity (red arrow). A moderately sized pericardial effusion is present (green star). Partially visualized tubular shaped filling defect within the right heart chambers, consistent with tumoral extension (yellow arrowheads).
Figure 6.
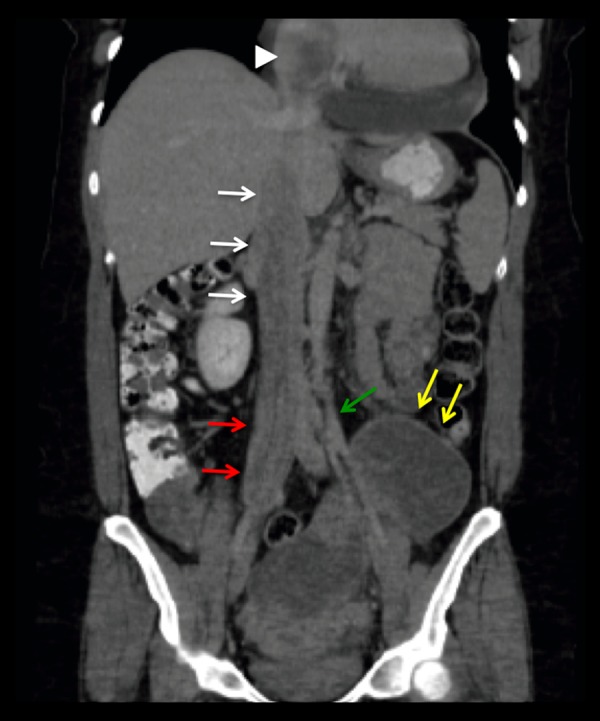
Coronal reformat image of a contrast-enhanced Abdomen and Pelvis CT scan. Again visualized, enlarged myomatous uterus with multiple large heterogeneous enhancing masses (yellow arrows). There is tumoral extension into the left gonadal vein (green arrow), right common iliac vein (red arrows), IVC (white arrows), and to the partially visualized right atrium (white arrowhead).
A preliminary diagnosis of intravenous leiomyomatosis (IVL) with a differential diagnosis of invasive hepatocellular carcinoma, as well as leiomyosarcoma, was given. Tissue biopsy and cardiac MRI were recommended for further evaluation.
Cardiac MRI was performed and showed an elongated tubular mass extending from the IVC to the right heart chambers and pulmonary arteries with associated mild dilation of the right heart chambers and no systolic dysfunction (Figures 7–9 and Videos 2, 3). There was no invasion of contiguous structures or enlarged lymph nodes; therefore, existence of a benign lesion was favored. At this point the main differential diagnosis given the patient’s radiologic findings was that of IVL.
Figure 7.
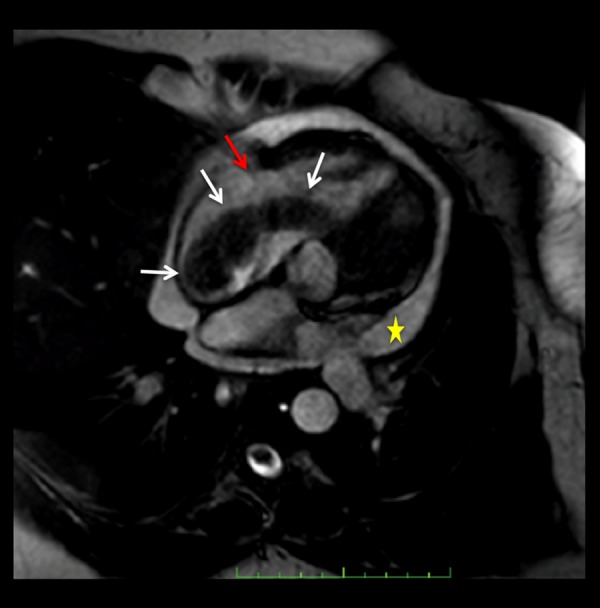
Static frame of a cine steady state free-precession (SSFP) 4 chamber cardiac MR image. Low signal intensity, tubular shaped, worm-like mass (white arrow) is visualized extending from the right atrium through the tricuspid valve (red arrow) to the right ventricle. Pericardial effusion (yellow star). The mass does not invade the myocardium.
Figure 8.
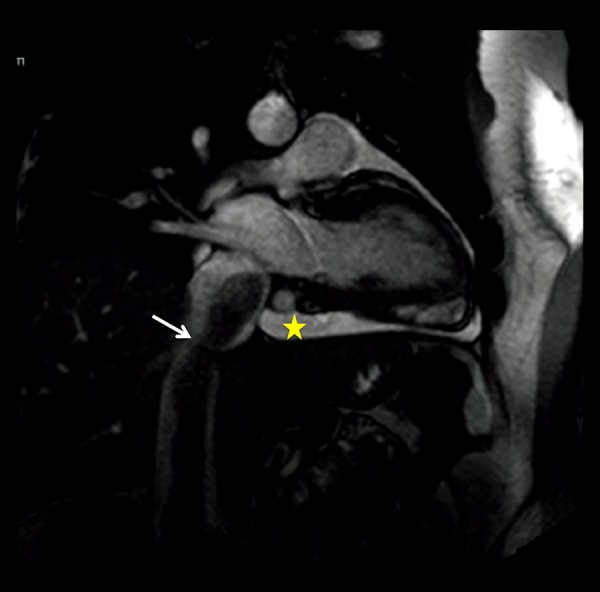
Static frame of a cine SSFP oblique vertical long axis cardiac MR image. Low-intensity tubular shaped, worm-like mass (white arrow) within the IVC extending into the right atrium. Pericardial effusion (yellow star). No attachment or invasion of the walls of the IVC was demonstrated.
Figure 9.
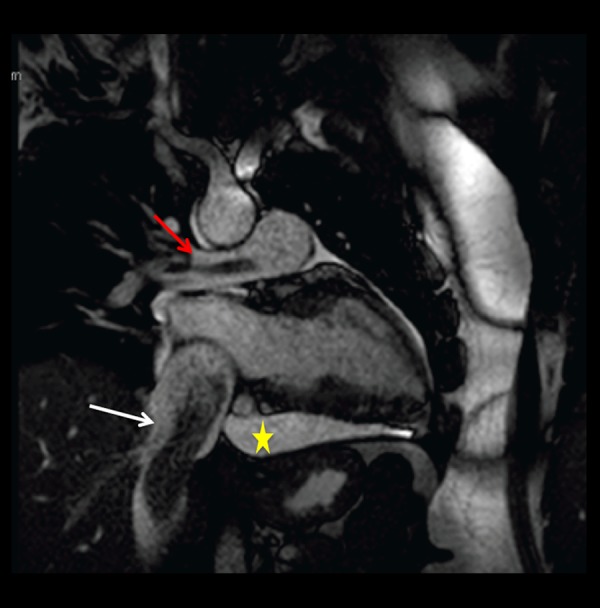
Static frame of a cine SSFP oblique vertical long axis cardiac MR image. Low signal intensity, tubular shaped, worm-like mass (white arrow) is seen within the IVC. A smaller tubular shaped mass of similar characteristics is visualized within the right pulmonary artery (red arrow), related to extension of tumor to the pulmonary vasculature. Pericardial effusion (yellow star).
Video 2.
Cine SSFP 4-chamber cardiac MR shows a low signal intensity tubular mass extending from the right atrium through the tricuspid valve into the right ventricle and to the right ventricular outflow tract. A pericardial effusion is also seen.
Video 3.
Cine SSFP oblique vertical long axis cardiac MR shows a low signal intensity tubular mass extending from the inferior vena cava into the right heart chambers. Extension into the pulmonary arteries is also seen. Pericardial effusion.
CT-guided biopsy by our IR department was scheduled and performed for tissue diagnosis, which upon histopathologic analysis yielded smooth muscle cell proliferation, consistent with intravascular leiomyomatosis. The patient was scheduled for a thoracotomy procedure by cardiothoracic surgery for mass excision. The surgery report revealed successful removal of an intra-cardiac solid mass involving the pulmonary arteries, right atrium, right ventricle, and IVC. The mass was excised in toto to below the hepatic veins. The procedure was performed without major complications.
The gross pathology report revealed an elongated, slightly lobulated, tan, rubbery mass measuring 31 cm in length by 2.5 cm in diameter at one end and 0.7 cm in diameter at the other end, with segments of hyperemia and cystic degeneration (Figure 10A–10C).
Figure 10.
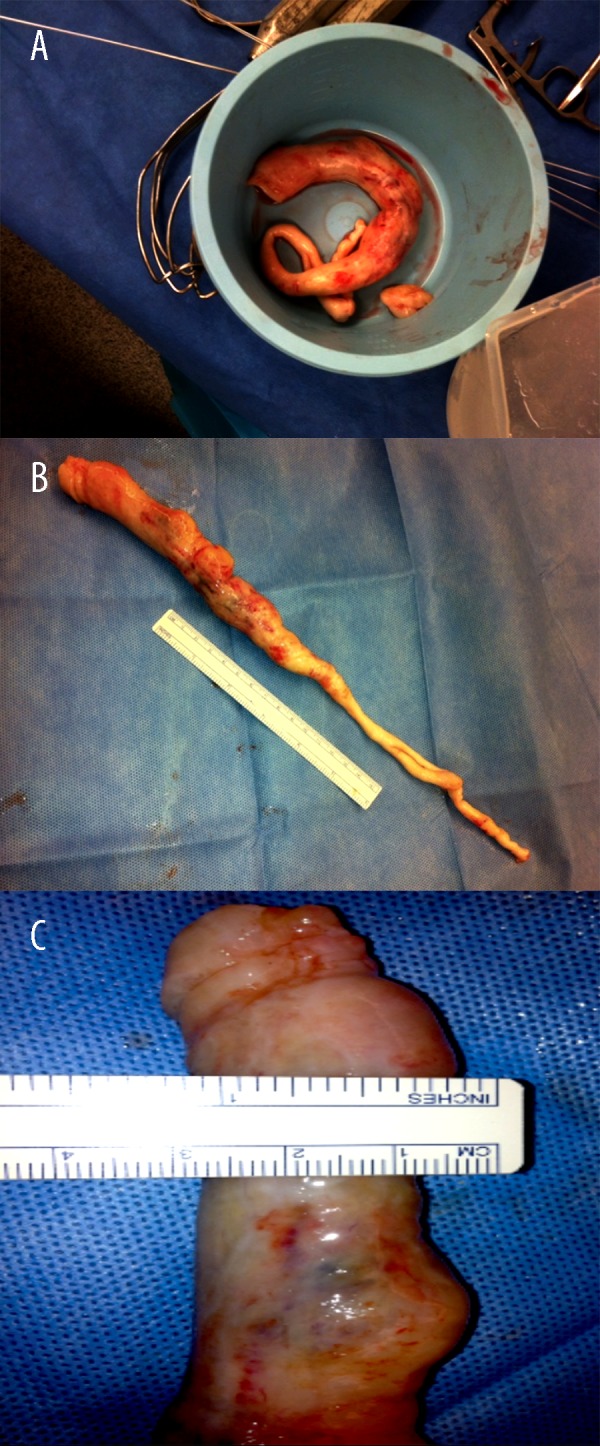
(A–C) Inferior vena cava, right atrium, right ventricle, and pulmonary artery mass excision. Gross pathology: elongated, tan, rubbery mass measuring 31 cm in length by 2.5 cm in diameter at one end and 0.7 cm in diameter at the other end. The external surface is tan and slightly lobulated. The cut surface is tan with a rubbery consistency. Also submitted is a fragment of a tan rubbery tissue with similar characteristics, as previously described, that measures 2.5×1.5×1.5 cm.
At histology, cigar-shaped elongated nuclei and muscle fibers without mitosis or atypia were identified, suggestive of smooth muscle cells, correlating with immunohistochemistry (Figure 11). Tissue fragments consistent with smooth muscle favored intravascular leiomyomatosis. The final diagnosis was intravenous leiomyomatosis.
Figure 11.
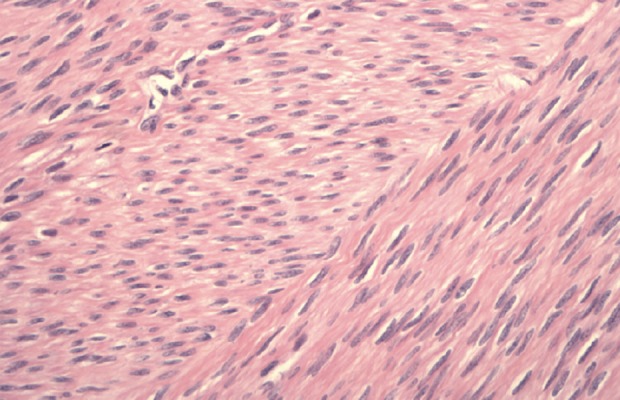
Inferior vena cava, right atrium, right ventricle, and pulmonary artery mass excision. Histology: Cigar-shaped elongated nuclei and muscle fibers without mitosis or atypia, suggestive of smooth muscle cell, correlating with immunohistochemistry.
The patient was discharged home after careful observations, and a follow-up appointment with OB/GYN service was schedule for evaluation for possible total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic mass resection. A follow-up referral for abdomen and pelvic CT scan study was also provided.
Discussion
Intravenous leiomyomatosis is a rare tumor, which usually is of uterine origin, characterized by intravascular nodular masses of histologically benign smooth muscle that may extend variable distances. This condition was first described by Birch-Hirschfeld in 1896 and by Dursk, who described the first case of IVL with intra-cardiac extension in 1907 [1,2]. To date, cases of IVL with intra-cardiac extension account for about 10% and less than 300 cases have been reported in the English literature [2,3]. In rare cases, the tumor may extend into the pulmonary arteries, as in the present case.
Two main theories have been proposed for its etiology. The first theory indicates that the tumor originates directly from the vein walls, whereas the second suggests that a uterine leiomyoma, as the primary tumor, causes intravascular projections into an adjacent venous channel [4].
IVL usually occurs in premenopausal women at age 20–70 years with a median age of 45 years, who have had multiple pregnancies [4,5]. Symptoms include shortness of breath, tachycardia, syncope, chest pain, pelvic pain, irregular menses, and abdominal mass, and may even result in sudden death [6,7]. However, patients may also be asymptomatic. Laboratory studies may show anemia, if abnormal uterine bleeding is present. Abnormalities in ECG may be related to enlargement of the cardiac chambers, systolic dysfunction, involvement of the cardiac valves, tumor emboli, and right heart strain, among others.
Histologic findings include endothelium-covered proliferations of benign smooth muscle within the lumen of vessels, with neoplastic cells showing minimal nuclear atypia and low mitotic index. Gross pathology shows a lobulated, worm-like, tubular mass or multi-nodular (grape-like) rubbery masses of a tan, grey, or reddish-blue color [4,8,9].
At imaging, IVL with cardiac extension may demonstrate a hyperechoic, elongated, mobile mass extending from the inferior vena cava to the right atrium, with or without evidence of protrusion into the right ventricle on echocardiography. The lesion usually enhances heterogeneously on post-contrast scans of CT and is of relatively lower density compared to the enhanced blood in the inferior vena cava and right atrium, with common iliac vein and the ipsilateral internal iliac and ovarian veins involved in some cases. The uterus may be enlarged and myomatous with heterogeneous enhancing mass or masses, which may be of different sizes, may calcify to different degrees, and may show cystic degeneration/necrosis. Enhancing tumors may extend to iliac, uterine, or gonadal veins, as well as to the IVC, heart, and pulmonary arteries (tumoral emboli are rare). Cardiac MRI or echocardiogram may show a tubular or worm-like structure extending from the IVC to the heart chambers and pulmonary arteries [10].
Differential diagnosis includes metastasis with IVC invasion (e.g., renal cell carcinoma, adrenal cortical carcinoma, hepatocellular carcinoma, and lymphoma), atrial myxoma, right-sided heart thrombus or embolus, and leiomyosarcoma. Atrial myxoma is usually the initial diagnosis in these patients given that it is the most common primary heart tumor. However, approximately 60–75% of atrial myxomas are located in the left atrium attached to atrial septum, usually at the fossa ovalis, and there is no IVC extension unless it arises from the IVC proper (in extremely rare cases) [11,12]. Another diagnosis to consider is benign metastasizing leiomyoma (BML). This disease is characterized by uterine leiomyoma in young adulthood, with pulmonary metastasis occurring in the pre-menopausal period [13]. Since there were no pulmonary nodules or masses, there was no evidence of BML in our patient.
Treatment of IVL is almost always surgical [7,10,14], which includes excision of the extra-uterine tumor and myomectomy or total hysterectomy, as necessary. Sternotomy with cardio-pulmonary bypass, as well as laparotomy in a single or 2-stage operation, may be performed for management of a mass that extends to the cardiovascular system. Avoidance of exogenous estrogens is recommended, since the tumor is often estrogen-dependent and estrogen may promote its growth.
The long-term prognosis is very good after complete surgical resection. Some patients may have persistent or continued growth of an incompletely excised intravenous tumor, with a recurrence rate as high as 30% [15,16]. In cases of incomplete resection or tumor recurrence, use of antiestrogens has been considered but their efficacy remains controversial [15,17]. Death from tumor is unusual and may be related to surgical complications.
Radiologists play a vital role in the diagnosis and follow-up of patients with the diagnosis of intravenous leiomyomatosis. Non-invasive, multimodality imaging evaluation facilitates and provides guidance for appropriate treatment and surgical planning.
Conclusions
Intravenous leiomyomatosis is a rare diagnosis that merits consideration in a young pre-menopausal female patient with cardiac symptoms associated with a right atrial mass. Radiologists play a vital role in the diagnosis and follow-up of patients with the diagnosis of intravenous leiomyomatosis. Differential diagnosis includes vascular thrombus as well as primary and metastatic tumors. Early detection and correct diagnosis is imperative for appropriate treatment and surgical planning.
References:
- 1.Birch-Hirschfeld FV. Lehrbuch der Pathologischen Anatomie. 5th ed. Leipzig: FCW Vogel; 1896. p. 226. [in German] [Google Scholar]
- 2.Xu ZF, Yong F, Chen YY, Pan AZ. uterine intravenous leiomyomatosis with cardiac extension: Imaging characteristics and literature review. World J Clin Oncol. 2013;4(1):25–28. doi: 10.5306/wjco.v4.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baca-López F, Martínez-Enriquez A, Castrejón-Aivar F, et al. Echocardiographic study of an intravenous leiomyoma: Case report and review of the literature. Echocardiography. 2003;20:723–25. doi: 10.1111/j.0742-2822.2003.02152.x. [DOI] [PubMed] [Google Scholar]
- 4.Norris HJ, Panmley T. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis: A clinical and pathological study of 14 cases. Cancer. 1975;36:2164–78. doi: 10.1002/cncr.2820360935. [DOI] [PubMed] [Google Scholar]
- 5.Kaszar-Seibert DJ, Gauvin GP, Rogoff PA, et al. Intracardiac extension of intravenous leiomyomatosis. Radiology. 1988;168:409–10. doi: 10.1148/radiology.168.2.3393658. [DOI] [PubMed] [Google Scholar]
- 6.Roman DA, Mirchandani H. Intravenous leiomyoma with intra-cardiac extension causing sudden death. Arch Pathology Lab Med. 1987;111:1176–78. [PubMed] [Google Scholar]
- 7.Sogabe M, Kawahito K, Aizawa K, et al. Uterine intravenous leiomyomatosis with right ventricular extension. Ann Thoracic Cardiovascular Surg. 2014;20(Suppl.):933–36. doi: 10.5761/atcs.cr.13-00309. [DOI] [PubMed] [Google Scholar]
- 8.Oliva E, Young RH, Clement PB, et al. Cellular benign mesenchymal tumors of the uterus. a comparative morphologic and immunohistochemical analysis of 33 highly cellular leiomyomas and six endometrial stromal nodules, two frequently confused tumors. Am J Surg Pathol. 1995;19:757–68. doi: 10.1097/00000478-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Suginami H, Kaura R, Ochi H, Matsuura S. Intravenous leiomyomatosis with cardiac extension: successful surgical management and histopathologic study. Obstetric Gynecoly. 1990;76:527–29. [PubMed] [Google Scholar]
- 10.Kang LQ, Zhang B, Liu BG, Liu FH. Diagnosis of intravenous leiomyomatosis extending to heart with emphasis on magnetic resonance imaging. Chin Med J (Engl) 2012;125(1):33–37. [PubMed] [Google Scholar]
- 11.Grebenc ML, Rosado-de-Christenson ML, Green CE, et al. Cardiac myxoma: imaging features in 83 patients. Radiographics. 2002;22:673–89. doi: 10.1148/radiographics.22.3.g02ma02673. [DOI] [PubMed] [Google Scholar]
- 12.Buckley O, Madan R, Kwong R, et al. Cardiac masses, Part 2: Key imaging features for diagnosis and surgical planning. Am J Roentology. 2011;197(5):W842–51. doi: 10.2214/AJR.11.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck MM, Biswas B, D’Souza A, Kumar R. Benign metastasizing leiomyoma after hysterectomy and bilateral salpingo-oophorectomy. Hong Kong Med J. 2012;18(2):153–55. [PubMed] [Google Scholar]
- 14.Clay TD, Dimitriou J, McNally OM, et al. Intravenous leiomyomatosis with intra-cardiac extension – a review of diagnosis and management with an illustrative case. Surg Oncol. 2013;22:e44–52. doi: 10.1016/j.suronc.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Moniaga NC, Randall LM. Uterine leiomyomatosis with intra-caval and intra-cardiac extension. Gynecologic Oncology Case Reports. 2012;2(4):130–32. doi: 10.1016/j.gynor.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed M, Zangos S, Bechstein WO. Intravenous leiomyomatosis. Eur Radiol. 2004;14:1316–17. doi: 10.1007/s00330-003-2186-z. [DOI] [PubMed] [Google Scholar]
- 17.Nam MS, Jeon MJ, Kim YT. Pelvic leiomyomatosis with intra-caval and intra-cardiac extension: a case report and review of the literature. Gynecol Oncol. 2003;89:175–80. doi: 10.1016/s0090-8258(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 18.Li B, Chen X, Chu YD, et al. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg. 2013;17:132–38. doi: 10.1093/icvts/ivt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorjani N, Kuo J, Ashley S, Hughes G. Intravenous uterine leiomyosarcomatosis with intra-cardiac extension. J Card Surg. 2005;20(4):382–85. doi: 10.1111/j.1540-8191.2005.200476.x. [DOI] [PubMed] [Google Scholar]
- 20.Koh DM, Burn PR, King DM. Benign metastasizing leiomyoma with intracaval leiomyomatosis. Br J Radiol. 2000;73:435–37. doi: 10.1259/bjr.73.868.10844871. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Yan X, Zhang X, et al. X-ray diffraction-enhanced imaging of uterine leiomyomas. Med Sci Monit. 2005;11(5):MT33–38. [PubMed] [Google Scholar]
- 22.Okamoto T, Koshiyama M, Yamamoto K. Treatment of huge uterine tumor thought to be benign in post-menopausal women. Med Sci Monit. 2004;10(2):CR43–45. [PubMed] [Google Scholar]


