Abstract
Background
Several observational studies suggested that vitamin E intake is related to the risk of kidney cancer; however, the results of published studies are inconsistent.
Material/Methods
A meta-analysis was performed to assess the relationship between vitamin E intake and the risk of kidney cancer by searching PubMed and Medline through August 2015. We computed pooled relative risks (RR) and 95%CI of kidney cancer for the highest versus lowest level of vitamin E intake.
Results
A total of 13 observational studies (7 case-control and 6 cohort) were included. The pooled RR (95%CI) of kidney cancer for the highest vs. the lowest level of vitamin E intake was 0.81 (0.69–0.94). In subgroup-analysis, this study found an inverse relationship between vitamin E intake and kidney cancer risk, which was not significantly modified by study design, study population, or sex distribution except in the cohort studies.
Conclusions
Results of the present study suggest an inverse relationship between vitamin E intake and kidney cancer risk. However, additional well designed cohort studies and randomized controlled trials that focus on the relationship between vitamin E intake and kidney cancer risk are needed.
MeSH Keywords: Kidney Neoplasms, Meta-Analysis, Vitamin E
Background
Kidney cancer is the third most common genitourinary malignancy [1]. The incidence of this cancer has been increasing rapidly globally [2]. For example, a study estimated that about 64 000 cases of kidney cancer were diagnosed in the United States, and 14 000 patients died in 2014 [3].
Many studies have suggested that heredity [4], environment [5], and lifestyle factors [6] influence the occurrence and development of kidney cancer. It has been hypothesized that the direct mechanism of metabolic disorders, as well as the presence of oxidative stress caused by external and internal factors, is closely related to the development of kidney cancer [7].
Vitamin E, a fat-soluble vitamin and a well known antioxidant, plays an important role in inhibiting cancer incidence and development in various organs of animal models by prevent DNA damage and mutagenesis [8]. However, some epidemiological observational studies showed an ambiguous relationship between vitamin E intake and kidney cancer risk [9]. Although a previous meta-analysis was conducted to assess the relationship between vitamin E intake and renal cell carcinoma risk and it found a significant inverse association, this study only assessed the renal call carcinoma risk but not all kidney cancer risk. Moreover, only 7 case-control studies were included, the evidence was limited, and it may lead to more confounding factors and biases. Hence, we conducted a meta-analysis that integrated all published observational studies (case-control and cohort studies) to assess the relationship between vitamin E intake and kidney cancer risk.
Material and Methods
Study identification
A literature search was conducted using the databases PubMed and Medline up to 2015/08/31 with the following search terms “vitamin E intake” or “dietary vitamin E” and “renal carcinoma” or “kidney cancer” were used as a combination of free text or as MeSH terms. Inclusion criteria were: studies that were conducted as case-control or cohort study designs, published as full-length articles in English, reported the association between vitamin E intake and kidney cancer risk, reported the relative risks (RRs) or odds ratios (ORs) or hazard ratios (HRs) and the corresponding 95% CIs of kidney cancer for the highest vs. the lowest level of vitamin E intake. Reference lists of the retrieved papers were checked to identify additional relevant studies.
Data collection
For each study we collected the following data extraction using a standardized data extraction form: first author’s last name, publication year, study population, sample size, RR and 95%CI of kidney cancer risk from the most fully adjusted model for the highest vs. the lowest level of vitamin E intake, and covariates adjusted in the statistical analysis.
Two independent authors performed all the above procedures, and any disagreements were resolved by discussion.
Statistical analysis
In this study, all statistical analyses were performed using STATA 12.0 (StataCorp LP, College Station, TX). The combined RR and the corresponding 95%CI were used to estimate the relationship between vitamin E intake and kidney cancer risk. We conducted a test based on the I2 and Q statistic to assess the heterogeneity across the included studies [23]. If there was evidence of heterogeneity, subgroup analysis was performed to identify the possible source of heterogeneity and the effect of potential factors on the overall risk assessment. Furthermore, we carried out a sensitivity analysis excluding each study in turn to investigate its influence on the overall risk estimate. Publication bias was detected using the funnel plot [24] and Egger’s test [25]. Results were considered as statistically significant when the P value was less than 0.05.
Results
Initially, 240 articles were searched from the 2 databases according to the inclusion criteria, after reviewing titles, abstracts even full-text. Most of them were excluded because of various reasons. Finally, 13 articles (6944 kidney cancer patients and 465 275 controls or participants) were included [19–21].
The main characteristics of the 13 studies are presented in Table 1. Seven were case-control studies [9–12,14,17,19] and 6 were cohort studies [13,15,16,18,20,21]. Five studies were conducted in the USA [9,13,15,16,21], 2 in Canada [14,19], 1 in Denmark [10], 1 in Sweden [12], 1 in Italy [17], one in the Netherlands [18], 1 in Finland [20], and 1 was conducted in a mixed population from several countries [11]. Most of the included studies adjusted for a wide range of potential confounders, such as age, sex, BMI, smoking, and alcohol use.
Table 1.
The characteristics of the included studies.
| Study (year, population) | Study design | Age of subjects | Sample size (n) case/control (total) | Adjusted RR (95% CI) (highest vs. lowest) | Variables used in multivariate model |
|---|---|---|---|---|---|
| Chow et al. (1994, American) | Case-control | 20–79 | 632/653 | 1.0 [0.6–1.8] | Age, sex, cigarette, dietary caloric intake, educational level, BMI |
| Mellemgaard et al. (1995, Danish) | Case-control | 20–79 | 351/340 | Men: 0.5 [0.2–1.0] Women: 1.1 [0.4–3.0] |
Age, total energy intake, smoking, socioeconomic status, BMI |
| Wolk et al. (1996, N) | Case-control | N | 1185/1526 | 0.9 [0.69–1.16] | Age, sex, study center, BMI, smoking, total calories |
| Lindblad et al. (1996, Swedish) | Case-control | 20–79 | 379/350 | 0.65 [0.42–1.01] | Age, sex, BMI, smoking, education, total energy |
| Prineas et al. (1997, American) | Cohort | 50–69 | 62/35129 | Women: 0.7 [0.38–1.31] | N |
| Hu et al. (2003, Canadian) | Case-control | 20–79 | 1279/5370 | Men: 0.7 [0.5–0.9] Women: 0.6 [0.4–0.8] |
Age, province, education, BMI, alcohol use, smoking |
| Nicodemus et al. (2004, American) | Cohort | 55–69 | 124/34637 | Women: 0.56 [0.3–1.03] | N |
| Lee et al. (2006, American) | Cohort | 40–75 | 248/136587 | 0.9 [0.51–1.6] | BMI, history of diabetes, history of hypertension, parity, smoking status, multivitamin use, alcohol, total energy intake |
| Bosetti et al. (2007, Italian) | Case-control | 22–79 | 767/1534 | 0.56 [0.41–0.75] | Age, sex, study center, smoking, education, BMI, alcohol intake, family history of kidney cancer, period of interview |
| van Dijk et al. (2008, Dutchman) | Cohort | 55–69 | 284/120852 | 1.0 [0.68–1.47] | Age, sex, smoking, BMI, history of hypertension |
| Hu et al. (2009, Canadian) | Case-control | 20–76 | 1138/5039 | 1.13 [0.89–1.45] | Age, sex, province, BMI, education, smoking, meat and fat intake, cholesterol and total energy intake |
| Bertoia et al. (2010, Finnish) | Cohort | 50–69 | 255/27062 | Men: 1.09 [0.73, 1.64] | Age, BMI, education level, history of hypertension, leisure-time physical activity, smoking, alcohol, total energy intake |
| Ho et al. (2015, American) | Cohort | 50–79 | 240/96196 | Women: 0.81 [0.49–1.33] | Age, micronutrients, race, clinical trial, smoking, BMI, education, hypertension, oral contraceptive use, hysterectomy, energy intake, oophorectomy, physical activity |
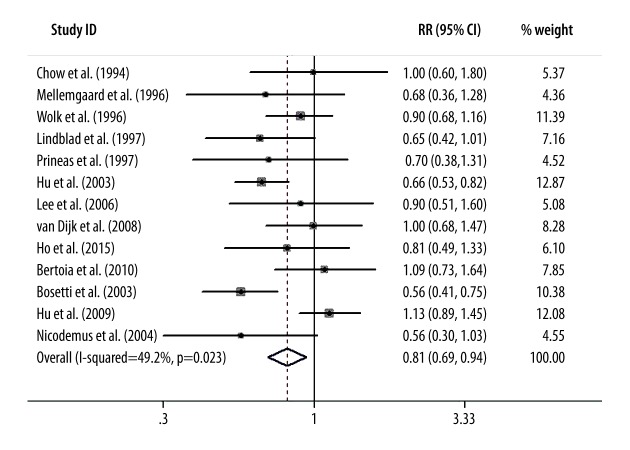
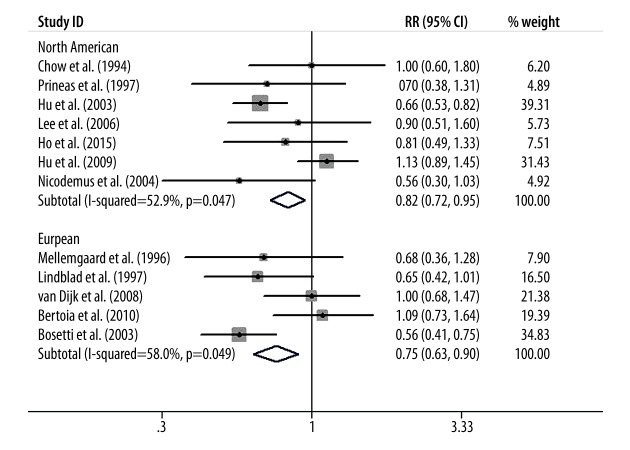
Figure 1 presents the summary RR of kidney cancer for the highest vs. the lowest level of vitamin E intake. Only 2 included studies showed an inverse association of vitamin E intake with kidney cancer risk [14,15]. The pooled RR (95%CI) from all the included studies was 0.81 (0.69–0.94); however, we found a significant evidence of heterogeneity (I2=49.2%, p=0.023).
Figure 1.
Forest plot shows the relationship between vitamin E intake and kidney cancer risk.
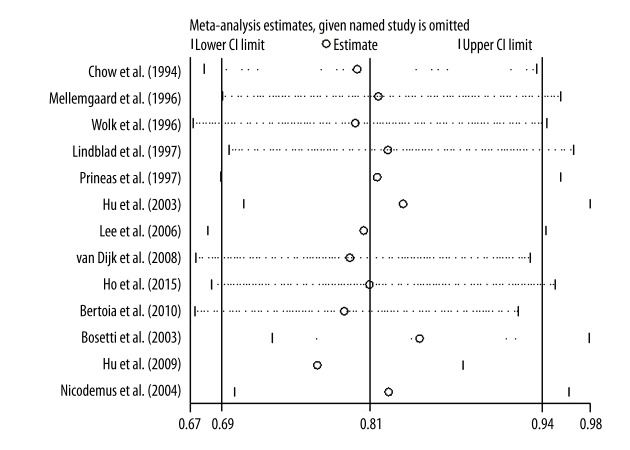
Figure 2 presents the results of sensitivity analysis. As removing each sequentially, the pooled RRs were similar and no individual study changed the pooled RR significantly.
Figure 2.
The result of sensitivity analysis.
Figure 3 presents the funnel plot of publication bias. The funnel plot and Egger’s regression test showed there was no evidence of publication bias in the present study (P=0.928).
Figure 3.
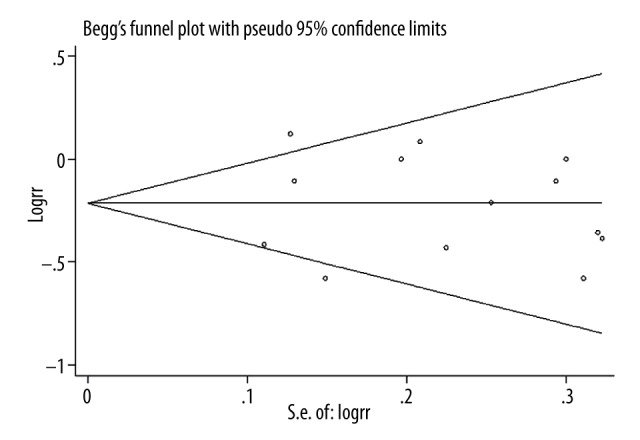
Forest plot for publication bias.
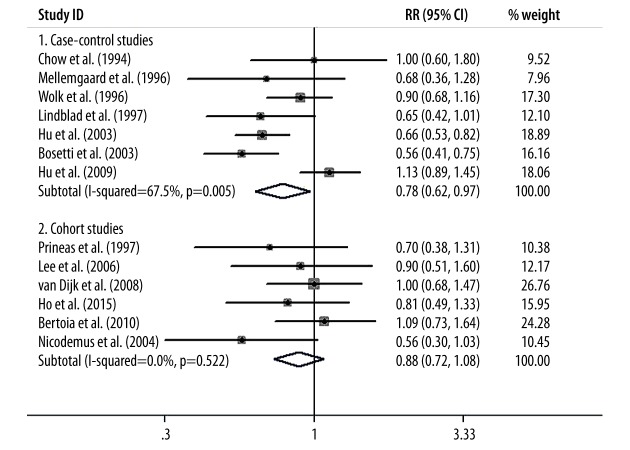
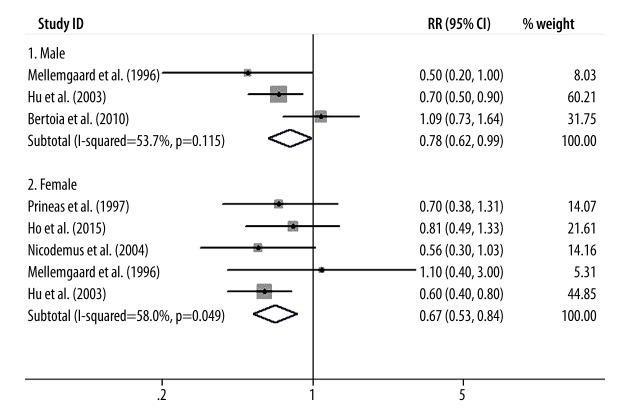
Finally, we conducted subgroup-analysis to assess the influence of various factors on the overall RR estimate according to study design, study population, and sex distribution. We found that the inverse relationship between vitamin E intake and kidney cancer risk was not significantly modified by study design, study population, or sex distribution except in the cohort studies. The results of subgroup-analyses are presented in Figures 4–6.
Figure 4.
The results of subgroup-analysis according to study design.
Figure 6.
The results of subgroup analysis according to sex distribution.
Discussion
Although a previous meta-analysis was conducted to assess the relationship between vitamin E intake and renal cell carcinoma risk, it only included case-control studies. The present meta-analysis was performed to estimate the association between vitamin E intake and kidney cancer risk by combining the published case-control and cohort studies to date, and we found a significant inverse association of vitamin E intake and kidney cancer risk, and this result was not changed by study design, study population, or sex distribution except in the cohort studies.
We assessed the association of vitamin E intake and kidney cancer risk by combining all included studies and by performing subgroup-analyses. We found significant evidence of heterogeneity in some of our estimates and we found that only sex distribution was a source of heterogeneity; however, several studies considered the influence of sex distribution on the results. The sources of heterogeneity, we thought, may be partly explained by the study design (some heterogeneity remained among case-control studies), population and other factors, such as type of pathology of kidney cancer.
We found a significant inverse association of vitamin E intake and kidney cancer risk, the concrete physiological mechanisms may be involved in that vitamin E effectively decreased the expressions of cyclooxygenase-2 and 8-hydroxydeoxyguanosine and type I insulin-like growth factor receptor, inhibited peroxidation and induced cell apoptosis, leading to suppressed cell proliferation [26,27]. A study indicated that in cancer patients, treatement with vitamin E induced cell apoptosis by restoring transforming growth factor-β and Fas signaling pathways, and also increasing the expression of peroxisome proliferator-activated receptor gamma [28]. A study suggested that γ-tocotrienol, a vitamin E derivative, reversed the deleterious impact of nicotine on embryo development; this result indicates that γ-tocotrienol may prevent the development of cancer by suppressing oxidative stress levels [29]. Vitamin E also preserves the balance of production of reactive oxygen species and thus prevents cellular nucleic acids, lipids, and proteins from the harmful effects of ROS [30].
Although another meta-analysis was conducted to assess the association between vitamin E intake and renal cell carcinoma risk, only 7 case-control studies were included and only risk of renal cell carcinoma was assessed. In the present study, we included all published observational studies to examine the association between vitamin E intake and risk of all kidney cancer. We conducted subgroup-analysis to assess the influence of various factors on the overall RR estimate, which was not performed in the previous study. The present study provided strong evidence of the association between vitamin E intake and risk of kidney cancer. However, several limitations of our study should be considered. First, although we combined all published studies, recall bias and selection bias of case-control studies were inevitable. Second, strong evidence of heterogeneity among included studies was found. We conducted subgroup-analyses to detect the source of heterogeneity; however, no stratified analysis was able to explain the heterogeneity. Third, most of the included studies assessed the vitamin E intake levels through questionnaires, which may yield some inaccurate reports. Fourth, the results of the present study were mainly based on populations in Western countries; whether our findings are supported in other populations is unclear. Fifth, vitamin E includes tocopherols (α, β, δ, and γ) and tocotrienols ((α, β, δ, and γ) [31]. Only 1 component is contained in non-enriched food, and thus it will present different biologic activities between dietary intake and supplements [32]. However, few of the included studies reported the effect of different vitamin E components on the risk of kidney cancer. Finally, we did not find evidence of publication bias, but we were not able to completely rule out such bias because of the limited number of studies.
Conclusions
Many randomized controlled trials have directly assessed the effects of dietary vitamin E or supplementation on incidence of several cancers and showed an important role in preventing cancer occurrence and development [33,34]. However, no randomized controlled trials definitely show that vitamin E intake can reduce the risk of kidney cancer; therefore, such studies are indispensable and more persuasive.
Figure 5.
The results of subgroup analysis according to study population.
Footnotes
Conflict of interest statement
None declared.
Source of support: Departmental sources
References
- 1.Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. A Cancer J Clin. 2008;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Moore L, Wilson R. Lifestyle factors, exposures, genetic susceptibility, and renal cell cancer risk: a review. Cancer Invest. 2005;23:240–55. doi: 10.1081/cnv-200055962. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Deckers IA, van den Brandt PA, van Engeland M, et al. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: interplay with hypertension and intakes of sodium, potassium and fluid. Int J Cancer. 2015;136:1104–16. doi: 10.1002/ijc.29060. [DOI] [PubMed] [Google Scholar]
- 5.Ashford NA, Bauman P, Brown HS, et al. Cancer risk: role of environment. Science. 2015;347:727. doi: 10.1126/science.aaa6246. [DOI] [PubMed] [Google Scholar]
- 6.Nicodemus KK, Sweeney C, Folsom AR. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer. 2004;108:115–21. doi: 10.1002/ijc.11532. [DOI] [PubMed] [Google Scholar]
- 7.Klinghoffer Z, Yang B, Kapoor A, et al. Obesity and renal cell carcinoma: epidemiology, underlying mechanisms and management considerations. Expert Rev Anticancer Ther. 2009;9:975–87. doi: 10.1586/era.09.51. [DOI] [PubMed] [Google Scholar]
- 8.Sharoni Y, Linnewiel-Hermoni K, Khanin M, et al. Carotenoids and apocarotenoids in cellular signaling related to cancer: a review. Mol Nutr Food Res. 2012;56:259–69. doi: 10.1002/mnfr.201100311. [DOI] [PubMed] [Google Scholar]
- 9.Chow WH, Gridley G, McLaughlin JK, et al. Protein intake and risk of renal cell cancer. J Natl Cancer Inst. 1994;86:1131–39. doi: 10.1093/jnci/86.15.1131. [DOI] [PubMed] [Google Scholar]
- 10.Mellemgaard A, McLaughlin JK, Overvad K, et al. Dietary risk factors for renal cell carcinoma in Denmark. Eur J Cancer. 1996;32A:673–82. doi: 10.1016/0959-8049(95)00633-8. [DOI] [PubMed] [Google Scholar]
- 11.Wolk A, Gridley G, Niwa S, et al. International renal cell cancer study. VII. Role of diet. Int J Cancer. 1996;65:67–73. doi: 10.1002/(SICI)1097-0215(19960103)65:1<67::AID-IJC12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Lindblad P, Wolk A, Bergström R, et al. Diet and risk of renal cell cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:215–23. [PubMed] [Google Scholar]
- 13.Prineas RJ, Folsom AR, Zhang ZM, et al. Nutrition and other risk factors for renal cell carcinoma in postmenopausal women. Epidemiology. 1997;8:31–36. doi: 10.1097/00001648-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Mao Y, White K. Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control. 2003;14:705–14. doi: 10.1023/a:1026310323882. [DOI] [PubMed] [Google Scholar]
- 15.Bosetti C, Scotti L, Maso LD, et al. Micronutrients and the risk of renal cell cancer: a case-control study from Italy. Int J Cancer. 2007;120:892–96. doi: 10.1002/ijc.22374. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, La Vecchia C, Negri E, et al. Dietary vitamin C, E, and carotenoid intake and risk of renal cell carcinoma. Cancer Causes Control. 2009;20:1451–58. doi: 10.1007/s10552-009-9371-6. [DOI] [PubMed] [Google Scholar]
- 17.Nicodemus KK, Sweeney C, Folsom AR. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer. 2004;108:115–21. doi: 10.1002/ijc.11532. [DOI] [PubMed] [Google Scholar]
- 18.Lee JE, Giovannucci E, Smith-Warner SA, et al. Intakes of fruits, vegetables, vitamins A, C, and E, and carotenoids and risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2445–52. doi: 10.1158/1055-9965.EPI-06-0553. [DOI] [PubMed] [Google Scholar]
- 19.van Dijk BA, Schouten LJ, Oosterwijk E, et al. Carotenoid and vitamin intake, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Cancer Causes Control. 2008;19:125–34. doi: 10.1007/s10552-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 20.Bertoia M, Albanes D, Mayne ST, et al. No association between fruit, vegetables, antioxidant nutrients and risk of renal cell carcinoma. Int J Cancer. 2010;126:1504–12. doi: 10.1002/ijc.24829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho WJ, Simon MS, Yildiz VO, et al. Antioxidant micronutrients and the risk of renal cell carcinoma in the Women’s Health Initiative cohort. Cancer. 2015;121:580–88. doi: 10.1002/cncr.29091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang Y, Yi S, Cui D, et al. Vitamin E intake and risk of renal cell carcinoma: a meta-analysis of 7 case-control studies. J Ren Nutr. 2015;25:339–44. doi: 10.1053/j.jrn.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 24.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–16. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SK, Kang JS, Jung da J, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008;216:180–88. doi: 10.1002/jcp.21391. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Okada S, Yu Y, et al. Vitamin E inhibits apoptosis, DNA modification, and cancer incidence induced by iron-mediated peroxidation in Wistar rat kidney. Cancer Res. 1997;57:2410–14. [PubMed] [Google Scholar]
- 28.Cui Y, Lu Z, Bai L, et al. beta-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer. 2007;43:2590–601. doi: 10.1016/j.ejca.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Kamsani YS, Rajikin MH, Mohamed Nor Khan NA, et al. Nicotine-induced cessation of embryonic development is reversed by γ-tocotrienol in mice. Med Sci Monit Basic Res. 2013;19:87–92. doi: 10.12659/MSMBR.883822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifried HE, Anderson DE, Fisher EI, et al. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Gibson RS. Principles of nutritional assessment. New York, NY: Oxford University Press Inc; 2005. Assessment of the status of vitamin A, D, and E; pp. 477–528. [Google Scholar]
- 32.Peng L, Liu X, Lu Q, et al. Vitamin E intake and pancreatic cancer risk: a meta- analysis of observational studies. Med Sci Monit. 2015;21:1249–55. doi: 10.12659/MSM.893792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazdak H, Zia H. Vitamin e reduces superficial bladder cancer recurrence: a randomized controlled trial. Int J Prev Med. 2012;3:110–15. [PMC free article] [PubMed] [Google Scholar]