Abstract
Left ventricular hypertrophy (LVH), a marker of cardiac end-organ damage, is a common complication of hypertension. Regression of LVH is achievable by sustained lowering of systolic blood pressure (SBP). However, it is unknown whether a strategy aimed at lowering BP beyond that recommended would lower the risk of LVH. We examined the effect of intensive (SBP<120 mmHg), compared to standard (SBP<140 mmHg), BP lowering on the risk of LVH in 4,331 patients with diabetes from the from the ACCORD BP trial, a randomized controlled trial. The outcomes measures were electrocardiographic LVH defined by Cornell voltage (binary variable) and mean Cornell index (continuous variable). The baseline prevalence of LVH (5.3% vs. 5.4%, p= 0.91) and the mean Cornell index (1456 µV vs. 1470 µV, p=0.45) were similar in the intensive (n=2154) and standard (n=2177) BP lowering arms, respectively. However, after median follow up of 4.4 years, intensive, compared to standard, BP lowering was associated with a 39% lower risk of LVH (odds ratio(95% CI):0.61(0.43, 0.88); p=0.008) and a significantly lower adjusted mean Cornell index (1352 µV vs. 1447 µV; p<0.001). The lower risk of LVH associated with intensive BP lowering during follow up was due to more regression of baseline LVH and lower rate of developing new LVH, compared to standard BP lowering. No interactions by age, sex, or race were observed. These results provide evidence that targeting a systolic BP<120 mmHg, as compared with <140 mm Hg, in patients with hypertension and diabetes produces a greater reduction in LVH.
Clinical Trial Registration: ClinicalTrials.gov number, NCT00000620
Keywords: Intensive Blood Pressure Lowering, Left Ventricular Hypertrophy, ACCORD
INTRODUCTION
Left ventricular hypertrophy (LVH), a marker of cardiac end-organ damage, is a common complication of hypertension and is associated with an increased risk of cardiovascular disease (CVD) morbidity and mortality (1–4). There is a strong line of evidence indicating that the risk of poor cardiovascular outcomes associated with LVH is significantly reduced with regression of LVH (5–12). Hence, regression of LVH is considered as a clinically useful intermediate target for assessing the efficacy of antihypertensive treatment (13).
LVH regression is achievable by sustained lowering of systolic blood pressure (SBP) by most antihypertensive agents, and selection of individual drugs appears to be not the key factor (14). It is not known, however, if lowering BP beyond what is recommended would be associated with more regression of LVH. Therefore, we examined the differential impact of intensive BP lowering (target SBP <120 mmHg) versus standard BP lowering (target SBP<140 mm Hg) on LVH in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Blood Pressure Trial, a randomized, multicenter trial involving middle-aged and older patients with type 2 diabetes (T2DM) who are at risk of CVD (15).
We hypothesized that, compared to standard BP lowering, a strategy aimed at intensive BP lowering will be associated with lower risk of LVH. This expected reduction in the risk of LVH will be due to regression of existing LVH and/or prevention of developing new LVH.
Given the common concomitant presence of hypertension and diabetes, and the established risk of poor outcomes associated with hypertension-induced LVH, results from this analysis could be of potential importance from both a clinical and public health perspectives.
METHODS
Study Population and Design
ACCORD was a randomized trial conducted at 77 clinical sites organized into 7 networks in the United States and Canada (15). The trial enrolled 10,251 patients with T2DM at high risk of CVD. Participants were eligible if they had T2DM and a glycated hemoglobin level ≥7.5% and were aged ≥40 years with CVD or ≥55 years with evidence of atherosclerosis, albuminuria, LVH, or ≥2 additional risk factors for CVD (dyslipidemia, hypertension, smoking, or obesity). All participants provided written informed consent.
All ACCORD participants were randomly assigned to either intensive or standard glycemic control (the ACCORD glycemia trial) (16). In addition, 5,518 participants were also randomly assigned (in a 2-by-2 factorial design) to either simvastatin plus fenofibrate or simvastatin plus placebo (the ACCORD lipid trial) (17), and the remaining 4733 participants were randomly assigned (in a 2-by-2 factorial design) to either intensive or standard BP lowering (the ACCORD BP trial) (18). This analysis is based on the ACCORD BP trial which included ACCORD participants with a SBP between 130 and 180 mmHg who were taking ≤3 antihypertensive medications and who had the equivalent of a 24-hour protein excretion rate of less than 1.0 g.
The ACCORD BP trial was a non-blinded trial in which participants were randomly assigned to intensive therapy that targeted SBP of <120 mmHg or standard therapy that targeted SBP of <140 mmHg. The ACCORD BP trial was a study of a treatment strategy to achieve specific SBP goals, rather than an evaluation of any specific drug regimen. Therefore, all available antihypertensive medications were used to lower BP. After the first year of therapy, the average SBP was 133.5 mmHg in the standard-therapy group and 119.3 mmHg in the intensive-therapy group, resulting in an average between-group difference of 14.2 mmHg (95% CI, 13.7–14.7). The corresponding mean diastolic blood pressures were 70.5 mmHg and 64.4 mmHg, for an average difference of 6.1 mmHg (95% CI, 5.7–6.5). These levels of BP control in the 2 groups were maintained throughout the study (18).
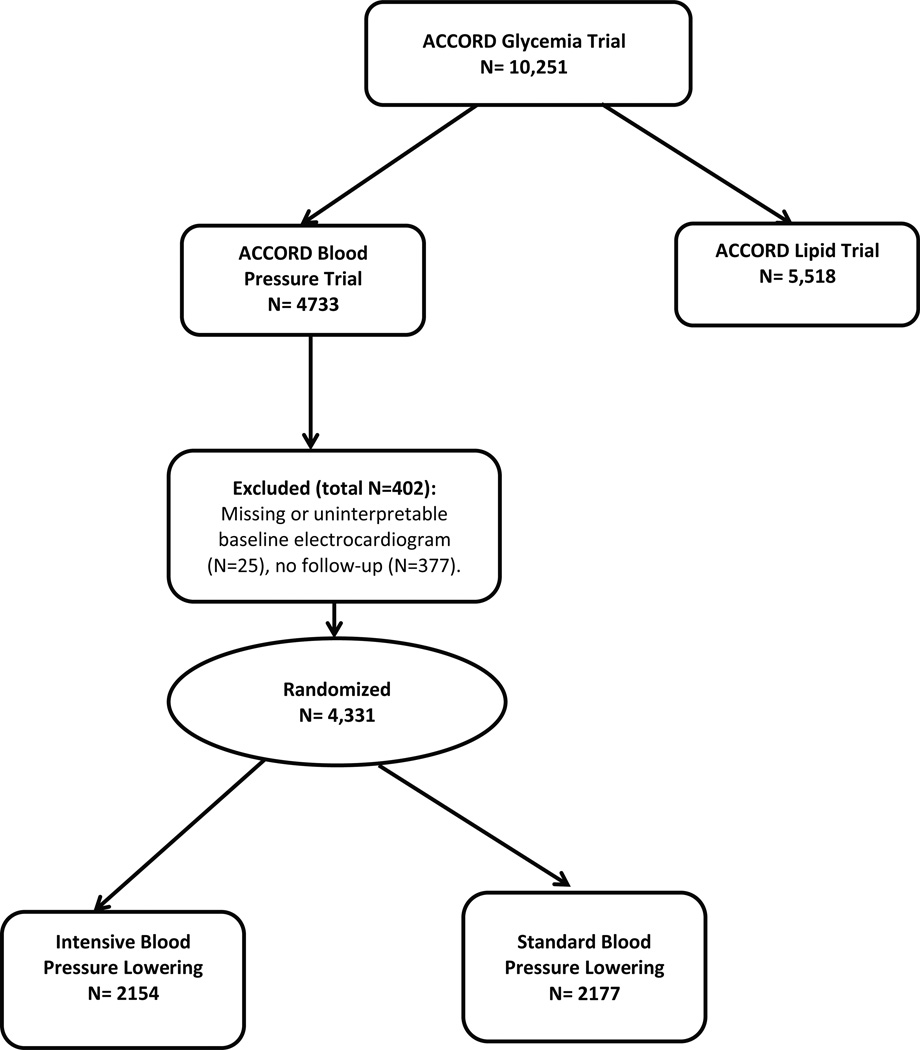
For the purpose of this analysis we excluded participants with missing or uninterpretable (missing leads, major background noise or lead location errors) baseline ECG (n=25), or without any follow-up (n=377) (Figure 1). Since the impact of intensive BP lowering would not be only on developing less new LVH but also regression of existing LVH, we opted not to exclude those with LVH at baseline. Also, since ECG diagnosis of LVH in the presence of major ventricular conduction delay need to be made with caution as recommended by the current guidelines (19), we conducted sensitivity analysis in which we excluded 202 participants with ECG conditions leading to major ventricular conduction delay manifested as prolonged QRS duration. This included complete left and right bundle branch blocks, Wolf-Parkinson-White Syndrome, pacemaker, or major non-specific conduction delay (QRS duration≥ 120 millisecond).
Figure 1.
Profile of ACCORD blood pressure trial
Ascertainment of LVH
LVH was ascertained from the 12-lead electrocardiograms (ECGs) obtained at the biennial ACCORD follow up visits and close out visit using Cornell voltage (RaVL amplitude + SV3 amplitude). LVH was considered present when Cornell voltage exceeded 2200 microvolt (µV) in women or 2800 µV in men (20). In addition to using LVH as a categorical/binary variable, Cornell voltage was also examined as a continuous variable, and referred to in this manuscript as Cornell index. Using Cornell index as a continuous variable has the advantage of being not dependent on the cut-points selected to define LVH, and is more sensitive to changes during follow up than a categorical variable such as LVH.
ECGs were digitally acquired using a GE MAC 1200 electrocardiograph (GE, Milwaukee, Wisconsin) at 10 mm/mV calibration and a speed of 25 mm/s. ECG reading was performed centrally at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, Winston Salem, North Carolina. All ECG tracings were initially inspected visually for technical errors and inadequate quality before being automatically processed using GE 12-SL Marquette version 2001 (GE, Milwaukee, Wisconsin).
Other data
Details of the assessment of BP, the adjustment of medication doses, and antihypertensive drug regimens in ACCORD BP trial are provided elsewhere (18). Race/ethnicity was self-reported. Blood samples were collected in the fasting state during visits, and were used to assess lipid profile, HbA1c, serum glucose and others. History of CVD included prior myocardial infarction (MI), stroke, arterial revascularization, angina with ischemic changes on ECG at rest, changes on a graded exercise test, and positive cardiac imaging test results.
Statistical Analyses
Baseline characteristics were compared between the two trial arms using chi-square test for categorical variables and two-sample t-test for continuous variables. Prevalence of LVH over time was examined and compared between the two trial arms. A generalized estimating equation approach that controls for within-subject correlations was used for this purpose while adjusting for baseline LVH status.
Mean Cornell index during follow-up was compared in the intensive BP lowering arm versus standard BP lowering arm using linear mixed-effects models that control for within-subject correlations between time points while adjusting for baseline values.
Similar to previous ACCORD papers, all models accounted for the assignment to the intensive glucose lowering intervention and each of the seven clinical center networks.
Subgroup analysis by age, race, sex, prior CVD and obesity (body mass index >30 kg/m2) were conducted to examine the consistency of the results in subgroups of the ACCORD participants.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). All P values reported were 2-sided, and statistical significance threshold was chosen as 5%.
Role of the Funding source
The funding source had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
RESULTS
A total of 4,331 participants (mean age 62.1 years, 47.1% women, 59.6% whites) from the ACCORD BP trial were included in the analysis; 2177 were randomly assigned to standard BP lowering and 2154 were assigned to intensive BP lowering. Table 1 shows the baseline characteristics of the participants, overall and by treatment assignment. As shown, baseline characteristics of the study participants did not differ by treatment arms.
Table 1.
Baseline characteristics of the study participants
| Characteristics | Total (n=4331) |
Standard BP Lowering (<140 mmHg) (n=2177) |
Intensive BP Lowering (<120 mmHg) (n=2154) |
p -value* |
|---|---|---|---|---|
| Age, (years) | 62.1 ± 6.8 | 62.2 ±6.9 | 62.1 ± 6.7 | 0.65 |
| Female sex | 2039 (47.1) | 1030 (47.3) | 1009 (46.8) | 0.76 |
| Race/ethnicity | 0.58 | |||
| White | 2582 (59.6) | 1272 (58.4) | 1310 (60.8) | |
| Latino | 293 (6.8) | 153 (7.0) | 140 (6.5) | |
| Black | 986 (22.8) | 510 (23.4) | 476 (22.1) | |
| Asian | 231 (5.3) | 116 (5.3) | 115 (5.3) | |
| Other | 239 (5.5) | 126 (5.8) | 113 (5.3) | |
| Smoking status | 0.87 | |||
| Never | 1947 (45.0) | 982 (45.1) | 965 (44.9) | |
| Past | 1826 (42.2) | 911 (41.9) | 915 (42.5) | |
| Current | 554 (12.8) | 283 (13.0) | 271 (12.6) | |
| Body mass index, (kg/m2) | 32.18 (5.6) | 32.10 (5.4) | 32.25 (5.7) | 0.40 |
| Systolic BP, (mmHg) | 139.07 (15.7) | 139.17 (15.3) | 138.98 (16.1) | 0.69 |
| Diastolic BP, (mmHg) | 75.97 (10.3) | 75.93 (10.1) | 76.02 (10.5) | 0.77 |
| History of cardiovascular disease | 1433 (33.1) | 719 (33.0) | 714 (33.2) | 0.93 |
| Intensive glycaemia lowering | 2152 (49.7) | 1095 (50.3) | 1057 (49.1) | 0.42 |
| Left ventricular hypertrophy | 233 (5.4) | 118 (5.4) | 115 (5.3) | 0.91 |
| Cornell index (µV) | 1463 ±594 | 1470±589 | 1456 ±598 | 0.45 |
Data are presented as number (%) or mean ± standard deviation.
p-value comparing participants’ characteristics in the standard vs. intensive blood pressure-lowering arms
The baseline prevalence of LVH in the intensive BP lowering arm was not different from that in the standard BP lowering arm (5.3%, n=115 vs. 5.4%, n=118, respectively; p-value 0.91). However, during a median follow up of 4.4 years, regression of LVH was more common in the intensive vs. standard therapy arm: 55.6% of the participants with baseline LVH in the intensive BP lowering arm no longer had LVH on their last follow up ECG compared to 49.6% of the participants with baseline LVH in the standard BP lowering arm (p<0.001). In parallel fashion, development of new LVH in those with no baseline LVH was significantly less common in the intensive BP lowering arm compared to standard BP lowering arm (1.7% vs. 3.0 %; p <0.001).
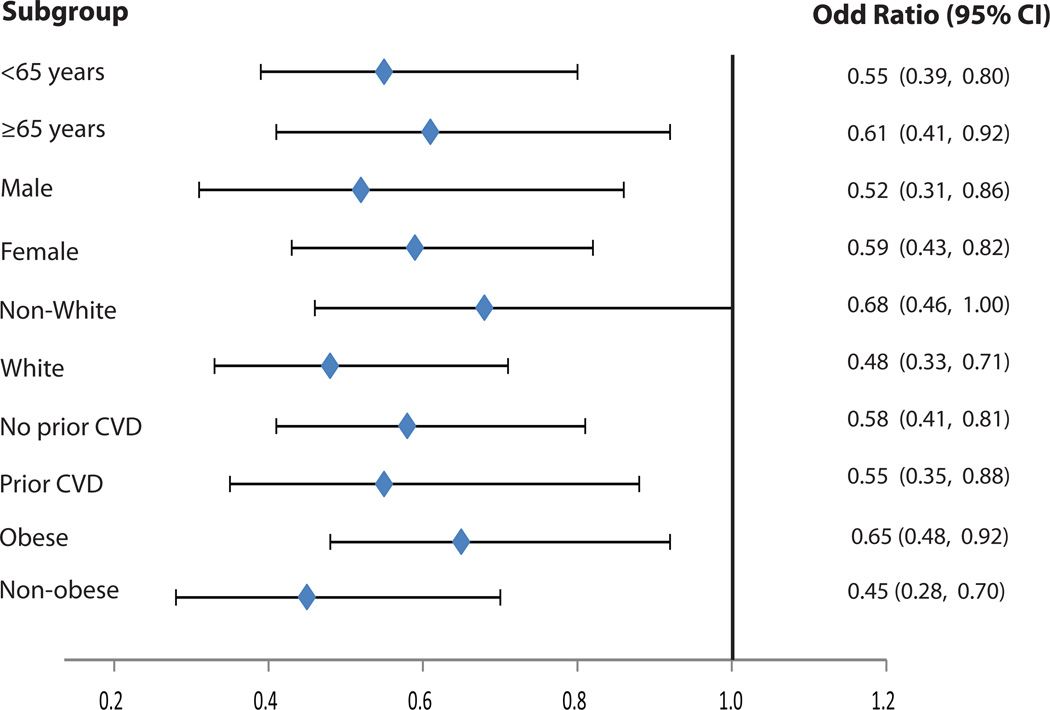
Table 2 shows the effect of intensive vs. standard BP lowering on the prevalence of LVH during follow up. As shown, intensive BP lowering was associated with a 39% lower risk of LVH compared to standard BP lowering (odds ratio (95% CI):0.61(0.43, 0.88); p=0.008). These results were consistent across subgroups of age, sex, race/ethnicity, prior CVD and obesity (Figure 2).
Table 2.
Effect of intensive versus standard blood pressure lowering on prevalence of left ventricular hypertrophy during follow up
| Treatment Arm |
Participants (N) |
LVH at baseline (%) |
New LVH during follow up (%) |
Odds ratio (95% CI)* |
P- value |
|---|---|---|---|---|---|
| Standard BP lowering | 2177 | 5.4 | 3.0 | Reference | N/A |
| Intensive BP lowering | 2154 | 5.3 | 1.7 | 0.61(0.43−0.88) | 0.0081 |
Odds ratio for prevalent LVH in intensive vs. standard blood pressure lowering. Model accounted for the assignment to the intensive glucose lowering intervention and each of the seven clinical center networks. Model adjusted for baseline left ventricular hypertrophy (LVH) LVH is defined from the study electrocardiogram using Cornell voltage criteria
Figure 2.
Effect of intensive vs. standard blood pressure lowering on prevalence of left ventricular hypertrophy in subgroups during follow up
All models accounted for the assignment to the intensive glucose lowering intervention and each of the seven clinical center networks. Model was also adjusted for baseline LVH status
No significant interaction between subgroups
LVH is defined from the study electrocardiogram using Cornell voltage criteria
CVD= Cardiovascular disease
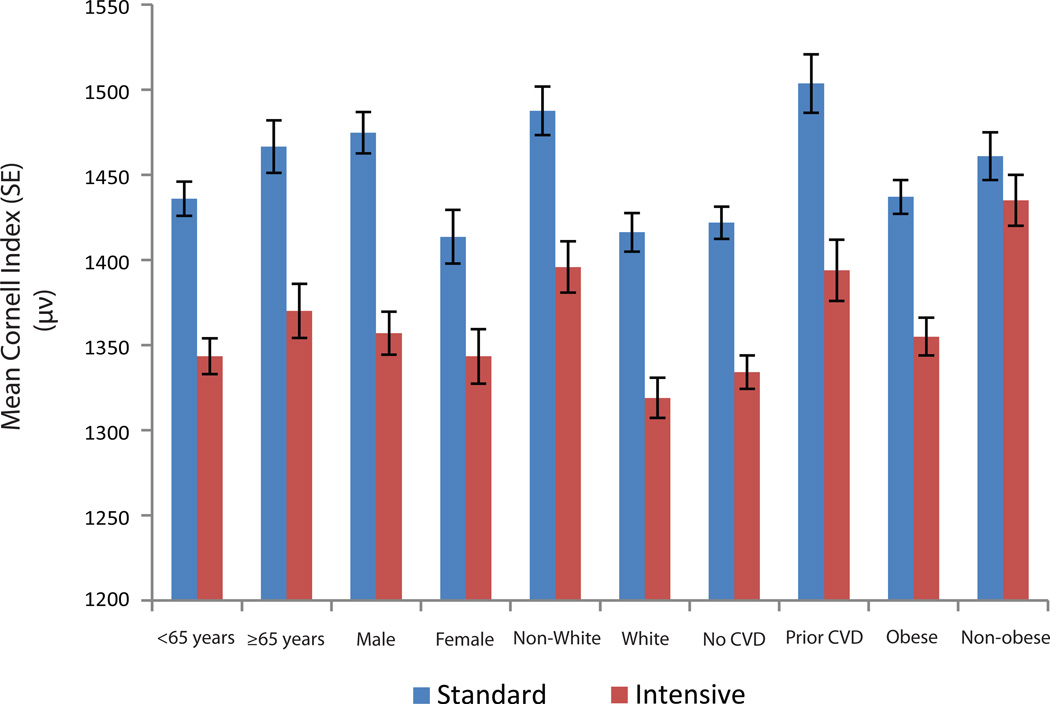
Similarly, there was no difference between the intensive and standard arms in terms of baseline mean Cornell index (1456 µV vs. 1470 µV, respectively; p-value=0.45). However, the adjusted mean Cornell index during follow up became significantly lower in the intensive arm compared to the standard arm (1352 µV vs. 1447 µV, respectively; p<0.001) (Table 3). These results were consistent across subgroups of age, sex, race/ethnicity, prior CVD and obesity (Figure 3). The trends of regression of Cornell voltage followed the trends in systolic blood pressure reduction during the trial (Supplemental Figure S1).
Table 3.
Effect of intensive versus standard blood pressure lowering on the mean Cornell index during follow up
| Study visit | Standard BP lowering Mean (SE) |
Intensive BP lowering Mean (SE) |
P- value |
|---|---|---|---|
| Baseline | 1470 (13)µV | 1456 (13) µV | 0.45 |
| Follow up | 1447 (8) µV | 1352 (9) µV | <.0001 |
All models accounted for the assignment to the intensive glucose lowering intervention and each of the seven clinical center networks. Model was also adjusted for baseline Cornell index values.
Cornell index is defined as the sum of the R amplitude in aVL and S amplitude in V3 in microvolt
Figure 3.
Effect of intensive vs. standard blood pressure lowering on the mean Cornell index during follow up
All models accounted for the assignment to the intensive glucose lowering intervention and each of the seven clinical center networks. Model was also adjusted for baseline Cornell index values.
p-value for the comparison of adjusted mean Cornell index in standard vs. intensive BP lowering was <0.01 in all subgroups. No significant interaction between subgroups
Cornell index is defined as the sum of the R amplitude in aVL and S amplitude in V3 in microvolt
In a sensitivity analysis in which we excluded 202 participants with major ventricular conduction delay, the impact of intensive vs standard blood pressure lowering on LVH (odds ratio (95% CI):0.55(0.41, 0.73); p=0.001) and Cornell index (1353 µV vs. 1449 µV; p<0.001) was similar to that observed in the main analysis.
DISCUSSION
In this analysis from the ACCORD BP trial, we examined the effect of intensive BP lowering (targeted SBP of <120 mmHg), compared with standard BP-lowering (targeted SBP of <140mm Hg), on electrocardiographic measures of LVH. We found that intensive BP lowering, compared with standard BP-lowering, resulted in lower risk of LVH. The lower risk of LVH in the intensive BP lowering arm was due to more regression of existing LVH as well as lower rate of developing new LVH during follow up, compared to standard BP lowering arm.
LVH is an adaptive response to the wall stress associated with increased impedance to ventricular emptying due to increase in peripheral resistance, the hallmark of established hypertension (21). Hence, successful BP lowering is expected to alter the chances of new occurrence of LVH and/or enhance regression of existing LVH. This is supported by results from several cohort studies and clinical trials indicating that LVH could be reversed by nonpharmacological and pharmacological interventions (22–37). However, none of these studies were designed to examine the impact of a strategy to lower BP beyond the recommended values (SBP <140 mmHg) on regression of LVH in patients with diabetes. One trial, however, compared a SBP goal of < 130 mmHg to a goal of < 140 mmHg in adults 55 years of age or older (n=1,111 participants). That trial, Cardio-Sis trial, concluded that lowering of SBP to < 130 mmHg in non-diabetic patients with at least one additional risk factor decreased the likelihood of electrocardiographic LVH by 39%, compared with usual lowering to SBP < 140 mmHg (38), similar to our results in patients with diabetes. This is despite the fact that Cardio-Sis used different ECG-LVH criteria; Perugia score which generally yields higher prevalence estimates of LVH. To our knowledge, our results from ACCORD BP trial is the first to provide evidence from a randomized clinical trial to suggest that intensive (SBP <120 mmHg) in patients with T2DM is associated with lower risk of LVH compared with standard BP lowering (SBP<140 mmHg).
Regression of ECG-LVH has been repeatedly shown to be associated with lower risk of cardiovascular morbidity and mortality (5–12). With our results in mind, intensive BP lowering compared to standard BP lowering should have been associated with better outcomes in the ACCORD BP trial. In contrary, however, intensive BP lowering did not significantly reduce the primary cardiovascular outcome (composite of nonfatal MI, nonfatal stroke, or death from cardiovascular causes) or the rate of death from any cause in the ACCORD BP trial (18). Nevertheless, intensive BP lowering did reduce the rate of total stroke and nonfatal stroke, two of the pre-specified secondary outcomes. Unlike a composite of CVD (39) or CHD (40), LVH is an established predictor of stroke, and a component of the Framingham stroke risk prediction score (41). This could explain why intensive BP lowering in ACCORD BP trial selectively reduced the risk of stroke but not CVD or CHD. It is unclear, however, why intensive BP lowering did not reduce the risk of fatal and non-fatal heart failure in ACCORD BP trial, although LVH is an established predictor of heart failure and a component of the Framingham heart failure risk prediction score (42) similar to stroke. This might be explained by the notion that in some pathological conditions, the development of mild levels of hypertrophy might be beneficial. For example, in myocardial infarction, presence of LVH worsens prognosis. However, it is the lack of an increase in wall thickness to compensate for the increase in chamber radius which leads to the progressively increased diastolic stress that begets the remodeling that is accompanied by LV systolic dysfunction and increased morbidity and mortality from heart failure (43). Evaluating the benefit of intensive BP lowering on different types of heart failure (preserved vs. low ejection fraction heart failure) may shed light as why intensive BP lowering did not reduce the risk of heart failure despite its favorable benefit on LVH and the established risk of heart failure associated with LVH.
Taken altogether considering our results showing favorable impact of intensive BP lowering on LVH and given the ACCORD BP trial results showing only benefit of intensive BP lowering on stroke, it might be reasonable to consider intensive BP lowering in selected patients with T2DM at higher risk of stroke. This suggestion is in agreement with a recent meta-analysis that showed reduction in risk of stroke, but not other cardiovascular outcomes, with BP lowering to < 130 mmHg in patients with diabetes. Notably, the same meta-analysis showed reduction in the risk of all cardiovascular outcomes with BP lowering below 140 but above 130 (44).
Our results should be read in the context of certain limitations and methodological considerations. By design, ACCORD BP trial included only patients with diabetes at high risk for CVD. Hence, our results may not be generalized to all patients with diabetes or non-diabetic populations. Also, ACCORD BP trial had an open-label design which could lead to some bias. However, it is unlikely that the open label design could have a significant impact on the ascertainment of LVH, which was measured from ECGs that were read centrally at an ECG core laboratory blinded to the treatment assignment.
In ACCORD BP trial, LVH was defined from ECG not imaging (echocardiography or cardiac magnetic resonance imaging). Although imaging provides a more accurate assessment of LVH than does the ECG, this does not obviate the clinical use of the ECG which is the most accessible cardiac investigation tool. More importantly, LVH detected by ECG has been shown to be predictive of poor outcomes in a similar way as LVH detected by imaging (45–48). Also, in addition to its established role as a predictor of poor outcome, regression of LVH defined by ECG has been shown to be associated with better prognosis (31–38). These findings along with its wide availability put ECG-LVH in a position to be an ideal tool to indicate a more advanced clinical state, predicts a more serious clinical course, and predicts improvement with therapy in patients with hypertension.
Despite these limitations, this is the first report from a well-designed large clinical trial in which the effect of intensive BP lowering on LVH in patients with hypertension and diabetes is examined. The strengths of our study include large sample size, racially/ethnically diverse population with representation of both sexes, random assignment of participants to treatment arms resulting in a balanced groups at baseline, standardized data collection including ECG data that were centrally read, and achievement and maintenance of an average between-group difference in SBP of 14 mmHg throughout the study.
PERSPECTIVES
This analysis from the ACCORD BP trial shows that intensive BP lowering (SBP <120 mmHg), compared to standard lowering (SBP <140 mmHg) reduces the risk of LVH in patients with hypertension and T2DM who are at high risk for CVD. The lower risk of LVH in the intensive BP lowering arm was due to more regression of existing LVH as well as lower rate of developing new LVH during follow up, compared to standard BP lowering arm. These findings suggest a potential benefit of intensive BP lowering in prevention of LVH-related comorbidities in patients with hypertension and T2DM.
Supplementary Material
Novelty and Significance.
What Is NEW
This is the first report from a well-designed large clinical trial in which the effect of intensive BP lowering on LVH in patients with hypertension and diabetes is examined.
What Is Relevant
Our findings indicate that intensive BP lowering (SBP <120 mmHg), compared to standard lowering (SBP <140 mmHg) reduces the risk of LVH in patients with hypertension and T2DM.
Summary
There is a potential benefit of intensive BP lowering in reducing the risk of LVH which could be reflected in prevention of LVH-related comorbidities in patients with hypertension and T2DM.
Acknowledgment
The authors thank the staff and participants of the ACCORD study for their important contributions.
Source of Funding: The ACCORD-BP trial was supported by contracts from the National Heart, Lung, and Blood Institute (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, and IAA#Y1-HC-9035 and IAA#Y1-HC-1010).
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, Castelli WP. Left ventricular mass and the risk of stroke in an elderly cohort: the Framingham Heart Study. JAMA. 1994;272:33–36. [PubMed] [Google Scholar]
- 4.Sokolow M, Perloff D. The prognosis of essential hypertension treated conservatively. Circulation. 1981;23:697–713. [Google Scholar]
- 5.Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the Hypertension Detection and Follow-up Program: prevention and reversal of left ventricular hypertrophy with antihypertensive drug therapy. Hypertension. 1985;7:105–112. [PubMed] [Google Scholar]
- 6.Prineas RJ, Rautaharju PM, Grandits G, Crow R MRFIT Research Group. Independent risk for cardiovascular disease predicted by modified continuous score electrocardiographic criteria for 6-year incidence and regression of left ventricular hypertrophy among clinically disease free men: 16-year follow-up for the Multiple Risk-Factor Intervention Trial. J Electrocardiol. 2001;34:91–101. doi: 10.1054/jelc.2001.23360. [DOI] [PubMed] [Google Scholar]
- 7.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 9.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 10.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 11.Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Lindholm LH, Dahlöf B LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint reduction in hypertension (LIFE) Study. Hypertension. 2007;50:984–990. doi: 10.1161/HYPERTENSIONAHA.107.096818. [DOI] [PubMed] [Google Scholar]
- 12.Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Dahlöf B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147:311–319. doi: 10.7326/0003-4819-147-5-200709040-00006. [DOI] [PubMed] [Google Scholar]
- 13.Cuspidi C, Esposito A, Negri F, Sala C, Masaidi M, Giudici V, Zanchetti A, Mancia G. Studies on Left Ventricular Hypertrophy Regression in Arterial Hypertension: A Clear Message for the Clinician? Am J Hypertens. 2008;21:458–463. doi: 10.1038/ajh.2007.85. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute National High Blood Pressure Education Program Coordinating Committee. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.ACCORD Study Group. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of Intensive Glucose Lowering in Type 2 Diabetes. New Eng J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy. J Am Coll Cardiol. 2009;53:992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Casale PN, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 21.Prisant LM. Hypertensive Heart Disease. J Clin Hypertens. 2005;7:231–238. doi: 10.1111/j.1524-6175.2005.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH, Jr, Neaton JD, Stamler J. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS) Circulation. 1995;91:698–706. doi: 10.1161/01.cir.91.3.698. [DOI] [PubMed] [Google Scholar]
- 23.Lièvre M, Guéret P, Gayet C, Roudaut R, Haugh MC, Delair S, Boissel JP. Ramipril-induced regression of left ventricular hypertrophy in treated hypertensive individuals. HYCAR Study Group. Hypertension. 1995;25:92–97. doi: 10.1161/01.hyp.25.1.92. [DOI] [PubMed] [Google Scholar]
- 24.Gottdiener JS, Reda DJ, Massie BM, Materson BJ, Williams DW, Anderson RJ. Effect of single-drug therapy on reduction of left ventricular mass in mild to moderate hypertension: comparison of six antihypertensive agents. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Circulation. 1997;95:2007–2014. doi: 10.1161/01.cir.95.8.2007. [DOI] [PubMed] [Google Scholar]
- 25.Dyadyk AI, Bagriy AE, Lebed IA, Yarovaya NF, Schukina EV, Taradin GG. ACE-inhibitors captopril and enalapril induce regression of left ventricular hypertrophy in hypertensive patients with chronic renal failure. Nephrol Dial Transplant. 1997;12:945–951. doi: 10.1093/ndt/12.5.945. [DOI] [PubMed] [Google Scholar]
- 26.Tedesco MA, Ratti G, Aquino D, Limongelli G, di Salvo G, Mennella S, Galzerano D, Iarussi D, Iacono A. Effects of losartan on hypertension and left ventricular mass: a long-term study. J Hum Hypertens. 1998;12:505–510. doi: 10.1038/sj.jhh.1000685. [DOI] [PubMed] [Google Scholar]
- 27.Thürmann PA, Kenedi P, Schmidt A, Harder S, Rietbrok N. Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension. Circulation. 1998;98:2037–2042. doi: 10.1161/01.cir.98.19.2037. [DOI] [PubMed] [Google Scholar]
- 28.Gerritsen TA, Bak AA, Stolk RP, Jonker JJ, Grobbee DE. Effects of nitrendipine and enalapril on left ventricular mass in patients with non-insulin-dependent diabetes mellitus and hypertension. J Hypertens. 1998;16:689–696. doi: 10.1097/00004872-199816050-00017. [DOI] [PubMed] [Google Scholar]
- 29.Sadowski Z, Szwed H, Kuch-Wocial A, Kubasik A, Januszewicz W, Krupa-Wojciechowska B, Polak G, Stejfa M, Dvorak I, Balazovjech I, Dubai G, Simon K. Regression of left ventricular hypertrophy in hypertensive patients after 1 year of treatment with rilmedine: a double-blind, randomized, controlled (versus nifedipine) study. J Hypertens Suppl. 1998;16:S55–S62. [PubMed] [Google Scholar]
- 30.Roman MJ, Alderman MH, Pickering TG, Pini R, Keating JO, Sealey JE, Devereux RB. Differential effects of angiotensin converting enzyme inhibition and diuretic therapy on reductions in ambulatory blood pressure, left ventricular mass, and vascular hypertrophy. Am J Hypertens. 1998;11:387–396. doi: 10.1016/s0895-7061(97)00492-5. [DOI] [PubMed] [Google Scholar]
- 31.Hernández D, Lacalzada J, Salido E, Linares J, Barragán A, Lorenzo V, Higueras L, Martín B, Rodríguez A, Laynez I, González-Posada JM, Torres A. Regression of left ventricular hypertrophy by lisinopril after renal transplantation: role of ACE gene polymorphism. Kidney Int. 2000;58:889–897. doi: 10.1046/j.1523-1755.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- 32.Gosse P, Sheridan DJ, Zannad F, Dubourg O, Guéret P, Karpov Y, de Leeuw PW, Palma-Gamiz JL, Pessina A, Motz W, Degaute JP, Chastang C. Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg versus enalapril 20 mg : the LIVE study. J Hypertens. 2000;18:1465–1475. doi: 10.1097/00004872-200018100-00015. [DOI] [PubMed] [Google Scholar]
- 33.Avanza AC, Jr, El Aouar LM, Mill JG. Reduction in left ventricular hypertrophy in hypertensive patients treated with enalapril, losartan or the combination of enalapril and losartan. Arq Bras Cardiol. 2000;74:103–117. [PubMed] [Google Scholar]
- 34.Midtvedt K, Ihlen H, Hartmann A, Bryde P, Bjerkely BL, Foss A, Fauchald P, Holdaas H. Reduction of left ventricular mass by lisinopril and nifedipine in hypertensive renal transplant recipients: a prospective randomized double-blind study. Transplantation. 2001;72:107–111. doi: 10.1097/00007890-200107150-00021. [DOI] [PubMed] [Google Scholar]
- 35.Terpstra WF, May JF, Smit AJ, de Graeff PA, Havinga TK, van den Veur E, Schuurman FH, Meyboom-de Jong B, Crijns HJ. Long-term effects of amlodipine and lisinopril on left ventricular mass and diastolic function in elderly, previously untreated hypertensive patients: the ELVERA trial. J Hypertens. 2001;19:303–309. doi: 10.1097/00004872-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Devereux RB, Palmieri V, Sharpe N, De Quattro V, Bella JN, de Simone G, Walker JF, Hahn RT, Dahlöf B. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (PRESERVE) trial. Circulation. 2001;104:1248–1254. doi: 10.1161/hc3601.095927. [DOI] [PubMed] [Google Scholar]
- 37.Malmqvist K, Kahan T, Edner M, Held C, Hägg A, Lind L, Müller-Brunotte R, Nyström F, Ohman KP, Osbakken MD, Ostergern J. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens. 2001;19:1167–1176. doi: 10.1097/00004872-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 39.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 40.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 41.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 43.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 44.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;10(313):603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 45.Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ, Folsom AR, Lima JA, Bluemke DA. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2010;159:652–658. doi: 10.1016/j.ahj.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rautaharju PM, LaCroix AZ, Savage DD, Haynes SG, Madans JH, Wolf HK, Hadden W, Keller J, Cornoni-Huntley J. Electrocardiographic estimate of left ventricular mass versus radiographic cardiac size and the risk of cardiovascular disease mortality in the epidemiological follow-up study of the first National Health and Nutrition Examination Survey. Am J Cardiol. 1988;62:59–66. doi: 10.1016/0002-9149(88)91365-3. [DOI] [PubMed] [Google Scholar]
- 47.Havranek EP, Emsermann CD, Froshaug DN, Masoudi FA, Krantz MJ, Hanratty R, Estacio RO, Dickinson LM, Steiner JF. Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electrocardiol. 2008;41:342–350. doi: 10.1016/j.jelectrocard.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bacharova L, Estes H, Bang L, Rowlandson I, Schillaci G, Verdecchia P, Macfarlane PW. The first statement of the Working Group on Electrocardiographic Diagnosis of Left Ventricular Hypertrophy. J Electrocardiol. 2010;43:197–199. doi: 10.1016/j.jelectrocard.2010.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.