Abstract
Background
Thrombin’s role in the nervous system is not well understood. Under conditions of blood-brain barrier compromise (e.g., neurosurgery or stroke), thrombin can result in neuroapoptosis and the formation of glial scars. Despite this, preconditioning with thrombin has been found to be neuroprotective in models of cerebral ischemia and intracerebral hemorrhage.
Methods
We investigated the effects of physiologically relevant concentrations of thrombin on cortical neurons using two culture-based assays. We examined thrombin’s effect on neurites by quantitative analysis of fluorescently labeled neurons. To characterize thrombin’s effects on neuron survival, we spectrophotometrically measured changes in enzymatic activity. Using receptor agonists and thrombin inhibitors, we separately examined the role of thrombin and its receptor in neuroprotection.
Results
We found that low concentrations of thrombin (1 nM) enhances neurite growth and branching, neuron viability, and protects against excitotoxic damage. In contrast, higher concentrations of thrombin (100 nM) are potentially detrimental to neuronal health as evidenced by inhibition of neurite growth. Lower concentrations of thrombin resulted in equivalent neuroprotection as the antifibrinolytic, aprotinin, and the direct thrombin inhibitor, argatroban. Interestingly, exogenous application of the species-specific thrombin inhibitor, antithrombin III, was detrimental to neuronal health; suggesting that some endogenous thrombin is necessary for optimal neuron health in our culture system. Activation of the thrombin receptor, protease-activated receptor - 1 (PAR-1), via micromolar concentrations of the thrombin receptor agonist peptide, TRAP, did not adversely affect neuronal viability.
Conclusions
An optimal concentration of thrombin exists to enhance neuronal health. Neurotoxic effects of thrombin do not involve activation of PAR receptors and thus separate pharmacologic manipulation of thrombin’s receptor in the setting of direct thrombin inhibitors could be a potential neuroprotective strategy.
Introduction
In addition to its central role in clot formation, thrombin is a promiscuous enzyme with multiple effects in systems besides coagulation; these include the immune system (1) and the nervous system (2). Thrombin’s role as a key modulator of cell growth, development, and response to injury in the nervous system appears to be dynamically modulated by concentration (3). Thrombin concentrations as low as 1–10 nM can influence glial cell mitosis and motoneuron outgrowth during the embryonic period, while higher concentrations (> 100 nM) have been shown to induce apoptosis (4). Although much of the work on thrombin’s central nervous system (CNS) effects have concentrated on trauma or stroke, there is evidence that brain-derived thrombin plays an important role in CNS function. For example, low concentrations (< 1 nM) have been associated with augmenting long-term potentiation, a synaptic correlate of memory (5). Furthermore, thrombin and its propeptide, prothrombin, are produced by neurons and glia in the CNS (6, 7). Clinical studies have postulated that thrombin regulation may play an important role in the neurodegeneration associated with Alzheimer’s Disease (8) and Multiple Sclerosis (9).
Thrombin’s cellular effects are mediated through the activation of protease activated receptors (PARs). To date, four PAR receptors (PAR1-4) have been identified. PAR1, PAR3, and PAR4 can all be activated by thrombin while PAR2 is activated by trypsin and mast cell tryptase (reviewed in (10)). Thrombin activates the receptor via proteolysis, causing release of a small peptide and an unmasking of a new N-terminal domain. After cleavage the new domain acts as a tethered ligand that binds to the receptor to initiate its G-protein coupled subunit signaling (11). Similar to its endogenous activator, thrombin, PAR-1 has been associated with both neurotoxicity (12) and neuroprotection (5).
Self-activation of the PAR receptors results in many downstream second messenger pathways such as stimulation of phosphoinositidase C activity, calcium mobilization, activation of protein kinase C, stimulation of Ras and Ras related proteins, stimulation of tyrosine kinases, inhibition of adenylyl cyclase, and activation of mitogen activated kinase (MAP kinase) and phosphoinositide 3-kinase (PI3 kinase) (11). Thrombin-mediated stimulation of the receptor is inactivated via phosphorylation of serine or threonine residues in the cytoplasmic tail which targets the receptor internalization and lysosomal digestion (13). The amino acids of this new N-terminal domain are SFLLRN (Ser-Phe-Leu-Leu-Arg-Asn). Several PARs can be activated by exogenous administration of the peptide sequence that resembles the tethered ligand. This short peptide is called thrombin receptor activation peptide (TRAP). Proteomic data suggests that TRAPs such as SFLLRN can mimic many of the cellular actions of thrombin through receptor activation and activate the same intracellular cascades (14). The role of TRAP in neuronal health remains largely unexplored.
In this paper, we investigated the effects of thrombin on cortical neuron survival and morphology using rat primary cortical neuron-enriched cultures Through the use of TRAP and thrombin inhibitors we examined the role of thrombin and its receptor in neuroprotection. Additionally, control of thrombin concentration via direct thrombin inhibitors may preserve neuronal health in conditions of high thrombin (neurotrauma or neurosurgery) and represent a novel avenue for pharmacologic therapies.
Materials and Methods
Materials
All cell culture reagents were from Sigma-Aldrich Inc. (St. Louis, MO, USA) unless otherwise specified. Enzymatic proteins were reconstituted in stock solutions as balanced salt solutions and freshly dissolved in media before application. Specific concentrations (see text) of bovine thrombin, argatroban, aprotinin, glutamate, rat antithrombin III, and thrombin receptor activating peptide (TRAP) were applied directly to the media in wells containing cortical neuron cultures (see below). Unless otherwise stated, all test substances and culture reagents were obtained from Sigma (Sigma, Inc., St. Louis, MO).
The physiologic concentrations of free thrombin during coagulation reactions is estimated to vary from 1 nM (0.1 U/mL) to over 500 nM (15). As several different units of measure exist for thrombin and other proteins related to blood coagulation, we represent concentrations of thrombin, argatroban and TRAP in molarity. For this study, we represent antithrombin III as a percentage of human serum levels. Our calculations are based on published plasma levels; 100% serum activity is equivalent to 20 mg/dL (16). Although the exact brain parenchymal levels of thrombin and other coagulation proteins are not known, we based our chosen concentrations on published results of circulating (serum) levels for thrombin (17) and antithrombin (18). Test concentrations for aprotinin were based on previous work focusing on neuroprotection (19). Argatroban levels are based on previous work focusing on in vitro thrombin binding assays (20).
Rat dissociated neuron cultures
All animal procedures were carried out according to the Atlanta VA Medical Center IACUC and adhered to the NIH guidelines for the care and use of laboratory animals. Cortical neurons were dissected from E18 Sprague Dawley rat embryos (Charles River Laboratory). For dendrite morphometrics, low-density neuronal cultures were prepared by plating dissociated primary neurons in 100 mm dishes (5 X 105 cells/100 mm dish) that contained poly-L-lysine (PLL) coated 15 mm diameter glass coverslips as described in (21) and incubated at 37°C with 5% CO2 in plating medium (MEM supplemented with 10% FBS, glucose 0.6% wt/vol, HEPES). Two-hours after plating, the low-density neuronal cultures on the glass coverslips were flipped over into 12 well dishes containing an astroglial monolayer in neurobasal medium (neurobasal medium supplemented with 2% B27 supplement and 1% GlutaMax) (all Invitrogen/Life Technologies; Grand Island, NY, USA). Cytosine-D-arabinoside (Ara-C) (1 μM) (Fluka/Sigma) was added at day 3 after plating to prevent glial cell proliferation. Media was exchanged every 7 days. For each drug tested, after 7 days DIV, the cell media was removed from the neurons and mixed with the experimental substance dissolved in fresh media. Coverslips (containing neurons) were removed from the astroglia support layer and placed in media with the experimental substance. After 24 hours, the neurons were rinsed with fresh media and stained for immunofluorescence. For MTT assay experiments, cortical neurons were plated at 30,000 cells/well in plating media in 96 well plates previously coated with. After 2 hours, plating media was exchanged for neurobasal medium, and glial proliferation was inhibited by the addition of cytosine β-D-arabinofuranoside to 1 μM for 24 hours during the second day in vitro. Experimental substances were added to wells 7 days post-plating.
MTT Assay
The yellow tetrazolium salt 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, or MTT, is enzymatically reduced by living cells into an insoluble purple formazan by mitochondria. A spectrophotometer is used to measure the absorption of the resulting colored solution (read absorbance at 595nm). Twenty-four hours after drug treatment, cell viability was determined as a percentage of control (vehicle-treated cells). A treatment can be considered detrimental to neuronal health if its administration results in fewer viable cells than control (22). Each experiment had a separate untreated control to which the drug applied groups were compared (represented as 100%, SD for controls varied between 7.4 – 12.8%). Much like previous work (19), we define an exposure to substances that results in more cellular death (and less mitochondrial respiration) as neurotoxic while neuroprotective substances prevent the anticipated amount of death (i.e., compared to untreated controls). We define an exposure to substances that result in more cellular death (and less mitochondrial respiration) as neurotoxic while neuroprotective substances prevent the anticipated amount of death. We do not use the term neuroprotection to describe the “rescue” of anticipated neuronal death due to co-application of a known toxic substance (i.e., glutamate; see Figure 3), unless the viability of cells was enhanced beyond untreated cells.
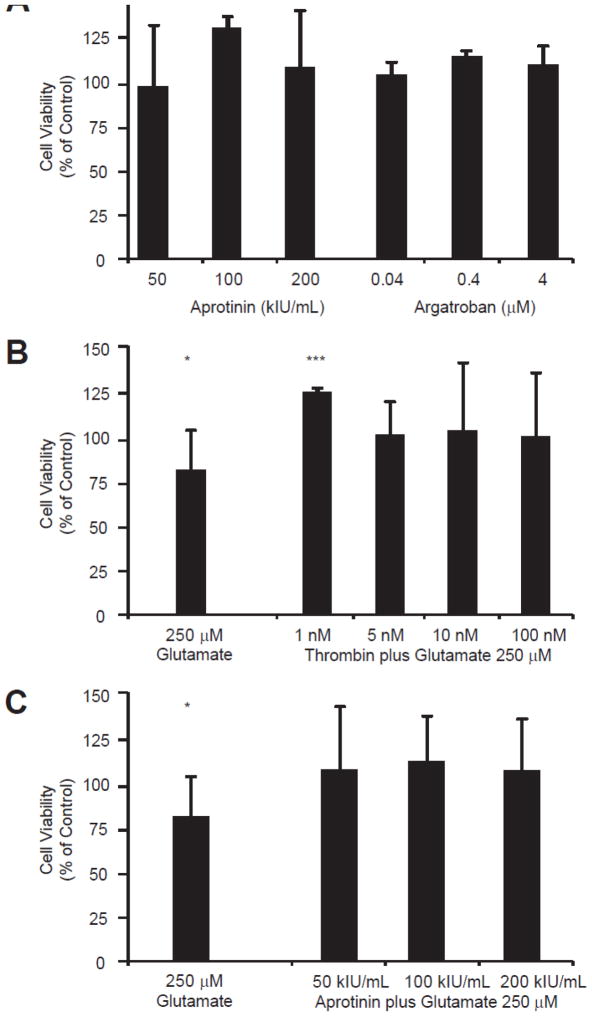
Figure 3.
A comparison of neuroprotection among exogenously applied aprotinin, argatroban, and thrombin in cultured neurons. A. The effect of applied aprotinin and argatroban on viability of cultured cortical neurons. Viability was measured using the MTT assay (see Methods). None of the tested concentrations of aprotinin or argatroban resulted in neurotoxicity. The largest neuroprotective effect was seen with the addition of 100 kIU/mL aprotinin (p-value = 0.0009, n = 4 for each condition). Exposure to all tested doses of argatroban resulted in a significant enhancement of neuronal viability (0.04 μM p-value = 0.0341, 0.4 μM p-value < 0.0001, 4 μM p-value = 0.0128; each n = 8). B. In the presence of a known neuronal insult, 24-hour exposure to 250 μM glutamate, low-dose thrombin enhances neuronal viability (p-value < 0.0001). C. In the presence of a known neuronal insult, 24-hour exposure to 250 μM glutamate, co-application of aprotinin doesn’t significantly enhance neuronal viability, but does appear to somewhat mitigate the detrimental effects of glutamate. The excitotoxic effects of 24 hour exposure to 250 μM glutamate are also shown as a comparison to control conditions (p-value = 0.0138). The bars depicting rescue of cell viability by aprotinin are indistinguishable from control conditions. Only at the aprotinin 100 kIU/mL concentration did the difference reach significance when compared to exposure to glutamate alone (not marked on graph, p = 0.0468).
Image acquisition and processing
After drug exposure, the cells were rinsed with fresh media and then washed in PBS before adding fixative (1% PBS, 4% PFA, 0.1M Sucrose, dH2O) for 20 mins. Coverslips were then rinsed in PBS and TBS50 before permeabilizing the cells in 0.3% Triton X-100/TBS50 for 5 mins. Cells were then rinsed in 0.1% Triton X-100/TBS50 before blocking in blocking buffer (2% BSA, 2% FBS (Sigma), 0.1% Triton X-100, in TBS50) for 1–2 hrs. Coverslips were then rinsed in buffer (2% BSA, 0.1% Triton X-100 in TBS50) and then incubated in primary anti-MAP2 antibody (EMD Millipore; Billerica, MA, USA) at 1:300 at room temperature for 1 hr. Secondary fluorescein goat anti-rabbit IgG (Vector Laboratories, Inc.; Burlingame, CA, USA) was then added at 1:500 in the dark after rinsing for 30 min. Coverslips were rinsed and placed on slides with Vectashield fluorescence protection (Vector Laboratories, Inc.; Burlingame, CA, USA). Neurons on slides were viewed and photographed using fluorescent-enabled microscopes (Olympus IX71, Olympus FV1000, Japan) by viewers blinded to experimental conditions. Similarly blinded experimenters were responsible for analyzing and scoring neuronal extensions using the software package ImagePro Express 6.0 (BioVision Technologies; Exton, PA, USA).
Statistical analysis
Values are expressed as percentage or mean ± SD. Statistical tests were performed with GraphPad InStat (GraphPad Software; La Jolla, CA, USA) and included the t-test and one-way ANOVA combined with post-ANOVA Tukey’s HSD tests for comparisons between groups. p values of < 0.05 are indicated by one asterisk (*), p values of < 0.01 are indicated by two asterisks (**), and three asterisks (***) for < 0.001.
Results
An optimal concentration of thrombin enhances the health of cortical neurons in culture
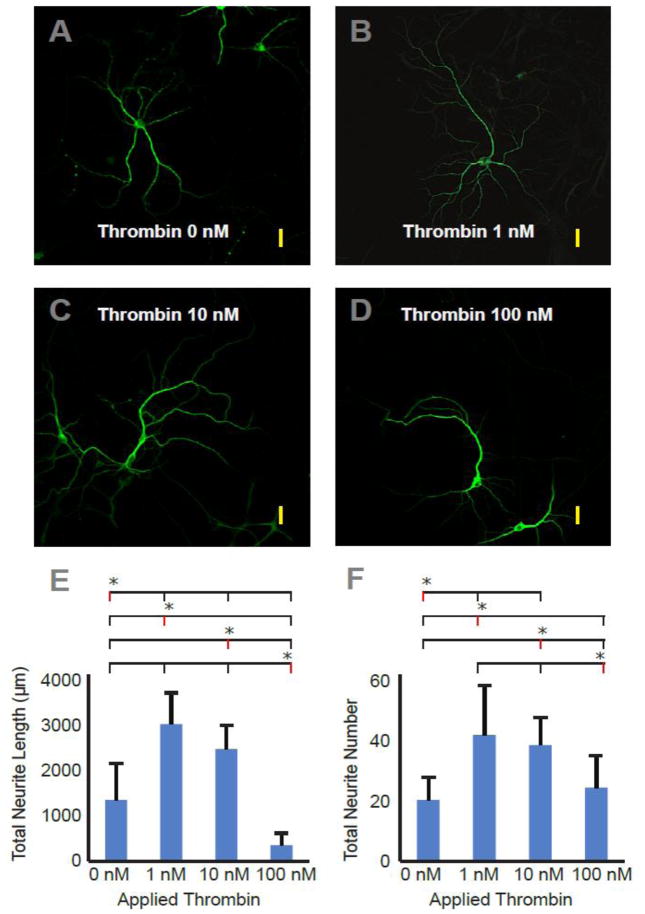
Neurite extension was influenced by thrombin concentration (Figure 1). Immunofluorescent staining of antibodies directed at microtubule-associated protein 2 (MAP2) demonstrate the effect of thrombin on neurons in culture. MAP2 is an important cytoskeletal protein that stabilizes dendritic shape and length by cross-linking microtubules to intermediate filaments. Total neurite lengths per neuron (in microns) for the tested thrombin concentrations were (mean±SD): control, 1315±748; 1 nM, 2941±646; 10 nM, 2403±518; 100 nM, 338±266 (p < 0.0001 (F = 21.96)). Number of branches per neuron for the tested thrombin concentrations were: control, 19.7±7.4; 1 nM, 40.6±15.9; 10 nM, 37.3±8.9; 100 nM, 23.6±10.4 (ANOVA, p < 0.0008 (F = 7.592)). At low and intermediate concentrations, (1nM, 10 nM) thrombin encouraged neurite growth as demonstrated by an increase in both neurite branches and total measured length of dendrites (p < 0.05 (Tukey’s post-test)). The intermediate concentration (10 nM) was indistinguishable from the low concentration with respect to neurite length and total neurite number (Tukey’s post-test analysis). Higher concentrations (100 nM) appeared to either impede neurite extension or cause neurite retraction when compared to control, although the number of neurites remained unchanged.
Figure 1.
The effect of exogenously applied thrombin on cortical neuron culture. Panels A – D. Photomicrographs of 16 – 20 day old cultured primary rat cortical neurons stained for the neuron-specific cytoskeletal protein, MAP-2, after 24 hours of exposure to varied concentrations of thrombin in the nM range (0, 1, 10, 100 nM). Yellow scale bars approximate 40 microns in length. E and F are graphical summaries of total measured neurite length and total number of neurites counted. All morphometric analysis was performed by experimenters blinded to experimental condition. Results are mean ± SD (n = 12, 8, 6, 5). Panel E. P-value is less than 0.0001 for group comparison of total length by one-way repeated measures analysis of variance (ANOVA); asterisks denote significance in post-hoc testing (as compared to the concentration marked with red line). With the exception of 1 nM vs. 10 nM each concentration is significantly different from all other concentrations at a p-value < 0.05 (Tukey’s post-test). Panel F. P-value is less than 0.0008 for group comparison of total number of neurites by one-way repeated measures analysis of variance (ANOVA); asterisks denote significance in post-hoc testing (as compared to the concentration marked with red line). With the exceptions of 1 nM vs. 10 nM and control vs. 100 nM, each concentration is significantly different from all other concentrations at a p-value < 0.05 (Tukey’s post-test).
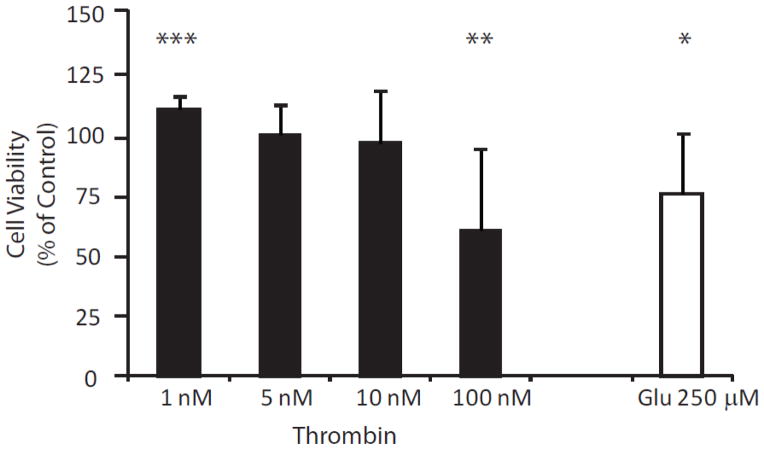
In order to determine if thrombin’s inverted U concentration effects on neurites was reflective of thrombin’s effect on neuronal viability, the MTT assay was used. Similar to our morphometric assays (Figure 1, Panels E and F), Figure 2 also demonstrates an inverted U effect of thrombin on neuronal health. After 24 hour exposure to low concentration (1 nM) thrombin, neuronal survival increased by approximately 10% (110.2±4.4%; p-value = 0.0004) as compared to control (100%), presumably through prevention of attrition of neurons in vitro. Moderate concentrations of thrombin (5 nM and 10 nM) did not significantly alter cell viability (99.5±11.7%, and 96.0±21.2%, respectively) and 24-hour exposure to high concentration of thrombin (100 nM) reduced viability by nearly 40% (60.2±27.5%; p-value = 0.0046). Similarly, lower concentrations of thrombin (0.1 to 0.001 nM) did not have an effect on neuron viability (data not shown). By comparison, 24-hour exposure to 250 μM glutamate decreased the amount of viable cells by about 25% (74.9±21.6% p-value = 0.0135).
Figure 2.
The effect of exogenously applied thrombin on viability of cultured cortical neurons. Viability was measured using the MTT assay (see Methods). Neuroprotection, represented as the presence of more living cells than control (100%), is present after 24 hour exposure to 1 nM thrombin (n = 8; p-value = 0.0004). Moderate concentrations of thrombin (5 nM and 10 nM) are neutral with regard to cell viability and exposure to high dose thrombin (100 nM) is neurotoxic (n = 8; p-value = 0.0046). For comparison, the excitotoxic effects of 24 hour exposure to 250 μM glutamate are also shown (n=8; p-value = 0.0135).
Argatroban and aprotinin have neuroprotective effects comparable to nanomolar concentrations of thrombin
Molecules involved in coagulation dynamics have been actively investigated for neuroprotection (23, 24). The effects of argatroban (a direct thrombin inhibitor) and the antifibinolytic, aprotinin, on neuronal survivability were examined and compared to thrombin using the MTT assay. Aprotinin inhibits plasmin and was previously used in cardiac surgery to decrease the need for blood transfusions until concerns over its renal effects resulted in restrictions in its use. No neurotoxicity was observed at any tested aprotinin concentration, and 100 kIU/mL aprotinin enhanced neuronal viability (50 kIU/mL, 99.4±34.4%; 100 kIU/mL, 133±5.7% (p < 0.001)); and 200 kIU/mL, 110.4±31.6%). Argatroban is used to prevent clot formation in heparin allergic patients or those at risk for heparin-induced thrombocytopenia. At all concentrations of argatroban a mild neuroprotective effect was noted (0.04 μM, 106±6.6% (p < 0.05); 0.4 μM, 116±2.6% (p < 0.0001); 4 μM, 111.7±10.0% (p < 0.05)). In summary, both afforded neuroprotection comparable to low concentrations of thrombin when exogenously applied in the micromolar and kIU/mL concentration range, respectively (Figure 3A).
When neurotoxicity was enhanced with the co-application of glutamate, nanomolar concentrations of thrombin (Figure 3B) produced a more pronounced neuroprotective effect than aprotinin (Figure 3C). In the presence of 24-hour exposure to 250 μM glutamate, low-concentration thrombin not only reversed the excitotoxic effects of glutamate but also maintained its ability to enhance neuronal viability (1 nM, 124.9±1.9% (p < 0.0001); 5 nM, 100.9±18.2%; 10 nM, 103±37.8%; 100 nM, 100.4±35.2%). Interestingly, in the setting of 24-hour exposure to both 250 μM glutamate and 100 nM thrombin, no adverse effects on neuronal health were noted. It was expected that the co-application of glutamate and the neurotoxic level (100 nM) of thrombin (see Figure 2) would have produced profound neuronal destruction in this assay. This result highlights the complex relationship of thrombin and neuronal health (see Discussion).
In the presence of the same excitotoxic insult (Figure 3C), aprotinin protected neurons against glutamate-induced neurotoxicity but did not enhance neuron viability above control conditions. In concordance with the experiments described in Figure 2, 24-hour exposure to glutamate resulted in a similar reduction in neuron viability relative to control (81.3±16.2%, p < 0.05)). At all tested concentrations, aprotinin appeared to rescue viability from damage due to glutamate, but in contrast to low-concentration thrombin (1 nM), did not result in an increase in cell viability (50 kIU/mL, 110.6±46.7%; 100 kIU/mL, 115.6±39.8%; 200 kIU/mL, 107.3±31.9%).
Serum levels of antithrombin III result in decreased neuron viability
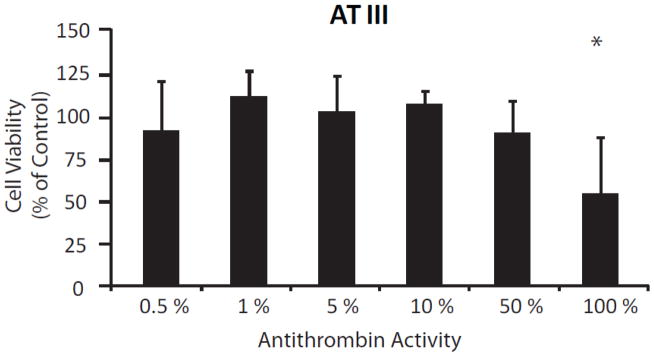
In order to determine if an endogenous thrombin inhibitor also exhibits neuroprotection, exogenous application of antithrombin III (AT III), the most abundant endogenous thrombin inhibitor, was investigated via MTT assays. AT III demonstrated no significant effects on cortical neuron survival after 24-hour exposure at concentrations 50% or lower than serum concentrations (Figure 4). However, a decrease in neuronal viability of nearly 45% (55.7±26.7%; p-value = 0.0452) was apparent when cultured neurons were exposed to normal serum concentrations (100%). This is in contrast to argatroban and aprotinin which showed an increase in cell viability. Although it is possible that serum levels of AT III exert neurotoxic effects on cultured neurons, it seems more plausible that this is evidence to support the notion that our cultured cortical neurons are producing and secreting a low-concentration of thrombin in vitro. Additionally, it is possible that the concentrations of argatroban and aprotinin applied resulted in a thrombin concentration within the optimum range. Although we did not directly measure thrombin levels in our culture media due to the difficulty in quantifying this labile enzyme, it has previously been shown by others that neurons express thrombin in vitro (25).
Figure 4.
The effect of exogenously applied antithrombin (AT III) on viability of cultured cortical neurons. Viability was measured using the MTT assay (see Methods). A neutral effect on neuron survival is present after 24 hour exposure to antithrombin at levels equivalent to 0–50% of normal serum activity (n = 4 for each condition). Exposure to normal serum levels of antithrombin result in a significant reduction in neuronal viability (n = 4; p-value = 0.045).
Thrombin receptor activation mediates neuroprotection and fails to produce neurotoxicity at high concentrations
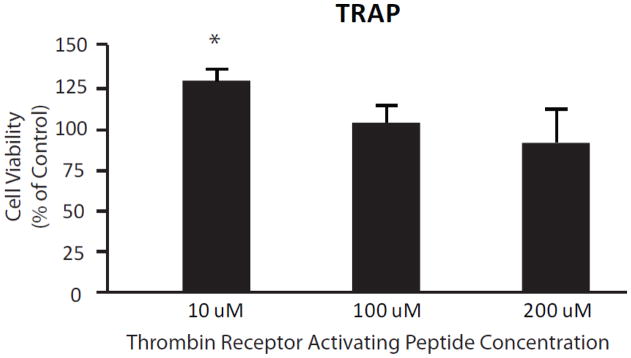
The PAR1 agonist, TRAP (SFLLRN), was used to attempt to identify the role of the thrombin receptor in mediating thrombin’s effects on neurons. We attempted to saturate the thrombin receptor with micromolar concentrations of TRAP, and found that neurons incubated for 24 hours with TRAP did not exhibit any detrimental effects on neuron survival (Figure 5). In fact, activation of the thrombin receptor by 10 μM TRAP appeared to be neuroprotective (116±7.9%; p-value = 0.0251). Higher concentrations of TRAP had no significant effects on cell viability (100 μM, 96.7±12.2%; 200 μM, 90.1±20.9%) (Figure 5). This is in contrast to the results seen in this assay by applying thrombin in nanomolar concentrations (see Figure 2); thereby, highlighting a distinction between thrombin’s receptor-dependent and receptor independent effects.
Figure 5.
The effect of thrombin receptor activation on viability of cultured cortical neurons. Viability was measured using the MTT assay (see Methods). Activation of the thrombin receptor by 10 μM TRAP (thrombin receptor activating peptide) appears to be neuroprotective (n = 4; p-value = 0.0251), while higher concentrations of TRAP are neutral with respect to viability as measured by the MTT assay (n = 4 for each condition).
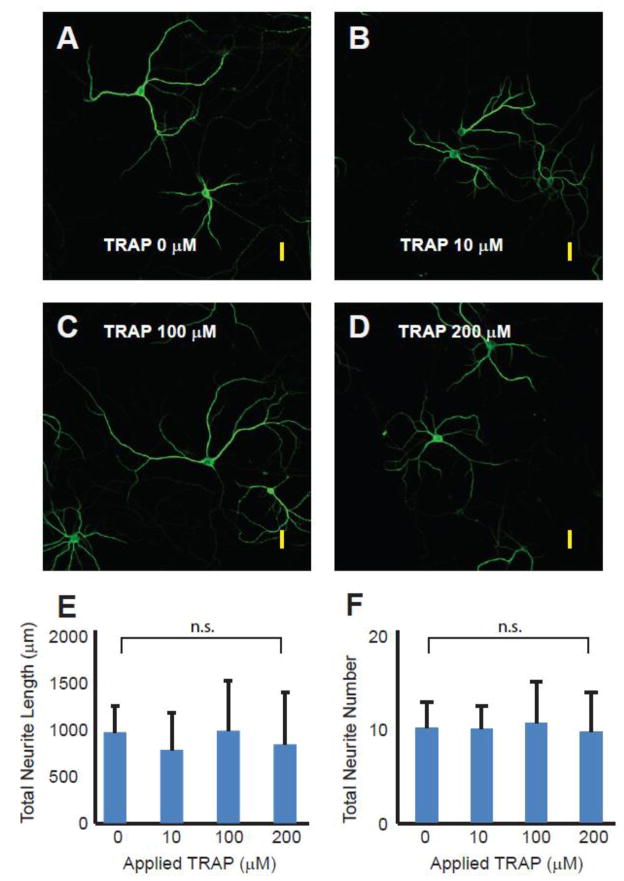
Thrombin Receptor Activating Peptide (TRAP) does not inhibit neurite extension
We examined the effects of these same concentrations of TRAP using the more sensitive indicator of neuronal health, morphometric analysis of dendritic extensions (Figure 6). In contrast to the effects of thrombin on dendritic growth, TRAP had a neutral effect. The total neurite lengths per neuron (in microns) for the tested TRAP concentrations were (mean±SD): control, 896±261; 10 μM, 719.2±371; 100 μM, 908.6±495; 200 μM, 774.9±514 (ANOVA, p > 0.98 (F = 0.066)). The number of branches per neuron for the tested TRAP concentrations were (mean +/−SD): control, 10.7±2.88; 10 μM, 10.5±2.67; 100 μM, 11.2±4.66; 200 μM, 10.2±.4.44 (ANOVA, p > 0.84 (F = 0.2842)).
Figure 6.
The effect of exogenously applied thrombin receptor activating peptide (TRAP) on cortical neuron culture. A – D. Photomicrographs of 16 – 20 day old cultured primary rat cortical neurons stained for the neuron-specific cytoskeletal protein, MAP-2, after 24 hours of exposure to varied concentrations of TRAP (0, 10, 100, 200 nM). Yellow scale bars approximate 40 microns in length. E and F are graphical summaries of total measured neurite length and total number of neurites counted. All morphometric analysis was performed by experimenters blinded to experimental condition. Results are mean ± SD (n = 6, 6, 5, 5). P-value is greater than 0.98 for group comparison of total length and greater than 0.84 for group comparison of total number of neurites by one-way repeated measures analysis of variance (ANOVA).
Discussion
This study demonstrates that 1) thrombin shows an inverted U effect on viability and morphology of rat cortical neuron enriched cultures, and 2) thrombin’s neurotoxic effects are complex, but do not appear to depend on signaling through PAR receptors. Using two different assays, we found that exposing cultured neurons to low concentrations of thrombin (1 nM) enhances neurite growth and branching, neuron viability, and protects against excitotoxic damage from glutamate exposure. In contrast, higher concentrations of thrombin (100 nM) are detrimental to neuronal health as evidenced by viability assays and inhibition of neurite growth. These concentrations are within the physiologic range of circulating thrombin which is estimated to vary from <1 nM to over 500 nM during hemostasis (15).
Previous studies have mainly focused on the detrimental effects of thrombin on neurons and the brain (26–29) and the beneficial effects of suppression of thrombin activity (23, 30, 31). However, thrombin’s role in regulating both neuronal development and the nervous system’s response to injury has also been highlighted (13, 32, 33). Additionally, thrombin has shown some potential therapeutic value via preconditioning (34). Our demonstration of a non-toxic neuroprotection mediated by low-concentration thrombin (1 nM) aligns well with studies on the peripheral nervous system, which showed important effects of low-concentration thrombin in neuromuscular junction development (35) and in promoting motoneuron regeneration after injury (32). An inverted U effect of thrombin concentration on brain cells experiencing hypoglycemic stress has been reported in the past (3); however our study is the first demonstration of neuroprotective effects of thrombin on viability and microarchitecture in primary cortical neurons (glial-reduced cultures) in response to excitotoxic damage.
Results from co-application of thrombin and glutamate were both expected and surprising. Low concentrations of thrombin blocked excitotoxic effects of glutamate. This may be consistent with previous observations that activated protein C (APC) blocks glutamate-induced neurotoxicity in cultured hippocampal and cortical neurons (36) since binding of thrombin is necessary to convert the inactive zymogen (protein C) to the activated form. Thus, low concentration thrombin might be expected to provide protection from glutamate. It is less clear why a high concentration of thrombin (100 nM) also blocked the excitotoxic effects of glutamate in our cells. It may be that neuronal mechanisms of thrombin toxicity are altered in the presence of glutamate such that protective effects of APC predominate.
Our experiments with the known inhibitors of thrombin (argatroban and AT III) and other serine proteases (aprotinin) provide a context to evaluate thrombin’s dual role in neuroprotection. First, micromolar application of the thrombin receptor agonist peptide, TRAP, in contrast to thrombin toward the high range of serum concentration (100 nM), did not adversely affect neuronal viability. TRAP did not reduce neurite outgrowth or branching and at low concentrations even enhanced neuronal viability in our MTT assay. Because PAR-1 activation did not completely reproduce the effects of exogenously applied thrombin on neuronal health, we suggest a need to delineate between thrombin’s cellular effects (mediated through activation of PAR-1) and thrombin’s general enzymatic effects (which may involve proteolytic activity at sites other than its receptor). Second, we provide evidence that the efficacy of low concentrations of thrombin in preserving neuronal health is comparable to the serpin aprotinin and to the direct thrombin inhibitor, argatroban. These effects mirror those by exogenous activation of the thrombin receptor via TRAP. As a direct thrombin inhibitor, argatroban blocks the active site of thrombin, and previous reports of neuroprotection by argatroban have focused on reversing thrombin’s detrimental effects (30). Our observations contribute to this body of work by demonstrating no adverse neuronal consequences by PAR-1 receptor activation (at concentrations as high as 200 μM) in brain cell cultures enriched for cortical neurons. This is consistent with thrombin’s protective effects being mediated through PAR-1 and its neurotoxic effects due possibly to off-target proteolysis by thrombin in high concentration. However, activation of the PAR-1 receptor by our highest concentrations of TRAP does not result in increased neuroprotection. The endogenous substance AT III, in contrast to argatroban, did not confer neuroprotection. As concentration of exogenous AT III increases, viability may be affected in our in vitro model through thrombin independent effects.
Our experiments cannot directly answer the mechanism of neurotoxicity via application of thrombin at high concentrations. Perhaps some of thrombin’s neurotoxic effects in high concentrations are mediated by less specific proteolytic cleavage of other membrane proteins besides the PAR-1 receptor. Future experiments using these proteolytic inhibitors in combination with accurate measures of thrombin concentration in PAR-1 knockout animals could aid in the investigation of mechanisms of thrombin’s neurotoxic effects independent of the receptor. Another mechanism to explore is calcium regulation in the cellular microenvironment, because both activation of PAR-1 (37) and glutamate (38)are known to increase intracellular calcium.
Despite preclinical evidence of potent neuroprotection, drugs that affect coagulation have yet to be clinically investigated for neuroprotection during ischemic stroke or other neurologic disorders. Our results must also be considered in light of the limitations of in vitro assays. We recognize that thrombin concentration is dynamic in the brain especially in the setting of injury, however we attempted to mimic physiological conditions by adding concentrations of exogenous thrombin in the measured physiologic range (15). At present, technical limitations prevent accurate measurement of rat thrombin produced in these cortical neuron cultures. However, we are fortunate to be able to apply species specific antithrombin III to our cultures. Based on other published results (39) we consider healthy neurite extension and branching as a sign of good neuronal health in our in vitro system focused on matured neurons. However developmental effects are difficult to isolate in this system and our work should not be considered with regard to thrombin’s effects on neuronal development. Care should be taken to not confuse our results in the context of neuroprotection by neuroserpin. While neuroserpin is a serine protease inhibitor actively investigated for neuroprotective potential, it does not appear to affect thrombin activity but instead binds and inactivates plasminogen activators like tPA/uPA (40). Similarly, although the MTT assay has been used by our lab and several others to assess neuronal viability in vitro, it gives no information about whether the cell death is due to necrosis or apoptosis. Future experiments that track specific markers of apoptosis or necrosis would be necessary to distinguish between these two possibilities.
With appropriate regulation, thrombin plays a normal and protective role in the brain. In situations of blood brain barrier compromise such as trauma, hemorrhagic stroke, cerebral ischemia, or status epilepticus, excess amounts of thrombin from local sources or from the systemic circulation can result in a worsening of pathologic effects on the neurons and glial cells. Thrombin stimulates astrocyte proliferation, angiogenesis, and attracts inflammatory cells. This leads to the inflammation and glial scar formation often seen in head injury. Less specific enzymatic activity of excess thrombin that does not involve its receptor could have direct negative consequences on neuronal function as suggested by our viability assays and morphometric results. Activation of thrombin receptors combined with specific inhibition of thrombin’s enzymatic activity (via direct inhibitors such as argatroban) could capitalize on the developmental regulatory functions and neuroprotective effects of thrombin receptor activation while minimizing pro-inflammatory and neurotoxic features of thrombin. Future translational studies could focus on the therapeutic combination of specific manipulation of thrombin concentration with direct receptor activation.
Acknowledgments
This work was supported by a grant (GRC 001) from the Atlanta Research and Education Foundation (AREF) and from departmental funds.
We gratefully acknowledge training in confocal microscopy by Shaojin You, MD, PhD; Director of Histology Core, Research Division, Atlanta VA Medical Center, Atlanta, Georgia. We also gratefully acknowledge technical help from Mayuri Maddi, Britany Raymond, and Cari Fritz-French Lessing.
Footnotes
Preliminary Results regarding this work were presented at ASA 2011, Chicago, Illinois.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Esmon CT. The impact of the inflammatory response on coagulation. Thrombosis research. 2004;114(5–6):321–7. doi: 10.1016/j.thromres.2004.06.028. Epub 2004/10/28. eng. [DOI] [PubMed] [Google Scholar]
- 2.Garcia PS, Gulati A, Levy JH. The role of thrombin and protease-activated receptors in pain mechanisms. Thrombosis and haemostasis. 2010 Jun;103(6):1145–51. doi: 10.1160/TH09-12-0848. Epub 2010/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995 Jul;15(7 Pt 2):5389–401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. Epub 1995/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turgeon VL, Milligan CE, Houenou LJ. Activation of the protease-activated thrombin receptor (PAR)-1 induces motoneuron degeneration in the developing avian embryo. Journal of neuropathology and experimental neurology. 1999 May;58(5):499–504. doi: 10.1097/00005072-199905000-00009. Epub 1999/05/20. eng. [DOI] [PubMed] [Google Scholar]
- 5.Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. J Neurosci. 2000 Jun 15;20(12):4582–95. doi: 10.1523/JNEUROSCI.20-12-04582.2000. Epub 2000/06/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deschepper CF, Bigornia V, Berens ME, Lapointe MC. Production of thrombin and antithrombin III by brain and astroglial cell cultures. Brain research Molecular brain research. 1991 Oct;11(3–4):355–8. doi: 10.1016/0169-328x(91)90045-y. Epub 1991/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 7.Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991 Apr;6(4):575–81. doi: 10.1016/0896-6273(91)90060-d. Epub 1991/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: implications for disease pathogenesis. Journal of Alzheimer’s disease : JAD. 2006 Mar;9(1):51–8. doi: 10.3233/jad-2006-9105. Epub 2006/04/22. eng. [DOI] [PubMed] [Google Scholar]
- 9.Shavit E, Beilin O, Korczyn AD, Sylantiev C, Aronovich R, Drory VE, et al. Thrombin receptor PAR-1 on myelin at the node of Ranvier: a new anatomy and physiology of conduction block. Brain : a journal of neurology. 2008 Apr;131(Pt 4):1113–22. doi: 10.1093/brain/awn005. Epub 2008/02/27. eng. [DOI] [PubMed] [Google Scholar]
- 10.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. Journal of thrombosis and haemostasis : JTH. 2005 Aug;3(8):1800–14. doi: 10.1111/j.1538-7836.2005.01377.x. Epub 2005/08/17. eng. [DOI] [PubMed] [Google Scholar]
- 11.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiological reviews. 2004 Apr;84(2):579–621. doi: 10.1152/physrev.00028.2003. Epub 2004/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 12.Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005 Apr 20;25(16):4082–90. doi: 10.1523/JNEUROSCI.4306-04.2005. Epub 2005/04/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito T, Bunnett NW. Protease-activated receptors: regulation of neuronal function. Neuromolecular medicine. 2005;7(1–2):79–99. doi: 10.1385/NMM:7:1-2:079. Epub 2005/07/30. eng. [DOI] [PubMed] [Google Scholar]
- 14.Garcia A, Prabhakar S, Hughan S, Anderson TW, Brock CJ, Pearce AC, et al. Differential proteome analysis of TRAP-activated platelets: involvement of DOK-2 and phosphorylation of RGS proteins. Blood. 2004 Mar 15;103(6):2088–95. doi: 10.1182/blood-2003-07-2392. Epub 2003/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 15.Allen GA, Wolberg AS, Oliver JA, Hoffman M, Roberts HR, Monroe DM. Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. Journal of thrombosis and haemostasis : JTH. 2004 Mar;2(3):402–13. doi: 10.1111/j.1538-7933.2003.00617.x. Epub 2004/03/11. eng. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Kato K. Determination of antithrombin III by sandwich enzymeimmunoassay technique. Thrombosis research. 1981 Apr 1–15;22(1–2):67–74. doi: 10.1016/0049-3848(81)90309-1. Epub 1981/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Szlam F, Levy JH, Tanaka KA. Tissue plasminogen activator and thrombin generation measurements using the Thrombinoscope. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2006 Oct;17(7):603–4. doi: 10.1097/01.mbc.0000245296.87515.fd. Epub 2006/09/22. eng. [DOI] [PubMed] [Google Scholar]
- 18.Abildgaard U, Lie M, Odegard OR. Antithrombin (heparin cofactor) assay with “new” chromogenic substrates (S-2238 and Chromozym TH) Thrombosis research. 1977 Oct;11(4):549–53. doi: 10.1016/0049-3848(77)90208-0. Epub 1977/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Sun HY, Szlam F, Levy JH, Csete ME, Tanaka KA. Antifibrinolytic agents reduce tissue plasminogen activator-mediated neuronal toxicity in vitro. Acta Anaesthesiol Scand. 2009 Mar;53(3):325–31. doi: 10.1111/j.1399-6576.2008.01858.x. Epub 2009/02/27. eng. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka KA, Szlam F, Katori N, Sato N, Vega JD, Levy JH. The effects of argatroban on thrombin generation and hemostatic activation in vitro. Anesth Analg. 2004 Nov;99(5):1283–9. doi: 10.1213/01.ANE.0000134685.75813.EB. table of contents. Epub 2004/10/27. eng. [DOI] [PubMed] [Google Scholar]
- 21.Kaech S, Banker G. Culturing hippocampal neurons. Nature protocols. 2006;1(5):2406–15. doi: 10.1038/nprot.2006.356. Epub 2007/04/05. eng. [DOI] [PubMed] [Google Scholar]
- 22.Giordano G, Hong S, Faustman EM, Costa LG. Measurements of cell death in neuronal and glial cells. Methods Mol Biol. 2011;758:171–8. doi: 10.1007/978-1-61779-170-3_11. Epub 2011/08/05. eng. [DOI] [PubMed] [Google Scholar]
- 23.Chen B, Friedman B, Whitney MA, Winkle JA, Lei IF, Olson ES, et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J Neurosci. 2012 May 30;32(22):7622–31. doi: 10.1523/JNEUROSCI.0369-12.2012. Epub 2012/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwata Y, Okamura T, Ishibashi N, Zurakowski D, Lidov HG, Jonas RA. Optimal dose of aprotinin for neuroprotection and renal function in a piglet survival model. The Journal of thoracic and cardiovascular surgery. 2009 Jun;137(6):1521–9. doi: 10.1016/j.jtcvs.2008.06.049. discussion 9. Epub 2009/05/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arai T, Miklossy J, Klegeris A, Guo JP, McGeer PL. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. Journal of neuropathology and experimental neurology. 2006 Jan;65(1):19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. Epub 2006/01/18. eng. [DOI] [PubMed] [Google Scholar]
- 26.Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. Journal of neurosurgery. 1996 Jan;84(1):91–6. doi: 10.3171/jns.1996.84.1.0091. Epub 1996/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 27.Motohashi O, Suzuki M, Shida N, Umezawa K, Sugai K, Yoshimoto T. Hirudin suppresses the invasion of inflammatory cells and the appearance of vimentin-positive astrocytes in the rat cerebral ablation model. Journal of neurotrauma. 1997 Oct;14(10):747–54. doi: 10.1089/neu.1997.14.747. Epub 1997/12/31. eng. [DOI] [PubMed] [Google Scholar]
- 28.Nishino A, Suzuki M, Ohtani H, Motohashi O, Umezawa K, Nagura H, et al. Thrombin may contribute to the pathophysiology of central nervous system injury. Journal of neurotrauma. 1993 Summer;10(2):167–79. doi: 10.1089/neu.1993.10.167. Epub 1993/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 29.Suo Z, Citron BA, Festoff BW. Thrombin: a potential proinflammatory mediator in neurotrauma and neurodegenerative disorders. Current drug targets Inflammation and allergy. 2004 Mar;3(1):105–14. doi: 10.2174/1568010043483953. Epub 2004/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009 Apr;40(4):1530–2. doi: 10.1161/STROKEAHA.108.531699. Epub 2009/02/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Zhao R, Qi J, Cong Y, Wang D, Liu T, et al. The expression and the role of protease nexin-1 on brain edema after intracerebral hemorrhage. Journal of the neurological sciences. 2008 Jul 15;270(1–2):172–83. doi: 10.1016/j.jns.2008.03.010. Epub 2008/04/30. eng. [DOI] [PubMed] [Google Scholar]
- 32.Smirnova IV, Zhang SX, Citron BA, Arnold PM, Festoff BW. Thrombin is an extracellular signal that activates intracellular death protease pathways inducing apoptosis in model motor neurons. Journal of neurobiology. 1998 Jul;36(1):64–80. Epub 1998/07/11. eng. [PubMed] [Google Scholar]
- 33.Turgeon VL, Salman N, Houenou LJ. Thrombin: a neuronal cell modulator. Thrombosis research. 2000 Sep 1;99(5):417–27. doi: 10.1016/s0049-3848(00)00300-5. Epub 2000/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 34.Henrich-Noack P, Striggow F, Reiser G, Reymann KG. Preconditioning with thrombin can be protective or worsen damage after endothelin-1-induced focal ischemia in rats. Journal of neuroscience research. 2006 Feb 15;83(3):469–75. doi: 10.1002/jnr.20746. Epub 2006/01/07. eng. [DOI] [PubMed] [Google Scholar]
- 35.Faraut B, Ravel-Chapuis A, Bonavaud S, Jandrot-Perrus M, Verdiere-Sahuque M, Schaeffer L, et al. Thrombin reduces MuSK and acetylcholine receptor expression along with neuromuscular contact size in vitro. Eur J Neurosci. 2004 Apr;19(8):2099–108. doi: 10.1111/j.1460-9568.2004.03300.x. Epub 2004/04/20. eng. [DOI] [PubMed] [Google Scholar]
- 36.Gorbacheva L, Davidova O, Sokolova E, Ishiwata S, Pinelis V, Strukova S, et al. Endothelial protein C receptor is expressed in rat cortical and hippocampal neurons and is necessary for protective effect of activated protein C at glutamate excitotoxicity. Journal of neurochemistry. 2009 Nov;111(4):967–75. doi: 10.1111/j.1471-4159.2009.06380.x. Epub 2009/09/29. eng. [DOI] [PubMed] [Google Scholar]
- 37.Smirnova IV, Vamos S, Wiegmann T, Citron BA, Arnold PM, Festoff BW. Calcium mobilization and protease-activated receptor cleavage after thrombin stimulation in motor neurons. Journal of molecular neuroscience : MN. 1998 Feb;10(1):31–44. doi: 10.1007/BF02737083. [DOI] [PubMed] [Google Scholar]
- 38.Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD. Researching glutamate - induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Frontiers in cellular neuroscience. 2015;9:91. doi: 10.3389/fncel.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Mar 25;29(12):3948–55. doi: 10.1523/JNEUROSCI.5595-08.2009. Epub 2009/03/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterwalder T, Cinelli P, Baici A, Pennella A, Krueger SR, Schrimpf SP, et al. The axonally secreted serine proteinase inhibitor, neuroserpin, inhibits plasminogen activators and plasmin but not thrombin. J Biol Chem. 1998 Jan 23;273(4):2312–21. doi: 10.1074/jbc.273.4.2312. Epub 1998/01/27. eng. [DOI] [PubMed] [Google Scholar]