Abstract
Primary central nervous system (CNS) lymphomas are relatively rare with the most common subtype being diffuse large B-cell lymphoma. Primary CNS T-cell lymphomas (PCNSTL) account for <5% of CNS lymphomas. We report the clinical, morphologic, immunophenotypic and molecular characteristics of 18 PCNSTLs. Fifteen cases were classified as peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS), 2 of which were of γδ T-cell derivation and 1 was TCR silent; there was 1 anaplastic large cell lymphoma (ALCL), ALK-positive and 2 ALCL, ALK-negative. Median age was 58.5 years (range 21-81), with M:F ratio of 11:7. By imaging 15 patients had supratentorial lesions. Regardless of subtype, necrosis and perivascular cuffing of tumor cells were frequently observed (11/18 cases). CD3 was positive in all cases but 1; 10/17 were CD8-positive and 5/17 were CD4-positive. Most cases studied had a cytotoxic phenotype with expression of TIA1 (13/15) and granzyme-B (9/13). PCR analysis of TRG rearrangement confirmed a T-cell clone in 14 cases with adequate DNA quality. Next Generation Sequencing (NGS) showed somatic mutations in 36% of cases studied; 2 had more than one mutation and none showed overlapping mutations. These included mutations in DNMT3A, KRAS, JAK3, STAT3, STAT5B, GNB1 and TET2 genes, genes implicated previously in other T-cell neoplasms. The outcome was heterogeneous; 2 patients are alive without disease, 4 are alive with disease and 6 died of disease. In conclusion, PCNSTL are histologically and genomically heterogeneous with frequent phenotypic aberrancy and a cytotoxic phenotype in most cases.
Keywords: T-cell lymphoma, central nervous system, next generation sequencing, gamma-delta T-cells, T-cell clonality, molecular diagnostics
INTRODUCTION
Primary central nervous system (CNS) lymphoma (PCNSL) is a relatively rare disease accounting for 2-6% of all primary brain malignancies and 1-2% of non-Hodgkin lymphoma (NHL) 1-5. These lymphomas are defined as being confined to the brain, spinal cord or the eye without extra CNS or lymph node manifestations at presentation (1-6). However, late relapses outside the CNS can occur 3, 6 . While diffuse large B-cell lymphoma (DLBCL) is the most common type of PCNSL (with primary DLBCL of CNS enjoying a separate category in the current WHO classification), other lymphomas including Burkitt lymphoma, MALT lymphomas (dura), follicular lymphoma and T-cell lymphomas, can present with intracranial disease 2, 3, 7, 8. The reported percentage of PCNSL of T-cell derivation (PCNSTL) varies from 3.6% (France), 8.5% (Japan) to 2% (8 cases out of 370 patients) in the largest PCNSL series from the western world 9. Choi et al. described a somewhat higher percentage of T-cell lymphomas (16.7%, 7/42 cases) in their series of primary CNS lymphomas from Korea 10.
The most recently published large case series from the International Primary CNS Lymphoma Collaborative Group described 45 patients with PCNSTL1. In this series, 20 patients (44%) had Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Twenty-six patients (58%) had involvement of cerebral hemispheres and 16 (36%) had lesions of deeper brain sites. The median disease-specific survival (DSS) was 25 months, and multivariate analyses demonstrated an association of better ECOG performance status and methotrexate use with longer survival. However, a detailed morphologic and immunophenotypic analysis is not available. In a separate study of primary CNS lymphoma other than DLBCL, outcomes for 7 patients with peripheral T-cell lymphomas (PTCL) were described; these T-cell lymphomas demonstrated similar or favorable clinical outcomes as compared to previously reported data on DLBCLs 7. While several case series (referenced above) have made valuable contributions to the understanding of the clinical characteristics of PCNSTL, an extensive pathologic analysis/description is lacking. Several case reports and smaller case series have described the pathology and immunophenotype in varying detail11-26.
The goal of this study was to describe in a comprehensive manner not only the clinical characteristics but also the histological, immunophenotypic and molecular characteristics of 18 PCNSTLs identified from the consultation files of the hematopathology division of the authors’ institution.
MATERIALS AND METHODS
Case selection
Nineteen cases of PCNSTLs were identified from the pathology database of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, between 2000 and 2014. Eighteen cases were submitted in consultation as brain biopsies. One additional autopsy case was contributed by 1 of the coauthors (DCM). None of the patients had lymphadenopathy or evidence of extra CNS disease at the time of CNS presentation. One patient had a soft tissue mass involved by PTCL 3 months after diagnosis of the CNS lesion; given the close proximity of these lesions, this case was excluded. This study was approved by the Institutional Review Board of the National Cancer Institute.
Immunohistochemistry studies
Immunohistochemical studies were performed on available formalin-fixed paraffin-embedded tissue (FFPE) sections using the following antibodies: CD2, CD3, CD4, CD5, CD7, CD8, CD30, CD56, βF1, TCRγ, TIA1, granzyme-B, perforin, LMP1, MIB-1 and ALK1. The panel of antibodies, clone, dilution and source are listed in Table 1. A case was scored as positive if more than 50% of the atypical lymphoid cells expressed the antigen. MIB-1 was scored as low (< 33%), moderate (33-66 %), and high (67-100%) based the percent of lymphoid cells positive.
Table 1.
Antibodies used in the immunophenotypic analysis
| Antigen | Clone | Dilution | Source |
|---|---|---|---|
| CD3 | Polyclonal | 1:100 | Dako |
| CD4 | 1F6 | 1:40 | Novocastra |
| CD8 | C8/144B | 1:50 | Dako |
| CD2 | AB75 | 1:160 | Novocastra |
| CD5 | 4C7 | 1:100 | Novocastra |
| CD7 | CD7-272 | 1:50 | Novocastra |
| βF1 | 8A3 | 1:20 | Endogen |
| TCRγ | γ3.20 | 1:100 | Thermo Scientific |
| CD30 | 1G12 | 1:50 | Novocastra |
| ALK1 | ALK1 | 1:400 | Dako |
| TIA1 | 2G9A10FS | 1:1000 | Immunotech |
| Granzyme-B | GrB-7+D170 | 1:100 | Monosan |
| Perforin | KM585 PI-8 | 1:10 | Vector |
| CD56 | 1B6 | 1:50 | Novocastra |
| LMP1 | CS1-4 | 1:400 | Dako |
| Ki-67 | MIB-1 | 1:50 | Abcam |
In situ hybridization for Epstein Barr virus (EBV) encoded RNA (EBER)
In situ hybridization was performed on FFPE tissue, using EBER1 DNP probe supplied by Ventana on an automated stainer (Ventana-Benchmark XT, Tucson, AZ). The ISH iView blue plus system with alkaline phosphatase and nitroblue tetrozolium and 5-bromo-4-chloro-3-indolyl phosphate substrate, with Fast Red contrast was used for visualization, with relevant controls.
Molecular studies
For T-cell receptor gamma (TRG) rearrangement, DNA was extracted from FFPE tissue blocks and either 1) single multiplexed PCR was done with primers directed against all known Vg family members, and the Jg1/2, JP1/2 and JP joining segments 27 or 2) three separate reactions were performed, one with primers Vg101, Vg11 and Jg12 (set 1), a second with primers Vg 101, Vg11 and Jp12 (set 2), and a third with primers Vg9 and Jg12 (set 3), the first 2 performed according to the method of Slack et al 28, and the third according to a validated in house method. Products were analyzed either via acrylamide gel electrophoresis or by capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). The results were interpreted as polyclonal, restricted, or clonal. The “restricted” TRG category was defined as an abnormal rearrangement pattern with small peaks that did not meet criteria for monoclonality, as previously described29.
Mutational analysis
DNA samples were analyzed for somatic mutations within genes previously implicated in the pathogenesis of mature T-cell lymphomas using a targeted next generation sequencing (NGS) strategy30. The mutation panel includes targeted regions of 38 genes previously reported to be mutated in T-cell lymphomas as well as targeted regions of genes involved in T-cell signaling focused on the JAK/STAT signaling pathway. The amplicon libraries were generated with two custom primer pools (total 227 amplicons) and were sequenced on an Ion Torrent Personal Genome Machine (PGM) (Life Technologies). The paraffin-embedded tissue sections were macrodissected to enrich for tumor cells with at least 20% tumor content. DNA was extracted using the Qiagen QIAamp DNA FFPE Tissue Kit and performed on a QIAcube according to the instructions of the manufacturer. Further details regarding the NGS methods (SDC1) and a list of the genes analyzed (SDC2) are included in Supplemental Digital Content files.
RESULTS
Clinical features
Eighteen confirmed cases of PCNSTL were identified. The clinical features of these cases are summarized in Table 2. There were 11 males and 7 females; with a median age of 58.5 years (range 21-81). The clinical manifestations of patients ranged from headache, aphasia, facial paralysis, facial and upper limb sensory abnormalities, speech abnormalities, ataxia, leg weakness, and difficulties in short term memory etc. By imaging studies, 15 patients had supratentorial lesions, 3 had cerebellar involvement. Solitary tumor was seen in 9 cases, multiple masses in 8 and one showed diffuse enhancement of meninges. None of the patients presented with or developed lymphadenopathy at any time point. One case of ALCL, ALK-positive diagnosed at autopsy (Case 16), had extensive dural, leptomeningeal and spinal disease. At autopsy one out of several lymph nodes tested showed rare scattered CD30 and ALK positive cells, which were interpreted as secondary lymph node involvement by virtue of the high burden of the disease in the CNS and the lack of lymphadenopathy or histologically confirmed disease elsewhere.
Table 2.
Clinical Features, Imaging, Treatment and Outcome of PCNSTLs
| Final No. | Age | Sex | Clinical presentation | Imaging | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | 21 | M | Headache | Solitary, right occipital mass (2 cm) | Steroids, Thiotepa+ HD MTX + XRT | na |
| 2 | 61 | M | NA | Solitary frontal mass | na | DOD |
| 3 | 81 | M | NA | Solitary, right occipital mass (2 cm) | Steroids | AwoD (64 mo) |
| 4 | 54 | M | Transient right facial and upper limb sensory symptoms, headaches, facial asymmetry and speech difficulties | Solitary, left frontal mass | HD MTX + XRT | AwD (47 mo) |
| 5 | 60 | M | Headache | Solitary, left cerebellar mass (2.2 cm) | Chemotherapy | DOD (3 mo) |
| 6 | 57 | M | Difficulties short term memory, ataxia, right leg weakness | Multiple lesions throughout the brain, largest left parietal lobe (3.5 cm) | na | na |
| 7 | 69 | M | na | Solitary, right parietal mass | MTX + AraC +Leucovorin + Procarbazine hydrocholoride | AwD |
| 8 | 81 | M | Altered mental status | Right occipital and temporal mass | 2×MTX + bendamustine | AwD (4 mo) |
| 9 | 63 | F | Left sided weakness | Periventricular and striatocapsular abnormalities | no treatment | DOD |
| 10 | 42 | F | Seizures | Right frontal and temporal masses | Dexametasone + HD AraC + 3×MTX + 17 × XRT | AwD (5 mo) |
| 11 | 21 | F | Pregnant, headaches, behavior changes | Solitary parietal mass (7×7×5 cm) | na | na |
| 12 | 67 | F | Aphasia and facial paralysis | Multiple bilateral frontal and occipital masses | 6× HD MTX + XRT | AwoD (56mo) |
| 13 | 31 | M | Seizure and slow (2 yrs) decline in mental function, weight loss, confusion | Bilateral temporal lobes enhancement | na | na |
| 14 | 56 | F | Headache | Solitary right frontal mass | na | na |
| 15 | 57 | M | Progressive neurologic decline | Multiple lesions in the basal ganglia, midbrain, brachium pontis and cerebellar hemispheres | na | DOD |
| 16 | 43 | M | Fever, nausea, vomiting (1 mo) | Multiple meningeal lesions (dural, leptomeningeal, spinal, superficial cortical parietal, right cerebellum and medulla involvement) | na | DOD |
| 17 | 61 | F | Weakness right superior extremity, paresthesia, mild paralysis | Diffuse enhancement | Dexamethasone | DOD (1 mo) |
| 18 | 62 | F | History multiple sclerosis, left lower extremity weakness (3 mo) | Solitary right frontal mass | na | na |
Abbreviations: HD- high dose; XRT- radiation; IT- intrathecal; MTX- Methotrexate; DOD - died of disease, AwoD - alive without disease; AwD - alive with disease; na - not available
Treatment information was available for 10 patients. 4 received chemotherapy and radiotherapy, 3 were treated with chemotherapy alone, 2 received only steroids and one patient did not received treatment due to a poor performance status. The outcome data was available for 12 patients. With a median follow-up of 5 months (range 1-64 months), 2 patients are alive without disease, 4 patients are alive with disease and 6 patients died of disease.
Morphologic findings
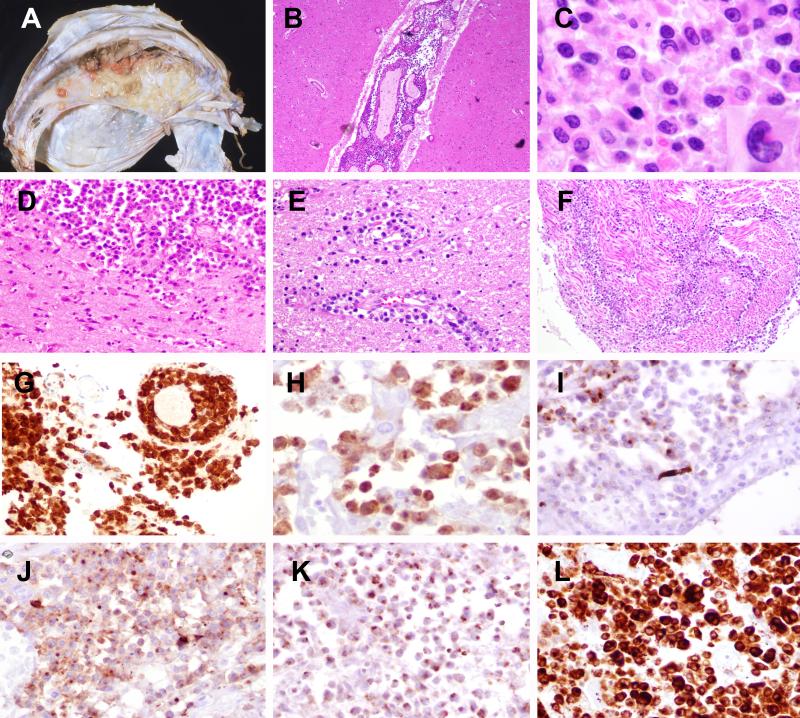
The PCNSTCL were classified as peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) (15 cases), anaplastic large cell lymphoma (ALCL), ALK-negative (2 cases), and ALCL, ALK-positive (1 case). The salient morphologic findings are summarized in Table 3. While most cases were submitted as small biopsies, detailed gross examination was available for one ALCL, ALK-positive diagnosed at autopsy (case 16). This case demonstrated tan-white nodules on the dural surface (mostly left-sided) with size ranging from 0.3-0.8 cm (Figure 1A). Leptomeningeal involvement was observed grossly in the lower thoracic and lumbar spinal cord with extension to the nerve roots of cauda equina. Overall, 5 cases had demonstrable leptomeningeal involvement.
Table 3.
Morphology, Immunophenotype, TCR clonality and Mutation analysis
| Final No |
Diagnosis | Size cells | Necrosis | Perivascular cuffing |
Meningeal spread |
CD2 | CD3 | CD4 | CD8 | CD5 | CD7 | CD56 | BF1 | TCRγ | TIA-1 | GrB | Perf | CD30 | ALK1 | EBV | KI67 | TRG PCR |
Ion Torrent TCLP39 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PTCL, NOS | small-medium | pos | pos | neg | na | pos | pos | neg | pos | na | equiv | na | na | na | na | na | na | na | neg (LPM1) | na | pos | na |
| 2 | PTCL, NOS | medium | pos | pos | pos | pos | pos | neg | pos | pos f | neg | neg | pos | na | pos | pos | na | neg | na | neg (LPM1) | na | pos | na |
| 3 | PTCL, NOS | small | neg | neg | neg | pos | pos | pos | neg | pos | pos f | neg | pos | na | pos | neg | neg | neg | na | neg | mod | susp | DNMT3A, c.2207G>T, p.Arg736Leu |
| 4 | PTCL, NOS | small-medium | pos f | pos | neg | pos f | pos | neg | pos | pos | pos | neg | pos | na | pos | neg | na | neg | neg | neg | high | pos | WT |
| 5 | PTCL, NOS | medium | pos | neg | neg | pos | pos | mix | mix | pos f | pos f | neg | pos | na | pos | neg | neg | neg | neg | rare | mod | rest | WT |
| 6 | PTCL, NOS | large | neg | neg | neg | pos | pos | pos | neg | pos f | pos f | not interp | pos | na | neg | neg | neg | neg | neg | neg | high | no amp | na |
| 7 | PTCL, NOS | medium-large | pos | pos | neg | pos | pos | pos | neg | pos | pos f | neg | pos | neg | pos | pos | pos f | neg | neg | neg | mod | pos | WT |
| 8 | PTCL, NOS | medium-large | pos | pos | neg | pos | pos | pos | neg | pos | pos f | neg | na | na | na | na | na | neg | na | neg | mod | pos | na |
| 9 | PTCL, NOS | small-medium | neg | neg | neg | neg | pos | neg | pos | neg | pos | neg | pos | neg | pos | pos | pos | neg | na | na | high | pos | KRAS, c.34G>A, p.Gly12Ser; STAT5B, c.1924A>C, p.Asn642His; JAK3, c.1533G>T, p.Met511Ile |
| 10 | PTCL, NOS | small-medium | pos | pos | pos | pos | pos | neg | pos | pos f | pos | neg | pos | neg | pos | pos | pos | neg | neg | neg | high | pos | WT |
| 11 | PTCL, NOS | medium-large | pos | pos | pos | pos | pos | neg | pos | pos f | na | na | pos | neg | pos | pos | na | neg | neg | neg | na | pos | WT |
| 12 | PTCL, NOS | small | pos | pos | pos | na | pos | neg | pos | pos f | na | neg | neg | na | pos | na | na | na | na | rare | high | pos | na |
| 13 | PTCL, NOS γδ | small-medium | neg | pos | neg | pos f | pos | neg | pos | pos | pos | na | neg | pos | pos | pos | na | neg | na | neg | mod | pos | WT |
| 14 | PTCL, NOS γδ | small-medium | neg | pos | neg | na | pos | neg | pos | neg | na | na | neg | pos | na | na | na | na | na | na | mod | pos | TET2, c.4034A>C, p.Tyr1345Ser |
| 15 | PTCL, NOS TCR silent | medium | neg | neg | neg | pos | pos | neg | pos | neg | pos | neg | neg | neg | pos | pos | na | neg | na | neg | high | pos | GNB1,c.232A>G, p.Lys78Glu; STAT3, c.1981G>C, p.Asp661His |
| 16 | ALCL, ALK pos | medium-large; “Hallmark” cells | pos | pos | pos* | neg | neg | neg | neg | neg | neg | neg | US | na | pos | pos | pos | pos | pos c | neg | high | pos | WT |
| 17 | ALCL, ALK neg | large, “hallmark” cells | neg | neg | neg | pos | pos | neg | pos w | pos | pos f | not interp | pos | neg | pos f | pos | na | pos | neg | na | mod | no amp | na |
| 18 | ALCL, ALK neg | large, “hallmark” cells | pos | neg | neg | na | pos w | na | na | neg | na | neg | na | na | neg | na | na | pos | neg | neg | high | pos | na |
Abbreviations: pos- positive; neg- negative; f - focal, w - weak, mix – mixed, both CD4 and CD8 positive cells present, na- not available; not interp - not interpretable, βF1- T-cell receptor beta F1; TCRγ- T-cell receptor gamma; GrB- Granzyme B; mod- moderate; TRG – T-cell receptor gene rearrangement; no amp- no amplification products; rest - restricted; susp - suspicious; EBV- Epstein-Barr virus; LMP1- EBV latent membrane protein, US – unsatisfactory.
Figure 1. Anaplastic large cell lymphoma, ALK-positive (Case 16).
A) Multiple tan-white lesions are attached to the left side of falx dura. B) The leptomeninges are extensively involved. C) The cells are medium to large with irregular nuclei; occasional larger cells have eccentric kidney or horse shoe shaped nuclei and abundant cytoplasm consistent with “hallmark cells”. D) Parenchymal involvement was also seen along with perivascular infiltrates (E) as well as extensive spinal and nerve root involvement (F). The cells are positive for CD30 (G), ALK, nuclear and cytoplasmic (H), focal EMA (I), CD43 (J), TIA (K) and granzyme-B (L).
Microscopically, most PTCL, NOS cases (11/15) were composed of atypical small and/or medium sized lymphocytes with dense, hyperchromatic nuclei, irregular nuclear outlines, occasional distinct nucleoli and scant cytoplasm (Figure 2). Medium to large cells (3 cases) or mostly large cells (1 case) were predominant in the remainder (Figure 3). Three cases showed features characteristic of ALCL, being composed of large cells with vesicular chromatin, evident nucleoli, and abundant cytoplasm; frequent “hallmark” cells were identified (Figure 1C). The tumor cells formed cohesive aggregates/sheets in two cases and were scattered throughout the white matter in one case.
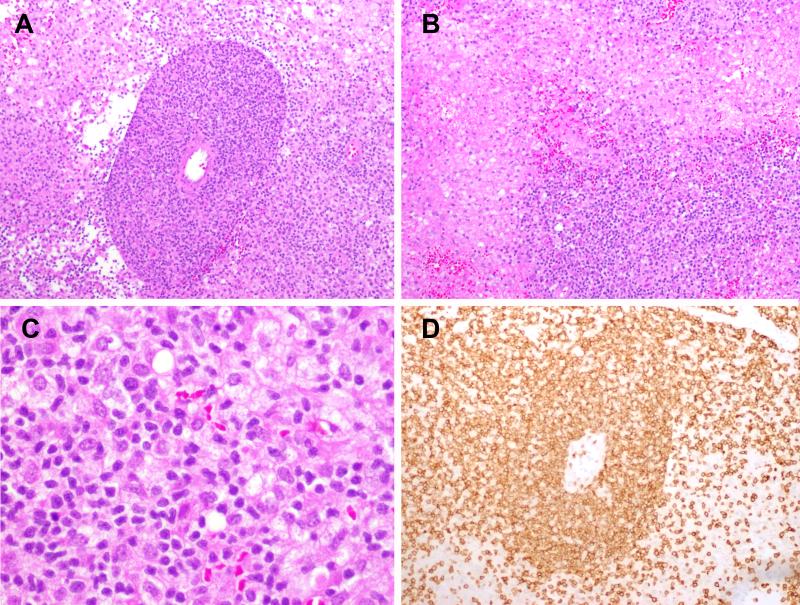
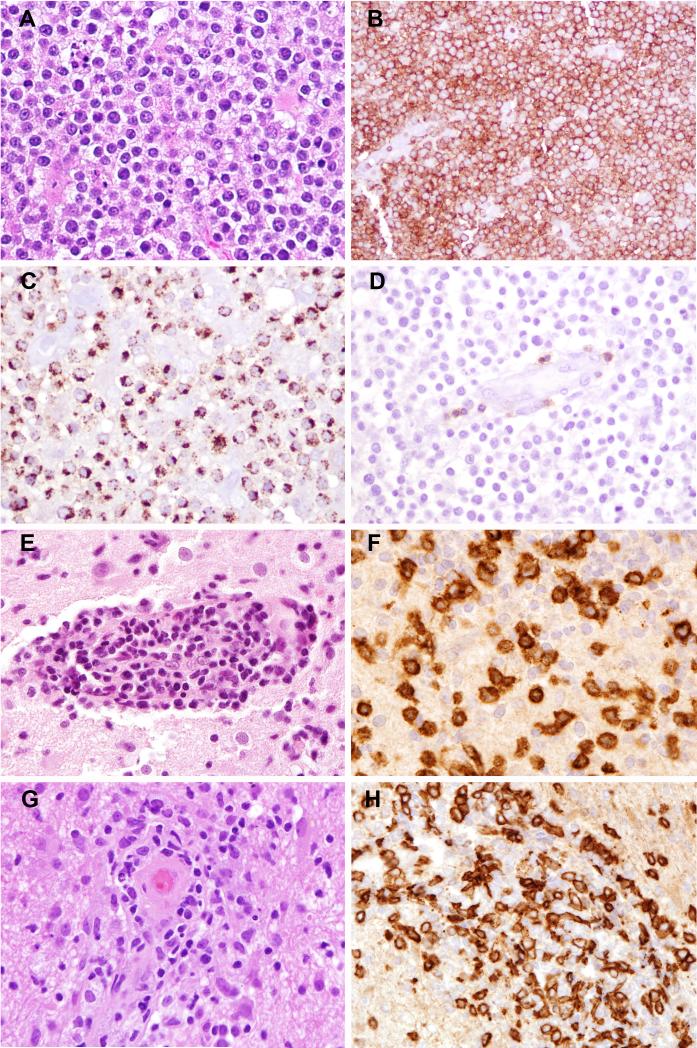
Figure 2. PTCL, NOS (Case 1).
A) Expansion of the Virchow-Robin space by an atypical lymphoid infiltrate. B) Broad areas of necrosis are visible. C) Neoplastic cells are small to medium in size with irregular nuclei; abundant admixed histiocytes are visible. D) The atypical cells are positive for CD3.
Figure 3. PTCL, NOS (Case 6).
A) A minority of cases, such as this one, contained large atypical cells with irregular nuclear contours, vesicular nuclei and basophilic nucleoli. The neoplastic cells are CD3-positive (B) and CD4-positive (C).
Prominent perivascular infiltration was evident in most cases (11/18) (Figure 4), with tumor cells expanding the Virchow-Robin space. Areas of necrosis were visible in 11 cases. Several cases demonstrated significant background gliosis and abundant histiocytes probably related to necrosis.
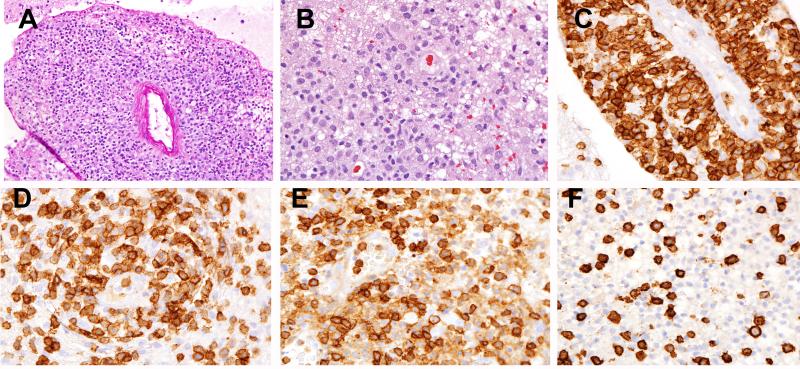
Figure 4. PTCL, NOS (Case 8).
(A) Marked perivascular infiltration is present. (B) The infiltrate is composed of small to medium atypical lymphocytes with significant background gliosis and abundant histiocytes. The atypical cells are strongly positive for CD2 (C), more variably positive for CD5 (D), positive for CD4 (E) and negative for CD8 (F).
Immunophenotype
The atypical lymphoid cells expressed CD3 in all cases except one (ALCL, ALK positive) (Table 3). 10/17 were CD8-positive and 5/17 were CD4-positive, with no case showing dual expression. Partial or total loss of T-cell antigens was seen in 4/15 cases for CD2, 11/18 cases for CD5 and 8/13 cases for CD7. No case expressed CD56, although high background staining for CD56 (as expected in CNS tissues) made interpretation difficult. 10/14 cases showed βF1 immunostaining. Among the 4 βF1-negative cases, a γδ T-cell derivation was confirmed in 2 by positivity for TCRγ (Figure 5); 1 case was TCR silent and 1 lacked material for TCRγ. Regardless of histological type, most of the cases showed a cytotoxic phenotype with expression of TIA1 in 13/15, granzyme-B in 9/13 and perforin in 4/7. Three (of 15) cases showed strong, uniform expression of CD30, corresponding to the diagnosis of ALCL, with one of these being positive for ALK, with both nuclear and cytoplasmic immunoreactivity. All 16 cases analyzed showed a brisk proliferation index as per Ki-67, with at least 50% of lymphoid cells positive. Rare EBV positive cells were present in 2/15 cases studied by EBER and/or LMP1. Stains for BCL6, CD10 and PD-1(CD279) were performed on a single CD4+ case (Case 5), and were negative.
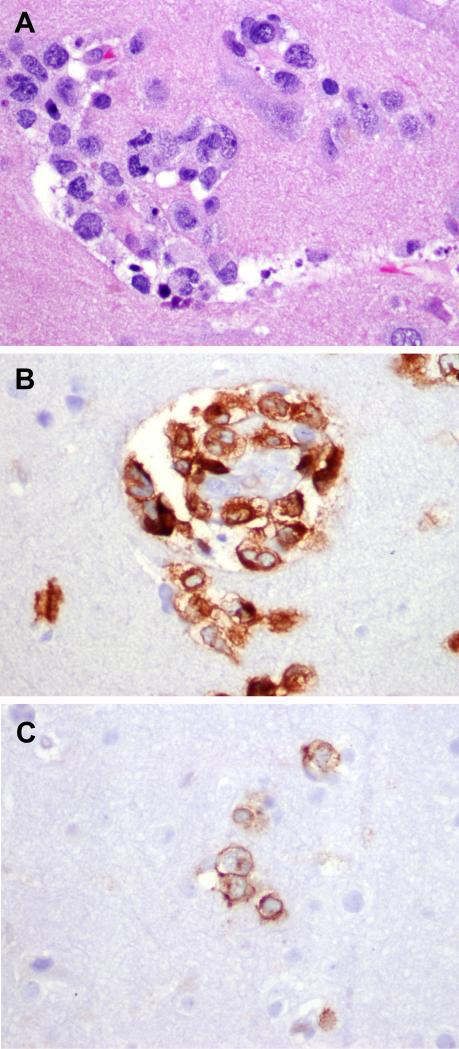
Figure 5. Phenotypic aberrancies in PCNSTCL.
A-D, PTCL, NOS with TCR silent phenotype (Case 15). A) Monomorphic medium sized atypical lymphocytes with irregular nuclear contours, vesicular chromatin and occasional nucleoli. The neoplastic T-cells are CD8-positive (B), TIA-1-positive (C) and beta-F1 (D) negative. TCR gamma was also negative (not shown). E-H) TCR gamma positive cases (Case 13 and 14). The cells are mostly small-medium with irregular nuclear contours, admixed occasional larger cells and demonstrate prominent perivascular cuffing (E and G). The cells are strongly positive for TCR-gamma immunostain (F and H).
T-cell Receptor Gene Rearrangement and Mutational analysis
The quality of DNA allowed for further analysis in 16/18 cases, with 2 cases showing no amplification products. A clonal rearrangement pattern was identified in 14 cases; one case showed a restricted pattern and 1 was considered suspicious for a significant clonal rearrangement.
Eleven of 18 cases (one of which was ALCL, ALK+) were analyzed with a custom NGS mutation panel targeting mutation hotspots in genes previously reported to be mutated in T-cell lymphomas, and in genes involved in T-cell signaling pathways. Four cases of PTCL-NOS were found to have somatic mutations (4/11, 36%); two had more than one mutation and none showed overlapping mutations. Case 3 displayed a DNMT3A (c.2207G>T; p.Arg736Leu) mutation. Case 9 was found to have KRAS (c.34G>A; p.Gly12Ser), STAT5B (c.1924A>C, p.Asn642His) and JAK3 (c.1533G>T; p.Met511Ile) mutations. Case 14, PTCL, NOS of γδ T-cell derivation showed a TET2 ( c.4034A>C; p.Tyr1345Ser) mutation and Case 15, silent for TCR expression by immunostains, contained both GNB1 (c.232A>G; p.Lys78Glu) and STAT3 (c.1981G>C; p.Asp661His) mutations. All remaining cases were wild type at the targeted sites in the 38 genes included in the panel.
DISCUSSION
Through this study, we describe in detail the histopathologic, immunophenotypic and molecular characteristics of 18 cases of primary CNS T-cell lymphomas. Diagnosis of these lesions is often a challenge, with the main differential being an inflammatory process, as the neoplastic T-cells were small to medium in size in the majority of cases, most of which were classified as PTCL, NOS. The diagnosis was more readily made in three cases of anaplastic large cell lymphoma, one of which was positive for ALK. A helpful feature was prominent perivascular infiltration; perivascular cuffing is common feature among both primary CNS B-cell and T-cell lymphomas. Additionally, necrosis, gliosis and histiocytic infiltration were seen in a significant number of cases. In contrast, abundant plasma cells, neutrophils or eosinophils were absent; when present, these would favor an inflammatory process.
Given the small cell size in many cases, immunohistochemical studies and molecular analysis were key in diagnosis. The most common antigenic aberrancies included complete or partial loss of CD5 (61%) and CD7 (62%). Loss of CD3 was very uncommon, restricted to 1 case of ALCL. More than half were CD8 positive. While most cases appeared to be derived from αβ-T cells, 4 cases were βF1 negative, suggesting a γδ T-cell derivation. However, only two were positive for TCRγ by immunohistochemistry; one case was noted to be TCR silent, also a major aberrancy31. The majority of cases had a cytotoxic phenotype, irrespective of histological subtype, as determined by staining with granzyme B, perforin, and TIA-1. Prior studies have shown a high incidence of a cytotoxic phenotype in extranodal as opposed to nodal T-cell lymphomas32.
Molecular testing for TCR γ chain gene rearrangement played an important role in the diagnosis of PCNSTL. A clonal process was confirmed in 14/16 cases with adequate DNA, while 2 others were either suspicious or showed a restricted pattern. PCNSTL have not previously been studied for molecular aberrations. Four of 11 PTCL, NOS studied (36%) had mutations involving, STAT3, STAT5B, JAK3, DNMT3A, KRAS, TET2 and GNB1 genes. Interestingly, no mutation was common to multiple cases, suggesting molecular heterogeneity. However, the findings in 2 cases with mutations in STAT5B, STAT3 and JAK3 support the importance of the JAK/STAT pathway in T-cell malignancies. Activating mutations of STAT3, STAT5B and JAKs have been reported with high frequency in large granular lymphocytic leukemia33, 34, γδ hepatosplenic T-cell lymphomas 30, T-prolymphocytic leukemia35, non-hepatosplenic γδ T-cell lymphomas36 and natural killer/T-cell lymphoma37. Other studies have demonstrated the importance of the JAK/STAT pathway in both ALK-positive and ALK-negative ALCL 38, 39. Thus, our data suggest that JAK and/or STAT inhibitors might represent potential treatment options in patients with PCNSTLs.
DNMT3A and TET2 mutations have been recently reported as important events in the pathobiology of mainly nodal lymphomas of TLH derivation40, 41. Interestingly, we found evidence of these mutations in PCNSTLs; one case with a mutation in DNMT3A had a CD4-positive phenotype, whereas a second case with a TET2 mutation was of γδ T-cell derivation. TET2 has not previously been implicated in the pathogenesis of γδ T-cell lymphomas. Clinically, most of our cases of PCNSTL had supratentorial disease and at presentation had solitary masses. Most patients received some form of chemotherapy combined with steroids with or without intrathecal methotrexate and/or brain irradiation. 6 patients had expired at the time of this study. In the largest series of PCNSTCL of the western world (International Primary CNS lymphoma collaborative group)1, the clinical characteristics were similar to those of primary CNS lymphomas in general 6, 9, 42 including the median age (approximately 60 years), propensity for supratentorial involvement, and male predominance. Similarly, primary CNS lymphomas of both B-cell and T-cell types are clinically aggressive, with median survivals of less than 2 years 1, 6, 9, 42.
Interestingly, a difference in prognosis based on morphology (i.e. small, medium versus large cells) was not present for PCNSTCL1. In a more recent study by Lim et al. 7, 9 patients with primary CNS PTCL were identified, and demonstrated relatively favorable clinical outcomes as compared to primary CNS DLBCL. However, other than CD3 positivity in these cases, further histologic, immunophenotypic and molecular data were not specified. Interestingly, a Korean study revealed a much higher percentage of PCNSTCL cases (16.7%) of all primary CNS lymphomas 10, significantly higher than that reported in western studies.
In a study by Levin et al 16, 5 patients out of a cohort of 100 patients with primary CNS lymphoma had T-cell lymphoma and all of them presented with isolated leptomeningeal involvement. However, in the study describing the largest primary CNS T-cell lymphoma cohort, only 1 out of 45 patients had leptomeningeal involvement 1. In the other cases described in their study, the parenchyma of the cerebral hemispheres (cortex and white matter) were the most frequent site (64%) followed by deep brain structures. This is similar to the known manifestations of B-cell PCNSL.
In our study leptomeningeal involvement was present in 5 cases and was extensive in one (case 16, ALCL, ALK+). Involvement of the leptomeninges in ALCL is a common feature. Of the 24 cases of primary CNS ALCL that have been described 21, 25, 43-61, 10 cases demonstrated some degree of dural or leptomeningeal involvement. In addition, there are 2 documented cases of primary dural ALCL without CNS parenchymal involvement 44, 45. In one case of ALCL from our series the bulk of the disease was in the leptomeninges, and the clinical syndrome was dominated by meningitic signs and symptoms. Of the reported ALCL cases, 13 were ALK positive, 10 were ALK negative, while data on three cases was unavailable. As expected, ALK positivity seems to correlate with a younger age and better prognosis (similar to that observed in systemic ALCL). Interestingly, leptomeningeal involvement does not seem to confer a worse prognosis (40). Secondary involvement of the CNS is very rare in most PTCL, being most often reported in ALCL in approximately 1% of cases62. The only PTCL that frequently involves the CNS is adult T-cell leukemia/lymphoma, which is a systemic disease in most patients63.
In conclusion, the diagnosis of T-cell lymphomas in the CNS is challenging, especially considering that the vast majority of these lymphomas have small or intermediate size cells with variable cytologic atypia. These need to be differentiated from reactive T-cell infiltrates and encephalitis caused by infections and autoimmune diseases. A combination of morphologic assessment, immunophenotypic aberrancies and demonstration of clonal T-cell receptor rearrangement helps in establishing the diagnosis of a T-cell lymphoma. Preliminary genetic analysis identified mutations in genes involved in other mature T-cell malignancies, but no common recurrent genetic events.
Supplementary Material
Acknowledgments
This study was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health
Dr. Miller has reported financial relationships with outside parties that have no direct bearing on the work reported in this manuscript.
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental Digital Content.
SDC1. Further information regarding the methods used for Next Generation Sequencing and the complete list of the 38 targeted genes is found in the supplemental content.
SDC2 lists the genes Included in the Sequencing Panel
REFERENCES
- 1.Shenkier TN, Blay JY, O'Neill BP, et al. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol. 2005;23:2233–2239. doi: 10.1200/JCO.2005.07.109. [DOI] [PubMed] [Google Scholar]
- 2.Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma. 2008;49(Suppl 1):43–51. doi: 10.1080/10428190802311441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller DC, Hochberg FH, Harris NL, et al. Pathology with clinical correlations of primary central nervous system non-Hodgkin's lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer. 1994;74:1383–1397. doi: 10.1002/1097-0142(19940815)74:4<1383::aid-cncr2820740432>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 5.DeAngelis LM. Primary CNS lymphoma: treatment with combined chemotherapy and radiotherapy. J Neurooncol. 1999;43:249–257. doi: 10.1023/a:1006258619757. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68:835–853. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- 7.Lim T, Kim SJ, Kim K, et al. Primary CNS lymphoma other than DLBCL: a descriptive analysis of clinical features and treatment outcomes. Ann Hematol. 2011;90:1391–1398. doi: 10.1007/s00277-011-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahnke K, Thiel E, Schilling A, et al. Low-grade primary central nervous system lymphoma in immunocompetent patients. Br J Haematol. 2005;128:616–624. doi: 10.1111/j.1365-2141.2004.05361.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 10.Choi JS, Nam DH, Ko YH, et al. Primary central nervous system lymphoma in Korea: comparison of B- and T-cell lymphomas. Am J Surg Pathol. 2003;27:919–928. doi: 10.1097/00000478-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kanavaros P, Mikol J, Nemeth J, et al. Primary T-cell malignant lymphoma of the central nervous system. Histological, immunohistochemical and ultrastructural study of a case. Pathol Res Pract. 1993;189:93–98. doi: 10.1016/S0344-0338(11)80123-8. discussion 98-101. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura T, Inamura T, Ikezaki K, et al. Primary Ki-1 lymphoma in the central nervous system. J Clin Neurosci. 2001;8:574–577. doi: 10.1054/jocn.2000.0854. [DOI] [PubMed] [Google Scholar]
- 13.Kleopa K, Becker G, Roggendorf W, et al. Primary T-cell lymphoma of the cerebellum. A case report. J Neurooncol. 1996;27:225–230. doi: 10.1007/BF00165478. [DOI] [PubMed] [Google Scholar]
- 14.Kuwata T, Funahashi K, Itakura T, et al. [Primary T-cell type lymphoma of the central nervous system: a case report]. No Shinkei Geka. 1987;15:657–661. [PubMed] [Google Scholar]
- 15.Lee DK, Chung CK, Kim HJ, et al. Multifocal primary CNS T cell lymphoma of the spinal cord. Clin Neuropathol. 2002;21:149–155. [PubMed] [Google Scholar]
- 16.Levin N, Soffer D, Grissaru S, et al. Primary T-cell CNS lymphoma presenting with leptomeningeal spread and neurolymphomatosis. J Neurooncol. 2008;90:77–83. doi: 10.1007/s11060-008-9633-2. [DOI] [PubMed] [Google Scholar]
- 17.Lueth M, Stein H, Spors B, et al. First case report of a peripheral T-cell lymphoma, not otherwise specified, of the central nervous system in a child. J Pediatr Hematol Oncol. 2012;34:e66–68. doi: 10.1097/MPH.0b013e318219f95e. [DOI] [PubMed] [Google Scholar]
- 18.Marsh WL, Jr., Stevenson DR, Long HJ., 3rd Primary leptomeningeal presentation of T-cell lymphoma. Report of a patient and review of the literature. Cancer. 1983;51:1125–1131. doi: 10.1002/1097-0142(19830315)51:6<1125::aid-cncr2820510625>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Matano S, Nakamura S, Ohtake S, et al. Primary T cell non-Hodgkin's lymphoma of the central nervous system. Case report and review of the literature. Acta Haematol. 1994;91:158–163. doi: 10.1159/000204323. [DOI] [PubMed] [Google Scholar]
- 20.McCue MP, Sandrock AW, Lee JM, et al. Primary T-cell lymphoma of the brainstem. Neurology. 1993;43:377–381. doi: 10.1212/wnl.43.2.377. [DOI] [PubMed] [Google Scholar]
- 21.Merlin E, Chabrier S, Verkarre V, et al. Primary leptomeningeal ALK+ lymphoma in a 13-year-old child. J Pediatr Hematol Oncol. 2008;30:963–967. doi: 10.1097/MPH.0b013e31818a959a. [DOI] [PubMed] [Google Scholar]
- 22.Ogura R, Aoki H, Natsumeda M, et al. Epstein-Barr virus-associated primary central nervous system cytotoxic T-cell lymphoma. Neuropathology. 2013;33:436–441. doi: 10.1111/neup.12005. [DOI] [PubMed] [Google Scholar]
- 23.Yeh KH, Cheng AL, Tien HF. Primary T cell leptomeningeal lymphoma--successful treatment with systemic chemotherapy. Oncology. 1995;52:501–504. doi: 10.1159/000227519. [DOI] [PubMed] [Google Scholar]
- 24.Gijtenbeek JM, Rosenblum MK, DeAngelis LM. Primary central nervous system T-cell lymphoma. Neurology. 2001;57:716–718. doi: 10.1212/wnl.57.4.716. [DOI] [PubMed] [Google Scholar]
- 25.Ponzoni M, Terreni MR, Ciceri F, et al. Primary brain CD30+ ALK1+ anaplastic large cell lymphoma (‘ALKoma’): the first case with a combination of ‘not common’ variants. Ann Oncol. 2002;13:1827–1832. doi: 10.1093/annonc/mdf300. [DOI] [PubMed] [Google Scholar]
- 26.Villegas E, Villa S, Lopez-Guillermo A, et al. Primary central nervous system lymphoma of T-cell origin: description of two cases and review of the literature. J Neurooncol. 1997;34:157–161. doi: 10.1023/a:1005754212792. [DOI] [PubMed] [Google Scholar]
- 27.Lawnicki LC, Rubocki RJ, Chan WC, et al. The Distribution of Gene Segments in T-Cell Receptor gamma Gene Rearrangements Demonstrates the Need for Multiple Primer Sets. J Mol Diagn. 2003;5:82–87. doi: 10.1016/s1525-1578(10)60456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slack DN, McCarthy KP, Wiedemann LM, et al. Evaluation of sensitivity, specificity, and reproducibility of an optimized method for detecting clonal rearrangements of immunoglobulin and T-cell receptor genes in formalin-fixed, paraffin-embedded sections. Diagn Mol Pathol. 1993;2:223–232. [PubMed] [Google Scholar]
- 29.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolae A, Xi L, Pittaluga S, et al. Frequent STAT5B mutations in gamma delta hepatosplenic T-cell lymphomas. Leukemia. 2014;28:2244–2248. doi: 10.1038/leu.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Herrera A, Song JY, Chuang SS, et al. Nonhepatosplenic gammadelta T-cell Lymphomas Represent a Spectrum of Aggressive Cytotoxic T-cell Lymphomas With a Mainly Extranodal Presentation. Am J Surg Pathol. 2011;35:1214–1225. doi: 10.1097/PAS.0b013e31822067d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swerdlow SH, Jaffe ES, Brousset P, et al. Cytotoxic T-cell and NK-cell Lymphomas: Current Questions and Controversies. Am J Surg Pathol. 2014;38:e60–71. doi: 10.1097/PAS.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koskela HLM, Eldfors S, Ellonen P, et al. Somatic STAT3 Mutations in Large Granular Lymphocytic Leukemia. New England Journal of Medicine. 2012;366:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048–3057. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiel MJ, Velusamy T, Rolland D, et al. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood. 2014;124:1460–1472. doi: 10.1182/blood-2014-03-559542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucuk C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo GC, Tan SY, Tang T, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2:591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 38.Anastasov N, Bonzheim I, Rudelius M, et al. C/EBPbeta expression in ALK-positive anaplastic large cell lymphomas is required for cell proliferation and is induced by the STAT3 signaling pathway. Haematologica. 2010;95:760–767. doi: 10.3324/haematol.2009.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crescenzo R, Abate F, Lasorsa E, et al. Convergent Mutations and Kinase Fusions Lead to Oncogenic STAT3 Activation in Anaplastic Large Cell Lymphoma. Cancer Cell. 2015;27:516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemonnier F, Couronne L, Parrens M, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120:1466–1469. doi: 10.1182/blood-2012-02-408542. [DOI] [PubMed] [Google Scholar]
- 41.Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012;366:95–96. doi: 10.1056/NEJMc1111708. [DOI] [PubMed] [Google Scholar]
- 42.Shibamoto Y, Ogino H, Suzuki G, et al. Primary central nervous system lymphoma in Japan: changes in clinical features, treatment, and prognosis during 1985-2004. Neuro Oncol. 2008;10:560–568. doi: 10.1215/15228517-2008-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JS, Park H, Park S, et al. Primary central nervous system ALK positive anaplastic large cell lymphoma with predominantly leptomeningeal involvement in an adult. Yonsei Med J. 2013;54:791–796. doi: 10.3349/ymj.2013.54.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MK, Cho CH, Sung WJ, et al. Primary anaplastic large cell lymphoma in the dura of the brain: case report and prediction of a favorable prognosis. Int J Clin Exp Pathol. 2013;6:1643–1651. [PMC free article] [PubMed] [Google Scholar]
- 45.George DH, Scheithauer BW, Aker FV, et al. Primary anaplastic large cell lymphoma of the central nervous system: prognostic effect of ALK-1 expression. Am J Surg Pathol. 2003;27:487–493. doi: 10.1097/00000478-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Feldges A, Gerhard L, Reinhardt V, et al. Primary cerebral anaplastic T-cell-lymphoma (type Ki-1): review and case report. Clin Neuropathol. 1992;11:55–59. [PubMed] [Google Scholar]
- 47.Sugino T, Mikami T, Akiyama Y, et al. Primary central nervous system anaplastic large-cell lymphoma mimicking lymphomatosis cerebri. Brain Tumor Pathol. 2013;30:61–65. doi: 10.1007/s10014-012-0094-0. [DOI] [PubMed] [Google Scholar]
- 48.Colen CB, Rayes M, Kupsky WJ, et al. Synchronous meningioma and anaplastic large cell lymphoma. Neuropathology. 2010;30:260–266. doi: 10.1111/j.1440-1789.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- 49.Kodama K, Hokama M, Kawaguchi K, et al. Primary ALK-1-negative anaplastic large cell lymphoma of the brain: case report and review of the literature. Neuropathology. 2009;29:166–171. doi: 10.1111/j.1440-1789.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 50.Rowsell EH, Zekry N, Liwnicz BH, et al. Primary anaplastic lymphoma kinase-negative anaplastic large cell lymphoma of the brain in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2004;128:324–327. doi: 10.5858/2004-128-324-PALKAL. [DOI] [PubMed] [Google Scholar]
- 51.Tajima Y, Miyazaki Y, Higashi T, et al. Primary CD30/Ki-1 positive anaplastic large cell lymphoma of the central nervous system occurring in a patient with a seventeen-year history of essential thrombocythemia. Leuk Lymphoma. 2003;44:1243–1245. doi: 10.1080/1042819031000077106. [DOI] [PubMed] [Google Scholar]
- 52.Chuang SS, Huang W, Lin CN, et al. Primary cerebral anaplastic large cell lymphoma containing abundant reactive histiocytes and eosinophils. A case report and literature review. Pathol Res Pract. 2001;197:647–652. doi: 10.1078/0344-0338-00140. [DOI] [PubMed] [Google Scholar]
- 53.Paulus W, Ott MM, Strik H, et al. Large cell anaplastic (KI-1) brain lymphoma of T-cell genotype. Hum Pathol. 1994;25:1253–1256. doi: 10.1016/0046-8177(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 54.Karikari IO, Thomas KK, Lagoo A, et al. Primary cerebral ALK-1-positive anaplastic large cell lymphoma in a child. Case report and literature review. Pediatr Neurosurg. 2007;43:516–521. doi: 10.1159/000108799. [DOI] [PubMed] [Google Scholar]
- 55.Carmichael MG. Central nervous system anaplastic large cell lymphoma in an adult: successful treatment with a combination of radiation and chemotherapy. Mil Med. 2007;172:673–675. doi: 10.7205/milmed.172.6.673. [DOI] [PubMed] [Google Scholar]
- 56.Cooper PB, Auerbach A, Aguilera NS, et al. Rare primary CNS anaplastic large cell lymphoma in an immunocompetent adult: a clinical-pathologic case report and review case of the literature. Clin Neuropathol. 2006;25:232–236. [PubMed] [Google Scholar]
- 57.Rupani A, Modi C, Desai S, et al. Primary anaplastic large cell lymphoma of central nervous system--a case report. J Postgrad Med. 2005;51:326–327. [PubMed] [Google Scholar]
- 58.Abdulkader I, Cameselle-Teijeiro J, Fraga M, et al. Primary anaplastic large cell lymphoma of the central nervous system. Hum Pathol. 1999;30:978–981. doi: 10.1016/s0046-8177(99)90253-8. [DOI] [PubMed] [Google Scholar]
- 59.Buxton N, Punt J, Hewitt M. Primary Ki-1-positive T-cell lymphoma of the brain in a child. Pediatr Neurosurg. 1998;29:250–252. doi: 10.1159/000028731. [DOI] [PubMed] [Google Scholar]
- 60.Havlioglu N, Manepalli A, Galindo L, et al. Primary Ki-1 (anaplastic large cell) lymphoma of the brain and spinal cord. Am J Clin Pathol. 1995;103:496–499. doi: 10.1093/ajcp/103.4.496. [DOI] [PubMed] [Google Scholar]
- 61.Goldbrunner R, Warmuth-Metz M, Tonn JC, et al. Primary Ki-1-positive T-cell lymphoma of the brain--an aggressive subtype of lymphoma: case report and review of the literature. Surg Neurol. 1996;46:37–41. doi: 10.1016/0090-3019(96)00033-x. [DOI] [PubMed] [Google Scholar]
- 62.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 63.Bunn PA, Jr., Schechter GP, Jaffe E, et al. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N Engl J Med. 1983;309:257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.