Abstract
Objective
To investigate the degree to which waist circumference (WC), BMI, and MRI measured abdominal fat deposition predict insulin resistance (IR) in non-obese girls of diverse racial and ethnic backgrounds.
Methods
Fifty-seven non-obese girls (12 African-American, 16 Hispanic White and 29 non-Hispanic White girls), aged 11–14 years old were assessed for WC, MRI hepatic proton density fat fraction, visceral and subcutaneous adipose tissue volume, BMI Z-score, fasting insulin, HOMA-IR, adiponectin, leptin, sex hormone binding globulin, HDL cholesterol, and triglycerides.
Results
Univariate and multivariate analyses adjusted for race and ethnicity indicated that only WC and visceral adipose tissue volume were independent predictors of fasting insulin and HOMA-IR, while dependent predictors were hepatic proton density fat fraction, BMI Z-score, and subcutaneous adipose tissue volume. Hispanic White girls showed significantly higher mean fasting insulin, HOMA-IR, and lower sex hormone binding globulin than non-Hispanic White girls (p-value <0.01).
Conclusions
In non-obese girls of diverse racial and ethnic backgrounds, WC, particularly when adjusted for race or ethnicity, is an independent predictor of IR comparable to MRI-derived measurements of fat and superior to BMI Z-score.
Keywords: Ethnicity, Race, Non-obese, HOMA-IR, Waist Circumference, MRI
Introduction
Comorbidity rates related to obesity, most notably type 2 diabetes mellitus, have increased in parallel to rises in pediatric obesity during past decades and are incurring profound health and economic burdens. In the U.S., approximately 3,700 youth are diagnosed with type 2 diabetes mellitus (T2DM) annually (1). Importantly, youth with T2DM exhibit more rapid deterioration of beta cell function than older adults, and often develop significant cardiovascular morbidity and mortality in the 3rd decade of life (2, 3). These findings highlight the need for reliable and cost-effective methods to identify youth at greatest risk for metabolic consequences of obesity. Currently, there is lack of consensus whether body mass index (BMI), waist circumference (WC), or another anthropometric measure provides a superior screening approach (4, 5). Development of consensus is complicated by evidence that the metabolic effects exerted by a given amount of fat vary with race and ethnicity (6, 7), and that WC and BMI fail to directly quantify site-specific fat deposition.
While WC and BMI are easily obtained estimates of adiposity, magnetic resonance imaging (MRI), can evaluate and quantify fat deposition with high accuracy and at specific anatomic sites. In obese adolescents, MRI demonstrates higher levels of visceral adipose tissue (VAT) and hepatic fat in subjects of Hispanic and European descent compared to those of African descent despite similar overall body fat percentage (8, 9). These imaging results suggest that racial and ethnic patterns of fat deposition may contribute to racial and ethnic disparities in rates and pathophysiology of T2DM and other metabolic disease (10). Importantly, these racial or ethnic tendencies for deleterious fat deposition can be detected at lower levels of accrued fat (11). For example, non-obese Hispanic adolescent girls exhibit higher levels of hepatic fat measured by MRI, with tighter correlations between insulin resistance (IR) and hepatic fat than non-obese, non-Hispanic white peers (7).
With its ability to accurately and rapidly quantify fat without the use of ionizing radiation, MRI holds promise for accurately defining pathologic connections between compartmentalization of fat deposition and metabolic health. However, MRI currently remains an impractical clinical screening tool. Instead, prevention and early intervention strategies are better served by clinically available measures that can identify those at risk for adverse metabolic effects of excess fat who should receive further laboratory or imaging. This study, innovative in its focus on non-obese girls of African-American (AA), Hispanic (H), and non-Hispanic white (NHW) ancestry, investigates whether anthropometric measures (i.e. WC and BMI Z-score) provide metabolic risk assessment comparable in value to MRI derived measurements of fat deposition (VAT, subcutaneous adipose tissue [SCAT], and hepatic proton density fat fraction [hepatic PDFF]).
Methods
Study Population
Non-obese female students who attended a local middle school fall registration were invited to participate in this cross-sectional study. Once written consent and assent were obtained for the HIPAA compliant and IRB approved study, personal and family medical history, and self-identified race and ethnicity (per NIH race and ethnicity criteria for subjects in clinical research) were collected at registration. Individuals of Asian descent were not included. Height was measured using a stadiometer and recorded to the nearest 0.5 cm. Waist circumference was measured twice and recorded to the nearest 1mm just above the iliac crests with Graham-Field® cloth woven measuring tape. Weight was measured without shoes in light clothes on a beam balance platform scale to the nearest 0.1 kg. BMI was then calculated. Self-assessment of Tanner staging for breast and pubic hair was performed (12). Study entrance criteria included a non-obese BMI percentile of < 90th percentile per CDC 2000 sex- and age-specific BMI growth charts and age 11–14 years old. Based on family self-identification, subjects were allocated to Hispanic White (H) or non-Hispanic White (NHW) or African American (AA) groups. To ensure recruitment of a non-obese study group, we set the upper limit for BMI percentile at the 90th percentile, rather than 95th. Exclusion criteria included: BMI greater than the 90th percentile for age and gender, Type 1 or Type 2 diabetes mellitus, chronic diseases (including infectious or inflammatory diseases), treatment with glucose metabolism altering (e.g. metformin) or lipid altering (e.g. statin) agents, or pregnancy.
Laboratory
Venipuncture was performed in the early morning after an overnight fast. Blood was processed immediately and analyzed at the University of Wisconsin Hospital and Clinics Laboratory for glucose, insulin, luteinizing hormone, estradiol, sex hormone binding globulin (SHBG), HDL cholesterol, and triglycerides. Glucose was determined by hexokinase method; insulin, luteinizing hormone, and estradiol by chemiluminescent immunoassay; triglycerides by enzymatic assay; HDL with direct homogeneous measures (University of Wisconsin Hospital and Clinics Laboratory, Madison, WI); and SHBG was measured by a quantitative electrochemiluminescent immunoassay (ARUP Laboratories, Salt Lake City, UT). A portion of the sample was analyzed at the University of Wisconsin National Primate Research Center for adiponectin and leptin by radioimmunoassay (Linco-Millipore, St. Charles, MO) both run per kit protocols. Homeostasis model of assessment-insulin resistance (HOMA-IR) was calculated from fasting glucose (mg/dL) and insulin (μU/ml) values: (fasting glucose × fasting insulin/405). Quantitative magnetic resonance imaging was performed at the Wisconsin Institute for Medical Research (WIMR). The Human Subjects Committee of the University of Wisconsin IRB approved all procedures.
Image Acquisition and Reconstruction
Volumetric 3D quantitative magnetic resonance imaging (MRI) was performed using a clinical 3 Tesla scanner (MR750, GE Healthcare, Waukesha, WI) and a 32-channel phased array body coil (Neocoil, Pewaukee WI), and all image acquisition and reconstruction was performed using established and accepted protocols (13–21). All images were determined using an investigational version of a confounder-corrected chemical shift encoded water-fat separation method (3D-IDEAL-SPGR) (13, 22). Two acquisitions were performed, including a sagittal 3D volume that covered from the liver dome to the pelvic floor (to quantify VAT and SCAT), and an axial 3D volume covering the liver to quantify hepatic PDFF. Acquisition parameters for the sagittal acquisition included: a single sagittal slab with 6 echoes- first echo time/echo spacing = 0.8/1.2ms, echo train length = 6 (1shot), flip angle/repetition time/bandwidth = 3°/9msc/±167kHz, field of view = 48×38×47cm, matrix size = 148×118×156. True spatial resolution = 3.2×3.2×3mm3, interpolated to 1.9×1.9×1.5mm3 through zero-filling. Scan time = 26s. The axial acquisition included a single sagittal slab with 6 echoes- first echo time/echo spacing = 1.2/2.0ms, echo train length = 6 (2 shots of 3 echoes), flip angle/repetition time/bandwidth = 3°/8.3msc/±125kHz, field of view = 44×40×22cm, matrix size = 230×160×14, Spatial resolution = 1.7×1.7×8mm3, 2D parallel imaging (ARC) with R=2.86. Scan time = 23s. For both acquisitions, separated water-only and fat-only images, as well as quantitative hepatic PDFF maps (23) were calculated using an automated on-line reconstruction algorithm. The acquisition and reconstruction are designed to avoid or correct for all known confounders, including spectral modeling of fat (24), corrects for eddy currents (15), T1 bias (25), T2* decay (16), and noise related bias (25). Because all known confounders have been addressed, the resulting PDFF map provides an accurate and fundamental measure of the fat concentration in tissue (13, 26).
Hepatic PDFF was determined by averaging PDFF value measured from 9 regions of interest placed in each of the 9 Couinaud segments of the liver (22, 27). Hepatic steatosis was defined as PDFF >5.56% (19).
Manual VAT and SCAT quantification was performed from the L3 to L5 vertebral levels. Segmentation was performed in SliceOmatic v5.0 as follows. Intestinal gas and bowel contents, as well as background voxels were masked prior to application a 45% threshold on the fat fraction images (20). Fat signal near the spine and bone marrow were manually erased.
Statistics
Metabolic lab parameters and subjects’ characteristics measured on a continuous scale are presented as means ± standard deviations and were compared for race and ethnicity groups using analysis of variance Nonparametric Spearman’s rank correlation analysis was performed to evaluate bivariate associations between variables. Categorical variables were compared between groups using Fisher’s exact test. Tukey’s Honestly Significance Difference (HSD) method was used to control the type I error when conducting multiple comparisons between the three groups. Multivariate analyses were conducted to examine whether BMI Z-score, WC, hepatic PDFF, SCAT and VAT measures predict IR, after adjusting for race and ethnicity. These analyses were conducted for each measure separately. Furthermore, multivariate regression analyses were conducted to identify independent predictors for IR. All potential predictors were included in an initial, non-parsimonious model. The backward and forward selection methods with a selection criterion of p<0.1 were then used to determine a parsimonious model which included only significant predictors. The identification of independent predictors was validated using the least absolute shrinkage and selection operator (LASSO) variable selection method. Fasting insulin concentration and HOMA IR values were log-transformed in the regression analyses to meet the assumption of normality. Statistical inference was performed with maximum likelihood estimation. All statistical analyses were performed with SAS software (version 8.2; SAS Institute, Cary, NC). All P values were 2-sided, and P values ≤ .05 were considered statistically significant.
Results
A summary of the characteristics of the 57 subjects (12 AA, 16 H, 29 NHW) is presented in Table 1. There were no statistically significant differences in mean age, BMI Z-score, WC, estradiol levels, or occurrence of menarche among the racial and ethnic groups when adjusting for multiple comparisons. For the unadjusted comparisons significant differences were detected when comparing WC between Hispanics vs. Non-Hispanics (p=0.029) and between African Americans vs. Non-Hispanics for LH (p=0.038). The significant difference in LH may have been attributable to the presence of an outlier in the AA group. With removal of the subject with a LH of 103.5, the difference was non-significant. Using the CDC 2000 sex- and age-specific BMI growth chart, the mean BMI Z-scores were consistent with mean BMI percentiles at the 63rd percentile in AA subjects, the 64th percentile in H subjects, and the 58th percentile in NHW subjects.
Table 1.
Characteristics of Study Participants
| African-American (AA) | Hispanic (H) | Non-Hispanic White (NHW) | |
|---|---|---|---|
| Number of Subjects | 12 | 16 | 29 |
| Age (years) | 12.41±0.91 | 12.40±1.03 | 12.57±1.17 |
| BMI Z-score | 0.34±0.98 | 0.36 ±0.70 | 0.21 ±0.67 |
| Waist Circumference (cm) | 69.05±6.12 | 75.50±10.66 | 65.15±15.66 |
| Estradiol (pg/mL) | 77.46±92.82 | 68.00±86.86 | 49.76±34.00 |
| Luteinizing Hormone (mIU/mL) | 14.85±29.57 | 6.15±8.08 | 4.37±4.13 |
| Self-Assessed Breast Tanner Stage | 33% (4/12) | 25% (4/16) | 24% (7/29) |
| Percent post-menarchal | 31% (4/13) | 56% (9/16) | 52% (14/29) |
No significant racial or ethnic differences among groups in above variables, with all p-values >0.05 when comparisons of ethnic and racial groups means were adjusted for multiple comparisons using Tukey’s Honestly Significance Difference method.
Comparison of the metabolic lab parameters by race and ethnicity, indicated that the H subjects had significantly higher mean fasting insulin (23.3±11.9μIU/mL vs. 12.6±6.14μIU/mL, p=0.002), higher mean HOMA-IR (5.18±3.20 vs. 2.76±1.42. p=0.006), and lower mean SHBG (44.0±24.1 nmol/L vs. 72.3±28.6nmol/L vs. p=0.004) than NHW subjects when adjusted for multiple comparisons (table 2). As shown in table 2, there were no statistically significant differences detected between H and NHW groups for any of the 5 other metabolic parameters (adiponectin, leptin, HDL, and triglycerides), and there were no statistically significant differences between AA and H groups or between AA and NHW groups for any of the 8 metabolic parameters (fasting insulin, HOMA-IR, SHBG, adiponectin, leptin, HDL, and triglycerides) when adjusted for multiple comparisons (table 2).
Table 2.
Metabolic Parameters of Study Participants
| African-American (AA) | Hispanic (H) | Non-Hispanic White (NHW) | |
|---|---|---|---|
| Fasting Insulin (μIU/mL) | 19.15±11.76 | 23.25±11.90* | 12.62±6.14* |
| HOMA-IR | 4.07±3.06 | 5.18±3.20* | 2.76±1.42* |
| SHBG (nmol/L) | 58.00±26.67 | 44.00±24.10* | 72.31±28.64* |
| Adiponectin (μg/mL) | 13.90±2.97 | 13.23±6.66 | 14.74±6.53 |
| Leptin (ng/mL) | 13.37±5.67 | 11.68±6.90 | 9.03±5.04 |
| HDL (mg/dL) | 53.15±8.69 | 52.00±10.25 | 53.14±13.88 |
| Triglycerides (mg/dL) | 67.31±24.44 | 87.75±39.28 | 77.66 ±23.42 |
Significant difference (p-value < 0.01) in mean fasting insulin, HOMA-IR, and SHBG between H and NHW groups; otherwise, no significant differences among racial and ethnic group means with all p-values >0.05 when adjusted for multiple comparisons using Tukey’s Honestly Significance Difference method. SHBG, sex hormone binding globulin.
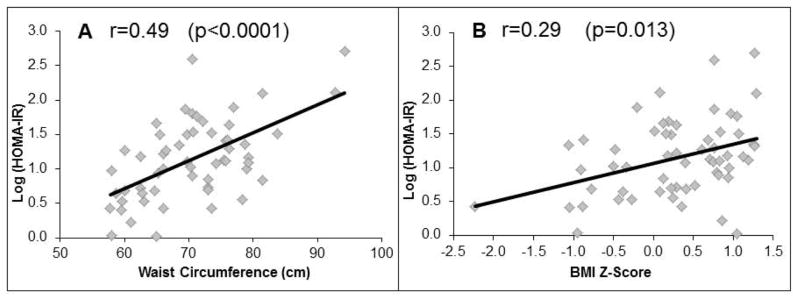
Overall, moderate to strong correlations of WC with metabolic parameters (fasting insulin, HOMA-IR, triglycerides, SHBG, adiponectin, and leptin) were observed, similar to those seen with VAT and SCAT (table 3). BMI Z-score correlations with metabolic parameters were only significant with fasting insulin, HOMA-IR, SHBG, adiponectin, and leptin (table 3). Waist circumference correlated more strongly with all of these metabolic parameters than BMI Z-score, however, the difference in r-values did not reach statistical significance. Figure 1 illustrates the correlations of waist circumference and BMI Z-score with HOMA-IR.
Table 3.
Correlations of Waist Circumference, BMI Z-score, Hepatic PDFF, VAT, and SCAT with Metabolic Parameters for All Study Participants (n=57)
| WC (cm) | BMI Z-score | Hepatic PDFF | VAT | SCAT | |
|---|---|---|---|---|---|
| Fasting Insulin (μIU/mL) | r=0.49** | r=0.31* | NS | r=0.41** | r=0.54** |
| HOMA-IR | r=0.49** | r=0.29* | NS | r=0.43** | r=0.54** |
| SHBG (nmol/L) | r=−0.55** | r=−0.30* | r=−0.54** | r=−0.37** | r=−0.69** |
| Adiponectin (μg/mL) | r=−0.41** | r=−0.34* | NS | r=−0.36** | r=−0.31** |
| Leptin (ng/mL) | r=0.66** | r=0.50** | r=0.3* | r=0.28** | r=0.71** |
| HDL (mg/dL) | r=−0.36** | NS | NS | r=−0.37** | r=−0.38** |
| Triglycerides (mg/dL) | r=0.35** | NS | NS | r=0.39** | r=0.28* |
p-value ≤ 0.05,
p-value ≤ 0.01,
NS = p-value not significant.
WC, waist circumference; PDFF, proton density fat fraction, VAT, visceral adipose tissue; SCAT, subcutaneous adipose tissue; NS, not significant; SHBG, sex hormone binding globulin.
Figure 1.
Correlations of Waist Circumference and BMI Z-score with HOMA-IR in non-obese girls.
In multivariate analyses, WC, BMI Z-score, Hepatic PDFF, VAT, and SCAT remained statistically significant predictors for fasting insulin and HOMA-IR, after adjusting for race and ethnicity. The partial Spearman’s rank correlations for predicting fasting insulin levels increased substantially for SCAT and VAT from 0.34 and 0.29 to 0.63 and 0.69, respectively, after adjusting for race and ethnicity.
In order to identify independent predictors for fasting insulin and HOMA-IR, multivariate regression analyses were conducted. In the initial non-parsimonious model, WC, BMI Z-score, hepatic PDFF, SCAT, VAT, age and race and ethnicity were included as independent variables the model., The backward and forward selection method was used to identify parsimonious models (See methods). In the parsimonious model, WC (p=0.012), VAT (p=0.0031) and race and ethnicity (AA vs. NHW, p=0.0092; H vs. NHW, p=0.022) were identified as statistically significant independent predictors for fasting insulin (table 4). The adjusted R square value for this model was 0.54. Analogously, WC (p=0.013), VAT (p=0.0024) and ethnicity (H vs. NHW, p=0.0049) were identified as statistically significant predictors for HOMA-IR with an adjusted R square value of 0.53 (table 4). There was no statistically significant difference in the slope of the correlation between WC and VAT (p=0.15 for fasting insulin and p=0.16 for HOMA-IR). These results were confirmed using the lasso variable selection method.
Table 4.
Parsimonious multivariate model for Waist Circumference and VAT (adjusted for race and ethnicity)
| Fasting Insulin | HOMA IR | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β(SE) | p-value | R2 | β(SE) | p-value | R2 | |
|
| ||||||
| Waist Circumference | 0.013 (0.005) | 0.012 | 0.54 | 0.013 (0.005) | 0.013 | 0.53 |
|
|
|
|||||
| VAT | 0.005 (0.002) | 0.0031 | 0.006 (0.002) | 0.0024 | ||
|
|
|
|||||
| Race/Ethnicity | ||||||
| AA vs. NHW | 0.41 (0.15) | 0.0092 | 0.31 (0.16) | 0.057 | ||
| H vs. NHW | 0.39 (0.12) | 0.022 | 0.37 (0.13) | 0.0049 | ||
| AA vs. H | 0.02 (0.16) | 0.898 | −0.06 (0.17) | 0.717 | ||
βslope parameter (non-standardized); SE, standard error; R2, coefficient of determination; PDFF, proton density fat fraction, VAT, visceral adipose tissue; SCAT, subcutaneous adipose tissue; AA, African American; NHW, non-Hispanic White; H, Hispanic.
Discussion
In this group of non-obese young females of African-American, Hispanic White, and non-Hispanic White background, WC independently predicted IR to a degree comparable to (i.e. not significantly different from) that of MRI derived VAT and stronger than BMI Z-score. While WC has been previously shown to correlate with MRI measured VAT in both obese and non-obese youth (28), this study adds the novel observation that, at non-obese levels of adiposity defined by BMI percentile, WC predict HOMA-IR and fasting insulin better than BMI Z-score and to a similar degree as MRI derived measures of fat (Hepatic PDFF, VAT, and SCAT).
These results suggest that, in non-obese girls, use of WC alone can improve pediatric risk stratification for IR risk over the current BMI-based approach. Evidence from other studies suggests that inclusion of both WC- and BMI-based measurements improves pediatric risk stratification into general categories of “healthy” and “unhealthy” obesity (5, 29, 30). A key finding of this study is the additional utility of WC in identifying non-obese (by BMI criteria) subjects who are already experiencing adverse health consequences from adiposity. Therefore, greater reliance on assessment of WC, easily obtained in clinical practice, could provide valuable information about a child’s fat distribution and risk for IR. Increasing use of electronic medical records may facilitate incorporation of WC and other anthropometric measures into clinically useful predictive equations (31).
Importantly, the predictive value of WC for fasting insulin and HOMA-IR was increased by racial and ethnic adjustments in the non-obese girls. While admittedly based on relatively few subjects, this finding suggests that adjustment of BMI and/or WC thresholds that would trigger concern for underlying IR based on an individual’s race or ethnicity may be appropriate, since it appears that certain ethnic or racial groups manifest adverse health effects at lower degrees of fat deposition (32, 33). In the US, WC percentile curves based on NHANES data for African-American and Mexican-American youth have greater absolute raw waist circumferences than White children, so a larger percentage of non-White children may be identified as high risk using WC criteria compared to BMI alone (34).
The waist circumference to height ratio has been proposed as an informative marker of abdominal obesity and metabolic risk because relative to WC or BMI it is more independent of patient age, pubertal status, and gender (35). Further, a waist circumference to height ratio of greater than 0.5 was proposed to define central obesity for all races and ethnicities instead of the use of various BMI or WC thresholds to assess habitus and metabolic risk—a threshold that additionally provides patients with a simple message to keep their WC less than ½ their height (36). However, use of the waist circumference to height ratio, rather than waist circumference, did not improve prediction of IR in our non-obese adolescent cohort (data not shown) and further study is needed in pediatric patients.
A fundamental issue is determining the best anatomic cross-section to measure WC. Interestingly, in youth, WC measurement at 4 different abdominal cross-sections (midpoint between iliac crest and lowest rib, top of the iliac crest, minimal abdominal circumference, and level of the umbilicus) does not affect WC correlations with VAT or fasting glucose and lipids (37). However, a standardized approach to WC measurements would minimize variability, improve reproducibility and thereby increase its clinical and research utility (38). This study, following the technique utilized for generation of the previously mentioned NHANES database, used the top of the iliac crests as markers, which provide bilateral bony landmarks to minimize variability of WC measurement.
This study has potential limitations, beginning with categorization of subjects into broad racial and ethnic groups (which include genetic and cultural heterogeneity among subjects) and inclusion of children with one or more parents with African or Hispanic ancestry into the African-American or Hispanic cohort, respectively. Enrollment for subgroup analysis was relatively small, so that observations describing differences based on race and ethnicity are preliminary and require confirmation with studies of greater numbers of subjects. In addition, whether the findings described here are applicable to male subjects, and adolescents of other races and ethnicities, remains uncertain. Further, future inclusion of subjects with BMI percentiles above the 90th percentile in would be expected to allow increase the level and variability of IR and allow analysis of how evolving obesity affects prediction of IR by BMI-, WC-, and MRI-based measures. In addition, although efforts were made to document similar pubertal maturation between groups (based on self-assessment and estradiol levels), variations in pubertal status could remain a potential confounding factor, given important effects of puberty on IR (39). Additionally, although verification of Tanner staging by a medical provider would have confirmed staging assessment, formal evaluation was not performed given the potential for reduced enrollment that mandatory physical examination would have created. Further, while fasting indices used in this study correlate closely with OGTT in assessing IR in obese adolescents (40), oral glucose tolerance testing (OGTT) may yield IR assessments more closely aligned with gold-standard hyperinsulinemic-euglycemic clamp testing. Strengths of this study include analysis of children of different races and ethnicities, and use of advanced quantitative MRI methods to quantify fat deposition and correlate with standard laboratory markers of IR. An additional strength was the unique focus on non-obese subjects from diverse racial and ethnic backgrounds (mean BMI Z-scores at the 63rd, 64th, and 58th percentiles for the African American, Hispanic, and non-Hispanic white, respectively).
Conclusion
Waist circumference measurement is an informative and inexpensive risk stratification tool for IR in children, even before progression of adiposity into obesity as defined by BMI percentile. Specifically, in a cohort of non-obese girls, waist circumference predicted IR better than BMI Z-score and to a similar extent as quantitative MRI measurements of fat. Additionally, WC and MRI VAT independently predicted IR, and this prediction was further strengthened when racial and ethnic background was taken into account. The fact these findings were evident in a cohort of non-obese adolescent girls strengthens the concept that adverse metabolic changes can occur early in the course of fat deposition, making early detection and promotion of healthy physical activity and nutrition of at-risk but still non-obese children paramount, particularly since obesity prevalence rises sharply during early grade school (41).
Acknowledgments
Funding: Support was provided by the NIH (R01DK083380, R01DK088925, RC1EB010384, T32DK07758604), Genentech Center for Clinical Research, Endocrine Fellows Foundation, and GE Healthcare. Study sponsors had no role in study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the manuscript for publication.
Abbreviations
- AA
African American
- BMI
body mass index
- H
Hispanic
- Hepatic PDFF
hepatic proton density fat fraction
- HDL
high density lipoprotein
- HOMA-IR
homeostatic model assessment of insulin resistance
- IR
insulin resistance
- MRI
magnetic resonance imaging
- NHW
non-Hispanic White
- T2DM
type 2 diabetes mellitus
- SCAT
subcutaneous adipose tissue
- SHBG
sex hormone binding globulin
- VAT
visceral adipose tissue
- WC
waist circumference
Footnotes
Disclosure: The authors have no potential, perceived, or real conflicts of interest.
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA : the journal of the American Medical Association. 2007;297(24):2716–24. doi: 10.1001/jama.297.24.2716. Epub 2007/06/28. [DOI] [PubMed] [Google Scholar]
- 2.Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes care. 2013;36(6):1749–57. doi: 10.2337/dc12-2393. Epub 2013/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes care. 2013;36(12):3863–9. doi: 10.2337/dc12-2455. Epub 2013/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotlyarevska K, Wolfgram P, Lee JM. Is waist circumference a better predictor of insulin resistance than body mass index in U.S. adolescents? The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2011;49(3):330–3. doi: 10.1016/j.jadohealth.2010.12.008. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. The Journal of pediatrics. 2006;149(6):809–16. doi: 10.1016/j.jpeds.2006.08.075. Epub 2006/12/02. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–7. doi: 10.1038/oby.2007.92. Epub 2008/02/02. [DOI] [PubMed] [Google Scholar]
- 7.Wolfgram PM, Connor EL, Rehm JL, Eickhoff JC, Reeder SB, Allen DB. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in nonobese middle school girls. Obesity (Silver Spring) 2014;22(1):243–8. doi: 10.1002/oby.20521. Epub 2013/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Adamo E, Northrup V, Weiss R, Santoro N, Pierpont B, Savoye M, et al. Ethnic differences in lipoprotein subclasses in obese adolescents: importance of liver and intraabdominal fat accretion. Am J Clin Nutr. 2010;92(3):500–8. doi: 10.3945/ajcn.2010.29270. Epub 2010/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2(6):e569. doi: 10.1371/journal.pone.0000569. Epub 2007/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maligie M, Crume T, Scherzinger A, Stamm E, Dabelea D. Adiposity, fat patterning, and the metabolic syndrome among diverse youth: the EPOCH study. The Journal of pediatrics. 2012;161(5):875–80. doi: 10.1016/j.jpeds.2012.05.003. Epub 2012/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. The Journal of clinical endocrinology and metabolism. 2014;99(7):2557–66. doi: 10.1210/jc.2013-4345. Epub 2014/04/10. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15(1):88–94. doi: 10.1046/j.1365-3016.2001.00317.x. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]
- 13.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258(3):767–75. doi: 10.1148/radiol.10100708. Epub 2011/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magnetic Resonance in Medicine. 2007;58(2):354–64. doi: 10.1002/mrm.21301. Epub 2007/07/27. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Shimakawa A, Hines CD, McKenzie CA, Hamilton G, Sirlin CB, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magnetic Resonance in Medicine. 2011;66(1):199–206. doi: 10.1002/mrm.22840. Epub 2011/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. Journal of Magnetic Resonance Imaging. 2007;26(4):1153–61. doi: 10.1002/jmri.21090. Epub 2007/09/27. [DOI] [PubMed] [Google Scholar]
- 17.Hines CD, Frydrychowicz A, Hamilton G, Tudorascu DL, Vigen KK, Yu H, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. Journal of Magnetic Resonance Imaging. 2011;33(4):873–81. doi: 10.1002/jmri.22514. Epub 2011/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. Journal of magnetic resonance imaging : JMRI. 2012;36(5):1011–4. doi: 10.1002/jmri.23741. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8. doi: 10.1152/ajpendo.00064.2004. Epub 2004/09/02. [DOI] [PubMed] [Google Scholar]
- 20.Poonawalla AH, Sjoberg BP, Rehm JL, Hernando D, Hines CD, Irarrazaval P, et al. Adipose tissue MRI for quantitative measurement of central obesity. Journal of magnetic resonance imaging : JMRI. 2013;37(3):707–16. doi: 10.1002/jmri.23846. Epub 2012/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artz NS, Haufe WM, Hooker CA, Hamilton G, Wolfson T, Campos GM, et al. Reproducibility of MR-based liver fat quantification across field strength: Same-day comparison between 1.5T and 3T in obese subjects. Journal of magnetic resonance imaging : JMRI. 2015 doi: 10.1002/jmri.24842. Epub 2015/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18(3):337–57. ix. doi: 10.1016/j.mric.2010.08.013. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: A standardized mr-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012 doi: 10.1002/jmri.23741. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60(5):1122–34. doi: 10.1002/mrm.21737. Epub 2008/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58(2):354–64. doi: 10.1002/mrm.21301. Epub 2007/07/27. [DOI] [PubMed] [Google Scholar]
- 26.Hines CD, Frydrychowicz A, Hamilton G, Tudorascu DL, Vigen KK, Yu H, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33(4):873–81. doi: 10.1002/jmri.22514. Epub 2011/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011–4. doi: 10.1002/jmri.23741. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR, et al. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes (Lond) 2006;30(1):23–30. doi: 10.1038/sj.ijo.0803163. Epub 2005/12/14. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SA, Hergenroeder AC. Waist circumference predicts increased cardiometabolic risk in normal weight adolescent males. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6(2–2):e307–11. doi: 10.3109/17477166.2011.575149. Epub 2011/06/09. [DOI] [PubMed] [Google Scholar]
- 30.Bassali R, Waller JL, Gower B, Allison J, Davis CL. Utility of waist circumference percentile for risk evaluation in obese children. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2010;5(1):97–101. doi: 10.3109/17477160903111722. Epub 2009/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgos MS, Burgos LT, Camargo MD, Franke SI, Pra D, Silva AM, et al. Relationship between anthropometric measures and cardiovascular risk factors in children and adolescents. Arquivos brasileiros de cardiologia. 2013;101(4):288–96. doi: 10.5935/abc.20130169. Epub 2013/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes care. 2011;34(8):1741–8. doi: 10.2337/dc10-2300. Epub 2011/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbaum M, Fennoy I, Accacha S, Altshuler L, Carey DE, Holleran S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21(10):2081–90. doi: 10.1002/oby.20483. Epub 2013/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. The Journal of pediatrics. 2004;145(4):439–44. doi: 10.1016/j.jpeds.2004.06.044. Epub 2004/10/14. [DOI] [PubMed] [Google Scholar]
- 35.Arnaiz P, Grob F, Cavada G, Dominguez A, Bancalari R, Cerda V, et al. Waist-to-height ratio does not change with gender, age and pubertal stage in elementary school children. Revista medica de Chile. 2014;142(5):574–8. doi: 10.4067/S0034-98872014000500004. Epub 2014/11/27. La razon cintura estatura en escolares no varia con el genero, la edad ni la maduracion puberal. [DOI] [PubMed] [Google Scholar]
- 36.Ashwell M, Gibson S. A proposal for a primary screening tool: ‘Keep your waist circumference to less than half your height’. BMC medicine. 2014;12:207. doi: 10.1186/s12916-014-0207-1. Epub 2014/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington DM, Staiano AE, Broyles ST, Gupta AK, Katzmarzyk PT. Waist circumference measurement site does not affect relationships with visceral adiposity and cardiometabolic risk factors in children. Pediatric obesity. 2013;8(3):199–206. doi: 10.1111/j.2047-6310.2012.00106.x. Epub 2012/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mason C, Katzmarzyk PT. Effect of the site of measurement of waist circumference on the prevalence of the metabolic syndrome. The American journal of cardiology. 2009;103(12):1716–20. doi: 10.1016/j.amjcard.2009.02.018. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 39.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and beta-cell function in overweight Latino youth. J Pediatr. 2011;158(3):442–6. doi: 10.1016/j.jpeds.2010.08.046. Epub 2010/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. Journal of Clinical Endocrinology and Metabolism. 2011;96(7):2136–45. doi: 10.1210/jc.2010-2813. Epub 2011/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. The New England journal of medicine. 2014;370(17):1660–1. doi: 10.1056/NEJMc1402397. Epub 2014/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]