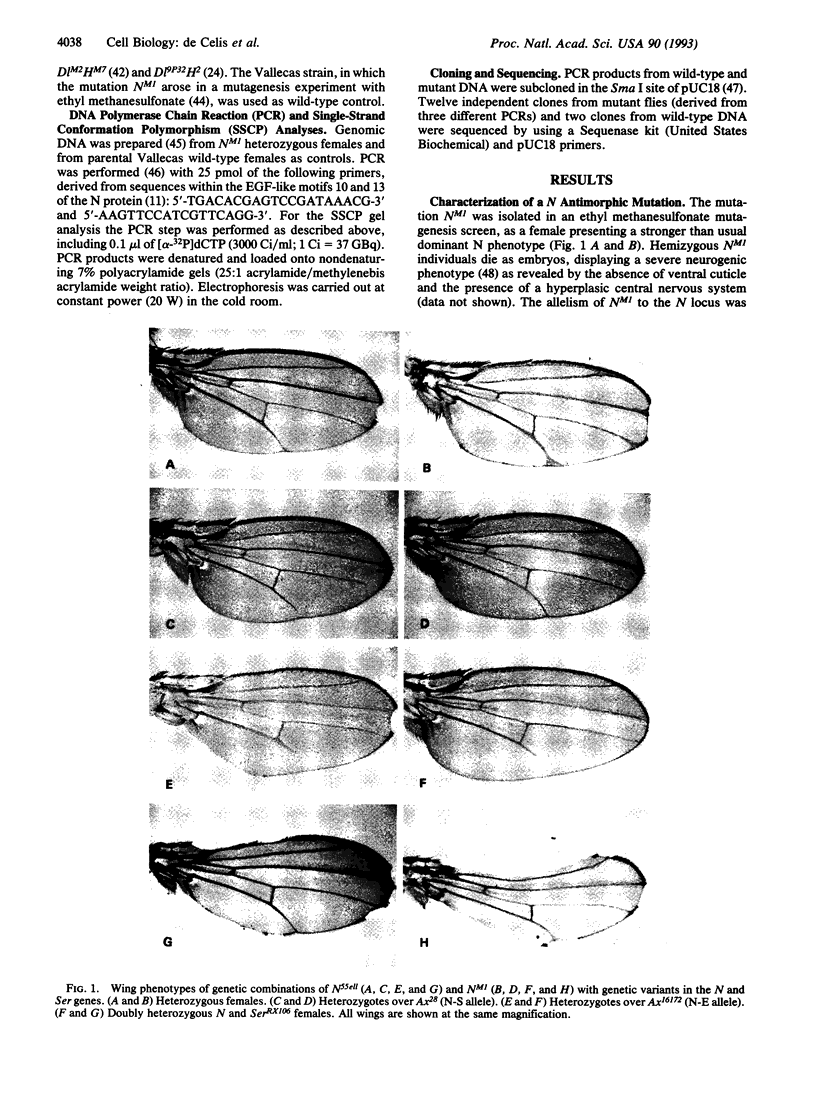
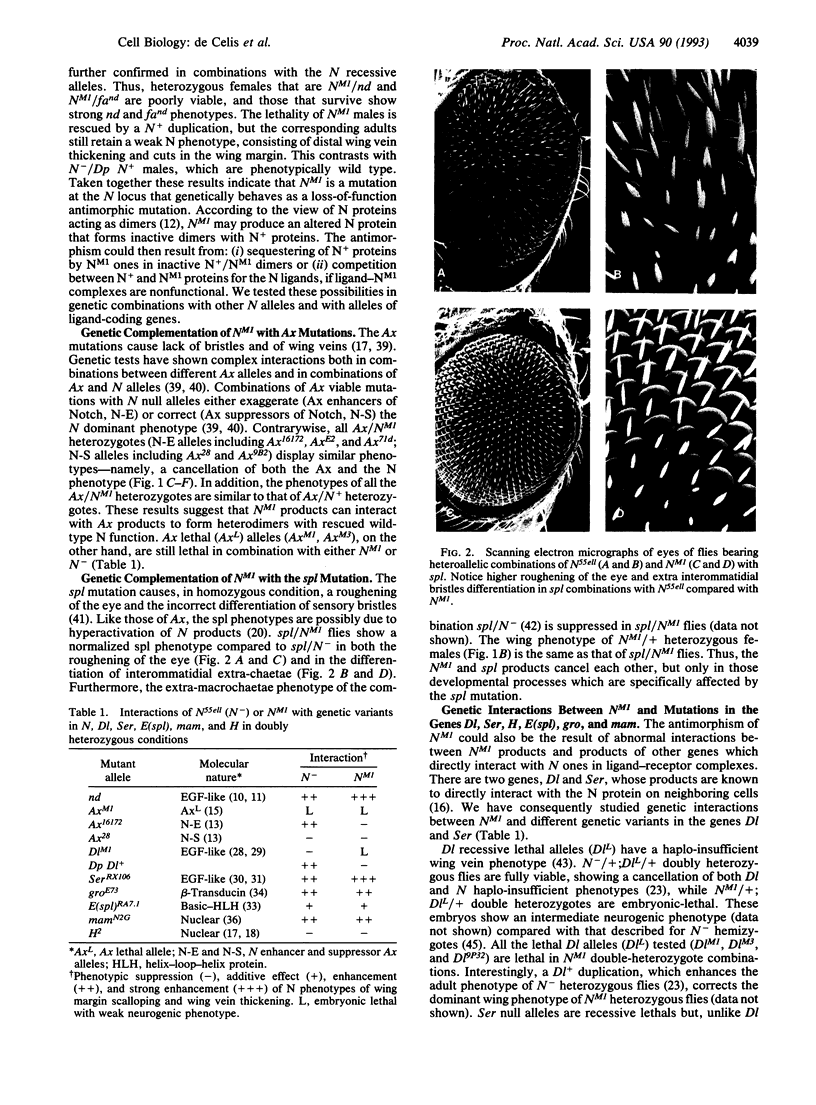
Abstract
The Drosophila Notch gene product is a transmembrane protein that functions as a receptor of intercellular signals in several Drosophila developmental processes. Two other transmembrane proteins, encoded by the genes Delta and Serrate, genetically and molecularly behave as Notch ligands. All these proteins share the presence of epidermal growth factor (EGF)-like repeats in their extracellular domain. The Notch protein has 36 EGF-like repeats, 2 of which, numbers 11 and 12, are required for the interaction with the Delta and Serrate ligands. We have isolated and molecularly characterized a Notch mutation in its Delta- and Serrate-binding domain that behaves genetically as both a Notch antimorphic and a loss-of-function mutation. This mutation, NM1, carries a Glu-->Val substitution in the Notch EGF repeat 12. The NM1 allele interacts with other Notch alleles such as Abruptex and split and with mutations in the Notch-ligand genes Delta and Serrate. The basis for the genetic antimorphism of NM1 seems to reside in the titration of Notch wild-type products into NM1/N+ nonfunctional dimers and/or the titration of Delta products into nonfunctional ligand-receptor complexes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artavanis-Tsakonas S., Delidakis C., Fehon R. G. The Notch locus and the cell biology of neuroblast segregation. Annu Rev Cell Biol. 1991;7:427–452. doi: 10.1146/annurev.cb.07.110191.002235. [DOI] [PubMed] [Google Scholar]
- Baker N. E., Mlodzik M., Rubin G. M. Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990 Dec 7;250(4986):1370–1377. doi: 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- Bang A. G., Posakony J. W. The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev. 1992 Sep;6(9):1752–1769. doi: 10.1101/gad.6.9.1752. [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989 Aug;3(8):1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Knust E. Genetics of early neurogenesis in Drosophila melanogaster. Annu Rev Genet. 1990;24:387–407. doi: 10.1146/annurev.ge.24.120190.002131. [DOI] [PubMed] [Google Scholar]
- Coffman C., Harris W., Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990 Sep 21;249(4975):1438–1441. doi: 10.1126/science.2402639. [DOI] [PubMed] [Google Scholar]
- Corbin V., Michelson A. M., Abmayr S. M., Neel V., Alcamo E., Maniatis T., Young M. W. A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell. 1991 Oct 18;67(2):311–323. doi: 10.1016/0092-8674(91)90183-y. [DOI] [PubMed] [Google Scholar]
- Ellisen L. W., Bird J., West D. C., Soreng A. L., Reynolds T. C., Smith S. D., Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991 Aug 23;66(4):649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Fleming R. J., Scottgale T. N., Diederich R. J., Artavanis-Tsakonas S. The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev. 1990 Dec;4(12A):2188–2201. doi: 10.1101/gad.4.12a.2188. [DOI] [PubMed] [Google Scholar]
- Foster G. G. Negative complementation at the notch locus of Drosophila melanogaster. Genetics. 1975 Sep;81(1):99–120. doi: 10.1093/genetics/81.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan R. J. The Notch gene, adhesion, and developmental fate in the Drosophila embryo. New Biol. 1990 Jul;2(7):595–600. [PubMed] [Google Scholar]
- Greenwald I., Rubin G. M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992 Jan 24;68(2):271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Xu T. A., Artavanis-Tsakonas S. The embryonic expression of the Notch locus of Drosophila melanogaster and the implications of point mutations in the extracellular EGF-like domain of the predicted protein. EMBO J. 1987 Nov;6(11):3407–3417. doi: 10.1002/j.1460-2075.1987.tb02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M. R., Kidd S., Deutsch W. A., Young M. W. Mutations altering the structure of epidermal growth factor-like coding sequences at the Drosophila Notch locus. Cell. 1987 Nov 20;51(4):539–548. doi: 10.1016/0092-8674(87)90123-1. [DOI] [PubMed] [Google Scholar]
- Kidd S., Baylies M. K., Gasic G. P., Young M. W. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 1989 Aug;3(8):1113–1129. doi: 10.1101/gad.3.8.1113. [DOI] [PubMed] [Google Scholar]
- Kidd S., Kelley M. R., Young M. W. Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol. 1986 Sep;6(9):3094–3108. doi: 10.1128/mcb.6.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Knust E., Schrons H., Grawe F., Campos-Ortega J. A. Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics. 1992 Oct;132(2):505–518. doi: 10.1093/genetics/132.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopczynski C. C., Alton A. K., Fechtel K., Kooh P. J., Muskavitch M. A. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988 Dec;2(12B):1723–1735. doi: 10.1101/gad.2.12b.1723. [DOI] [PubMed] [Google Scholar]
- Lifschytz E., Falk R. Fine structure analysis of a chromosome segment in Drosophila melanogaster: analysis of ethyl methanesulphonate-induced lethals. Mutat Res. 1969 Jul-Aug;8(1):147–155. doi: 10.1016/0027-5107(69)90149-3. [DOI] [PubMed] [Google Scholar]
- Maier D., Stumm G., Kuhn K., Preiss A. Hairless, a Drosophila gene involved in neural development, encodes a novel, serine rich protein. Mech Dev. 1992 Aug;38(2):143–156. doi: 10.1016/0925-4773(92)90006-6. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Baker N. E., Rubin G. M. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990 Nov;4(11):1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- Palka J., Schubiger M., Schwaninger H. Neurogenic and antineurogenic effects from modifications at the Notch locus. Development. 1990 May;109(1):167–175. doi: 10.1242/dev.109.1.167. [DOI] [PubMed] [Google Scholar]
- Portin P. Allelic negative complementation at the Abruptex locus of Drosophila melanogaster. Genetics. 1975 Sep;81(1):121–133. doi: 10.1093/genetics/81.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss A., Hartley D. A., Artavanis-Tsakonas S. The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J. 1988 Dec 1;7(12):3917–3927. doi: 10.1002/j.1460-2075.1988.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinow L., Birchler J. A. Interactions of vestigial and scabrous with the Notch locus of Drosophila melanogaster. Genetics. 1990 May;125(1):41–50. doi: 10.1093/genetics/125.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay I., Fleming R. J., Fehon R. G., Cherbas L., Cherbas P., Artavanis-Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell. 1991 Nov 15;67(4):687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- Ruohola H., Bremer K. A., Baker D., Swedlow J. R., Jan L. Y., Jan Y. N. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991 Aug 9;66(3):433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- Shellenbarger D. L., Mohler J. D. Temperature-sensitive periods and autonomy of pleiotropic effects of l(1)Nts1, a conditional notch lethal in Drosophila. Dev Biol. 1978 Feb;62(2):432–446. doi: 10.1016/0012-1606(78)90226-9. [DOI] [PubMed] [Google Scholar]
- Smoller D., Friedel C., Schmid A., Bettler D., Lam L., Yedvobnick B. The Drosophila neurogenic locus mastermind encodes a nuclear protein unusually rich in amino acid homopolymers. Genes Dev. 1990 Oct;4(10):1688–1700. doi: 10.1101/gad.4.10.1688. [DOI] [PubMed] [Google Scholar]
- Thomas U., Speicher S. A., Knust E. The Drosophila gene Serrate encodes an EGF-like transmembrane protein with a complex expression pattern in embryos and wing discs. Development. 1991 Mar;111(3):749–761. doi: 10.1242/dev.111.3.749. [DOI] [PubMed] [Google Scholar]
- Vässin H., Bremer K. A., Knust E., Campos-Ortega J. A. The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. EMBO J. 1987 Nov;6(11):3431–3440. doi: 10.1002/j.1460-2075.1987.tb02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vässin H., Campos-Ortega J. A. Genetic Analysis of Delta, a Neurogenic Gene of Drosophila melanogaster. Genetics. 1987 Jul;116(3):433–445. doi: 10.1093/genetics/116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vässin H., Vielmetter J., Campos-Ortega J. A. Genetic interactions in early neurogenesis of Drosophila melanogaster. J Neurogenet. 1985 Nov;2(5):291–308. doi: 10.3109/01677068509102325. [DOI] [PubMed] [Google Scholar]
- Welshons W. J. Analysis of a gene in drosophila. Science. 1965 Nov 26;150(3700):1122–1129. doi: 10.1126/science.150.3700.1122. [DOI] [PubMed] [Google Scholar]
- Welshons W. J. Genetic basis for two types of recessive lethality at the notch locus of Drosophila. Genetics. 1971 Jun;68(2):259–268. doi: 10.1093/genetics/68.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Bell J. B., Carroll S. B. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes Dev. 1991 Dec;5(12B):2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Xu T., Caron L. A., Fehon R. G., Artavanis-Tsakonas S. The involvement of the Notch locus in Drosophila oogenesis. Development. 1992 Aug;115(4):913–922. doi: 10.1242/dev.115.4.913. [DOI] [PubMed] [Google Scholar]
- Xu T., Rebay I., Fleming R. J., Scottgale T. N., Artavanis-Tsakonas S. The Notch locus and the genetic circuitry involved in early Drosophila neurogenesis. Genes Dev. 1990 Mar;4(3):464–475. doi: 10.1101/gad.4.3.464. [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Marí-Beffa M., García-Bellido A. Cell-autonomous role of Notch, an epidermal growth factor homologue, in sensory organ differentiation in Drosophila. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):632–636. doi: 10.1073/pnas.88.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-la-Concha A., Dietrich U., Weigel D., Campos-Ortega J. A. Functional interactions of neurogenic genes of Drosophila melanogaster. Genetics. 1988 Mar;118(3):499–508. doi: 10.1093/genetics/118.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]