Abstract
Importance
Screening mammography intervals remain under debate in the United States.
Objective
To compare the proportion of breast cancers with less versus more favorable prognostic characteristics in women screening annually versus biennially by age, menopausal status, and postmenopausal hormone therapy use.
Design
Prospective cohort from 1996-2012.
Setting
Breast Cancer Surveillance Consortium facilities.
Participants
We included 15,440 women aged 40-85 years with breast cancer diagnosed within one year of an annual or within two years of a biennial screening mammogram.
Exposure
We updated previous analyses by using narrower intervals for defining annual (11-14 months) and biennial (23-26 months) screening.
Main outcomes and measures
We defined less-favorable prognostic characteristics as stage IIB or higher, size >15 millimeters, positive nodes, and any one or more of these characteristics. We used log-binomial regression to model the proportion of breast cancers with less-favorable characteristics following an annual versus biennial screen by 10-year age groups and by menopausal status and current postmenopausal hormone therapy use.
Results
Among premenopausal women, biennial screeners had higher proportions of tumors stage IIB+ (relative risk [RR]=1.28, 95% confidence interval [CI]=1.01-1.63, p=0.040), size >15 mm (RR=1.21, 95% CI=1.07-1.37, p=0.002), and with any less-favorable prognostic characteristic (RR=1.11, 95% CI=1.00-1.22, p=0.047) compared with annual screeners. Among women on postmenopausal hormone therapy, biennial screeners tended to have tumors with less-favorable prognostic characteristics compared to annual screeners; however, CIs were wide and differences had only borderline significance. The proportions of tumors with less-favorable prognostic characteristics were not significantly larger for biennial versus annual screeners among postmenopausal women not on hormone therapy, postmenopausal hormone therapy users after subdividing by type of hormone use, or any 10-year age group.
Conclusions and relevance
Premenopausal women diagnosed with breast cancer following biennial versus annual screening mammography are more likely to have tumors with less-favorable prognostic characteristics. Postmenopausal women not using hormone therapy who are diagnosed with breast cancer following a biennial or annual screen have similar proportions of tumors with less-favorable prognostic characteristics.
Introduction
The frequency at which women should receive screening mammography remains controversial in the United States. In 2009, the US Preventive Services Task Force (USPSTF) updated their breast cancer screening guidelines to recommend routine biennial mammography for women aged 50-74 years, based on modeling evidence suggesting that the harms of more frequent screening outweigh the small estimated added benefit of annual screening.1,2 In contrast, some organizations such as the American Cancer Society3 and other groups4-6 have recommended annual screening starting at age 40 for decades. However, during this time, mammography accuracy has improved,7,8 new breast cancer treatments have been developed, and interest in tailoring screening recommendations to individual risk to maximize the balance of benefits versus harms has increased.9-13
No head-to-head randomized controlled trials (RCTs) have compared annual to biennial screening. Thus, recommended screening intervals have mainly been influenced by interval cancer rates14 and inferential evidence on tumor growth rates observed in trials.15 Based on tumor biology, some have argued that screening intervals should be shorter for younger women, whereas less frequent screening may be sufficient for women aged 50 years and older.16-19 New RCTs comparing screening mammography intervals with mortality endpoints are impractical; thus today, screening interval guidelines must rely on observational data20-28 and modeling.2,13,29-31
The Breast Cancer Surveillance Consortium (BCSC) has published several large empirical studies comparing the benefits and harms of different screening intervals.20-25 These observational data suggest no difference in the proportion of advanced-stage invasive cancers with annual compared to biennial screening overall or for women aged 50 and older. These analyses classified all women with two screening mammograms 9-30 months apart into annual (median 13 months, range 9-18 months) versus biennial (median 24 months, range 19-30 months) screeners. Given the broad ranges used, these prior studies may not address subgroups of women who closely adhere to screening guidelines or evaluate whether screening at intervals more closely approximating 12 versus 24 months influences tumor characteristics in subgroups of women undergoing screening. To more specifically determine if annual versus biennial screening is associated with more favorable prognostic characteristics in younger or older women, we updated our prior analyses using more recent data and narrower definitions for annual (11-14 months) and biennial (23-26 months) screening. We evaluated whether proportions of tumors with less-favorable versus more-favorable prognostic characteristics differed by annual versus biennial screening in subgroups of women identified by age, menopausal status, and postmenopausal hormone therapy (HT) use.
Methods
Study Setting and Data Sources
We used data from the BCSC (http://breastscreening.cancer.gov).32 BCSC registries collect patient and clinical information from community radiology facilities with populations similar to the US population.33 Breast cancer diagnoses and tumor characteristics are obtained by linking with pathology databases; regional Surveillance, Epidemiology, and End Results (SEER) programs; and state tumor registries, with estimated completeness of reporting >94.3%.34 BCSC registries and the Statistical Coordinating Center received Institutional Review Board approval for active or passive consenting processes or a waiver of consent to enroll participants, link and pool data, and perform analysis. All procedures were Health Insurance Portability and Accountability Act compliant, and registries and the Coordinating Center received a Federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
Participants and Study Design
Women aged 40-85 years were included if diagnosed between 1996 and 2012 with an incident invasive breast cancer or ductal carcinoma in situ (DCIS), either as a screen-detected or interval cancer, and who had at least two screening mammography examinations 11-14 or 23-26 months apart before diagnosis. The time between the two screening examinations was used to classify women as annual (11-14 months) or biennial (23-26 months) screeners.
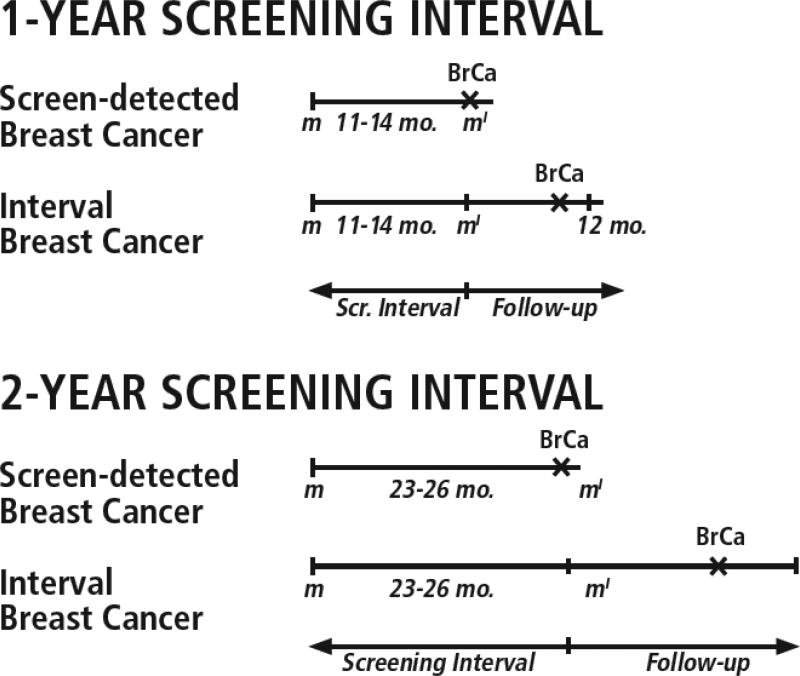
We aimed to capture two mechanisms by which breast cancers with less-favorable characteristics might result from a longer versus a shorter screening interval: (a) more tumor growth between two screening mammograms, leading to more advanced disease at screen detection, and (b) more time for a tumor to become symptomatic and clinically detected, and therefore more likely to be advanced, after a negative screening mammogram (Figure 1). Thus, we included both screen-detected and interval breast cancers diagnosed within one year of an annual screening mammogram or two years of a biennial screening mammogram, as would be done in the analysis of an RCT. Breast cancers following a positive screening mammogram were considered screen detected and those following a negative screening mammogram were considered interval cancers using standard BCSC definitions for classifying mammography results.35 Only mammograms that occurred at least one year before the end of complete capture of cancers by the BCSC for annual mammograms and at least two years for biennial mammograms were included.
Figure 1. Overview of study design.
This study captured two mechanisms by which a longer versus shorter screening interval might lead to breast cancers with less-favorable characteristics: (1) more time for tumor growth between the index screening mammogram m′ and the previous screen m; and (2) more time for a tumor to become symptomatic and clinically detected after a negative screening mammogram m′. BrCa indicates breast cancer; follow-up, follow-up period for cancer ascertainment.
Measures and Definitions
Screening mammograms were defined using the indication reported by the radiologist or technologist. To minimize misclassification of diagnostic mammography as screening, we excluded examinations that were unilateral or were preceded by a mammogram or breast ultrasound within nine months.
Women completed a questionnaire at each mammography examination to collect information on race and ethnicity, history of first-degree relatives (mother, sister, or daughter) with breast cancer, menopausal status, current postmenopausal HT use, and history of hysterectomy. If self-reported race/ethnicity was missing, we used information from cancer registries. Women were considered postmenopausal if they reported removal of both ovaries, periods that stopped naturally or no period for more than 365 days, current HT use, or age 55 or older.36 Women were considered premenopausal if they reported currently having periods or using oral contraceptives.36 Women were considered to have missing menopausal status if they were under 55 years and reported having had a hysterectomy without bilateral oophorectomy and were not using HT, or if menopausal status could not be determined based on available information. Postmenopausal women were classified by HT use. Women using HT with non-missing hysterectomy information (53%) were included in subanalysis by HT type. Women with a uterus using HT were classified as using estrogen plus progestogen; women without a uterus using HT were classified as using estrogen only, based on clinical practice, as previously described.22,37
Four outcomes measured less-favorable prognostic characteristics: American Joint Committee on Cancer (AJCC)38 stage IIB or higher; size >15 millimeters; positive nodes; and a measure of any one or more of these characteristics. For 262 women missing AJCC stage (3% of invasive cancers), stage IIB or higher was imputed based on tumor size or extension, nodal status, metastasis, or SEER summary stage, as previously described.22 In sensitivity analyses to evaluate our choices for stage and size thresholds, we classified tumors as stage IIA or higher and size >20 mm.
Statistical Analysis
We described the participant population by screening interval. We estimated the proportion of women with invasive cancer versus DCIS. Among women with invasive cancer, we estimated the distribution of tumor characteristics (stage, size, lymph node status) at diagnosis by screening interval, and separately by age group and by menopausal status and HT use. Among women with invasive breast cancer, we used log-binomial regression39 to estimate relative risks (RR) and 95% confidence intervals (CIs) of less-favorable versus more-favorable invasive tumor characteristics associated with screening interval by age group and by menopausal status and postmenopausal HT use, adjusting for race/ethnicity, first-degree family history of breast cancer, and BCSC registry. In one case for which the log-binomial model could not be estimated, we used Poisson regression with robust error variances. This approach gave results very similar to log-binomial regression in cases that could be estimated using both methods. Based on the observed numbers of women with invasive breast cancer, we had 80% power with a two-sided alpha of 0.05 to detect RRs within age and menopausal status groups of approximately 1.25-1.35 for stage IIB or higher, 1.20-1.30 for positive nodes, and 1.10-1.20 for tumors >15 mm and the measure of any one or more characteristics. For analyses subdivided by HT type, we had 80% power to detect an RR of 1.25-1.55. Analyses were performed in SAS® software, Version 9.2 (SAS Institute, Cary, NC).
Results
Among 15,440 women with breast cancer, most were ≥50 years old, white, and postmenopausal (Table 1). Biennial screeners were more likely to be in the youngest (40-49 years) or oldest (70-85 years) age groups and less likely than annual screeners to have a family history of breast cancer. Among annual screeners, 77.8% of cancers were screen detected compared to 72.8% for biennial screeners.
Table 1.
Population Characteristics by Screening Interval for Women With Breast Cancer Who Underwent Screening Mammography Between 1996 and 2011.
| Screening Intervala |
||
|---|---|---|
| Characteristic | Annual | Biennial |
| Total number of women | 12,070 | 3,370 |
| Screening interval time, median, months | 13 | 24 |
| Age, % | ||
| 40-49 y | 13.6 | 18.2 |
| 50-59 y | 29.7 | 27.4 |
| 60-69 y | 29.4 | 25.3 |
| 70-85 y | 27.3 | 29.1 |
| Race/ethnicity, % | ||
| White, Non-Hispanic | 78.4 | 77.2 |
| Black, Non-Hispanic | 4.5 | 4.5 |
| Hispanic | 4.5 | 5.3 |
| Asian/Pacific Islander | 4.9 | 7.3 |
| American Indian/Alaska Native | 0.4 | 0.7 |
| Other or mixed race | 1.1 | 1.4 |
| Unknown | 6.3 | 3.7 |
| Menopausal Status, % | ||
| Premenopausal | 12.6 | 14.9 |
| Postmenopausal without hormone therapy use | 42.5 | 40.1 |
| Postmenopausal with hormone therapy use | 21.8 | 21.0 |
| Surgical menopausal or unknown | 23.1 | 23.9 |
| Type of postmenopausal hormone therapy use at screen, %b | ||
| Estrogen + progestogen | 46.3 | 53.8 |
| Estrogen only | 53.7 | 46.2 |
| First-degree family history of breast cancer, % | ||
| Yes | 23.3 | 18.3 |
| No | 67.4 | 73.0 |
| Unknown | 9.3 | 8.8 |
| Type of detection, %c | ||
| Screen detected (True positive screen) | 77.8 | 72.8 |
| Interval detected (False negative screen) | 22.2 | 27.2 |
Annual cancers diagnosed within 12 months of screening exam performed 11-14 months after prior mammogram; Biennial cancers diagnosed within 24 months of screening exam performed 23-26 months after prior mammogram.
Restricted to 1767 women with known hysterectomy status: women with uterus assumed to use estrogen plus progestogen; without uterus assumed to use estrogen only.
Screen-detected breast cancer diagnosed after a positive screening mammography result and interval breast cancer detected after a negative screening mammography result and before the next screening examination.
The proportion of DCIS versus invasive cancers and the proportion of invasive tumors associated with less-favorable versus more-favorable prognostic characteristics decreased with age (Table 2). For example, 21.3-24.2% of women age 40-49 diagnosed with an invasive cancer after a annual or biennial screen were stage IIB or higher, compared to 16.4% or less among women 60 and older. Within age groups, the proportions of invasive tumors versus DCIS were similar among annual versus biennial screeners. Only small and inconsistent differences were seen in the proportions of invasive tumors with more-favorable versus less-favorable characteristics for annual versus biennial screeners.
Table 2.
Distribution of Tumor Characteristics by Age and Screening Interval
| Age |
||||||||
|---|---|---|---|---|---|---|---|---|
| 40-49 y Screening Intervala | 50-59 y Screening Intervala | 60-69 y Screening Intervala | 70-85 y Screening Intervala | |||||
| Tumor Characteristic | Annual | Biennial | Annual | Biennial | Annual | Biennial | Annual | Biennial |
| No. of breast cancers (N=15,440) | 1,645 | 613 | 3,579 | 923 | 3,549 | 853 | 3,297 | 981 |
| DCIS, % (n=3,340) | 26.3 | 27.4 | 24.4 | 22.0 | 20.7 | 18.6 | 18.6 | 16.0 |
| Invasive, % (n=12,100) | 73.7 | 72.6 | 75.6 | 78.0 | 79.3 | 81.4 | 81.4 | 84.0 |
| AJCC stage | ||||||||
| No. of invasive cancers | 1,137 | 416 | 2,460 | 665 | 2,542 | 644 | 2,429 | 735 |
| Stage I, % | 54.9 | 51.0 | 57.2 | 56.4 | 62.3 | 64.0 | 68.2 | 65.7 |
| Stage IIA, % | 23.6 | 24.3 | 22.7 | 24.4 | 21.0 | 20.8 | 17.9 | 21.6 |
| Stage IIB, % | 12.3 | 13.2 | 10.9 | 9.6 | 8.9 | 7.5 | 7.3 | 6.4 |
| Stage III or IV, % | 9.2 | 11.5 | 9.2 | 9.6 | 7.8 | 7.8 | 6.6 | 6.3 |
| AJCC stage IIB or higherb | ||||||||
| No. of invasive cancers | 1,155 | 425 | 2,532 | 680 | 2,616 | 666 | 2,506 | 782 |
| Yes, % | 21.3 | 24.2 | 19.7 | 19.0 | 16.4 | 14.7 | 13.6 | 12.1 |
| No, % | 78.7 | 75.8 | 80.3 | 81.0 | 83.6 | 85.3 | 86.4 | 87.9 |
| Tumor Size | ||||||||
| No. of invasive cancers | 1,171 | 426 | 2,597 | 690 | 2,673 | 668 | 2,569 | 776 |
| <10 mm, % | 23.7 | 20.2 | 27.8 | 23.9 | 30.6 | 25.9 | 33.8 | 28.2 |
| 10 to <15 mm, % | 22.6 | 17.1 | 22.9 | 24.2 | 24.3 | 23.8 | 24.8 | 26.3 |
| 15 to 20mm, % | 23.0 | 28.6 | 21.0 | 24.1 | 20.4 | 26.2 | 20.0 | 24.2 |
| >20 mm, % | 30.7 | 34.0 | 28.2 | 27.8 | 24.8 | 24.1 | 21.4 | 21.3 |
| Lymph node | ||||||||
| No. of invasive cancers | 1,189 | 435 | 2,621 | 692 | 2,725 | 672 | 2,603 | 800 |
| Positive, % | 32.5 | 35.9 | 28.9 | 30.5 | 24.3 | 22.6 | 19.2 | 18.6 |
| Negative, % | 67.5 | 64.1 | 71.1 | 69.5 | 75.7 | 77.4 | 80.8 | 81.4 |
| Any one or more less-favorable characteristicc | ||||||||
| No. of invasive cancers | 1,171 | 425 | 2,545 | 685 | 2,627 | 662 | 2,505 | 774 |
| Any, % | 59.1 | 63.1 | 54.0 | 53.7 | 48.6 | 49.7 | 44.0 | 44.6 |
| None, % | 40.9 | 36.9 | 46.0 | 46.3 | 51.4 | 50.3 | 56.0 | 55.4 |
Abbreviation: DCIS, ductal carcinoma in situ; AJCC, American Joint Committee on Cancer.
Annual includes cancers diagnosed within 12 months of screening exam performed 11-14 months after prior mammogram; Biennial includes cancers diagnosed within 24 months of screening exam performed 23-26 months after prior mammogram.
AJCC stage IIB or higher was imputed based on tumor size or extension, nodal status, metastasis, or SEER summary stage, when available, for women missing AJCC stage.
Stage IIB or higher, tumor size >15 mm, or positive nodes.
Premenopausal women had higher proportions of DCIS versus invasive cancers and invasive tumors with less-favorable prognostic characteristics than postmenopausal women (Table 3). For example, 19.8-25.7% of premenopausal women diagnosed with an invasive cancer after a annual or biennial screen were stage IIB or higher compared to 13.2-15.8% of postmenopausal women not using HT and 16.1-18.4% of HT users. Within most groups, the proportions of invasive tumors versus DCIS were similar among annual versus biennial screeners; however, postmenopausal women not using HT had a higher proportion of invasive cancers if they were screened biennially compared to annually. Among premenopausal women, women screened biennially versus annually had a higher proportion of stage IIB or higher tumors (25.7% vs. 19.8%), tumors >15 mm (65.3% vs. 54.6%), and node-positive disease (36.6% vs. 31.3%). Differences in these tumor characteristics among postmenopausal women were small and inconsistent, regardless of HT use.
Table 3.
Distribution of Tumor Characteristics by Screening Interval, Menopausal Status, and Postmenopausal Hormone Therapy Use
| Menopausal Status | Type of Hormone Therapy Usea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal | Postmenopausal, without hormone therapy use | Postmenopausal, with hormone therapy use | Estrogen plus progestogen use | Estrogen only use | ||||||
| Screening Intervalb | Screening Intervalb | Screening Intervalb | Screening Intervalb | Screening Intervalb | ||||||
| Tumor Characteristic | Annual | Biennial | Annual | Biennial | Annual | Biennial | Annual | Biennial | Annual | Biennial |
| No. of breast cancers (N=11,850) | 1,525 | 502 | 5,130 | 1,353 | 2,632 | 708 | 584 | 273 | 676 | 236 |
| DCIS, % (n=2,469) | 24.1 | 27.7 | 21.6 | 16.9 | 18.9 | 18.4 | 14.4 | 15.4 | 20.3 | 19.9 |
| Invasive, % (n=9,381) | 75.9 | 72.3 | 78.4 | 83.1 | 81.1 | 81.6 | 85.6 | 84.6 | 79.7 | 80.1 |
| AJCC stage | ||||||||||
| No. of invasive cancers | 1,074 | 339 | 3,605 | 1,027 | 1,930 | 524 | 444 | 211 | 463 | 166 |
| Stage I, % | 54.2 | 46.9 | 64.4 | 63.5 | 61.9 | 61.6 | 61.0 | 62.1 | 60.0 | 60.8 |
| Stage IIA, % | 25.8 | 26.8 | 19.5 | 22.9 | 20.9 | 20.2 | 22.5 | 21.3 | 21.8 | 19.9 |
| Stage IIB, % | 10.5 | 13.6 | 9.0 | 6.8 | 8.7 | 8.4 | 8.8 | 8.5 | 9.9 | 9.0 |
| Stage III or IV, % | 9.5 | 12.7 | 7.1 | 6.8 | 8.5 | 9.7 | 7.7 | 8.1 | 8.2 | 10.2 |
| AJCC stage IIB or higherc | ||||||||||
| No. of invasive cancers | 1,095 | 346 | 3,720 | 1,071 | 1,982 | 547 | 454 | 224 | 482 | 174 |
| Yes, % | 19.8 | 25.7 | 15.8 | 13.2 | 16.9 | 17.6 | 16.1 | 16.1 | 17.6 | 18.4 |
| No, % | 80.2 | 74.3 | 84.2 | 86.8 | 83.1 | 82.4 | 83.9 | 83.9 | 82.4 | 81.6 |
| Tumor Size | ||||||||||
| No. of invasive cancers | 1,123 | 349 | 3,841 | 1,073 | 2,040 | 546 | 475 | 220 | 508 | 176 |
| <10 mm, % | 22.4 | 21.2 | 33.2 | 29.1 | 28.0 | 23.1 | 29.3 | 25.5 | 25.8 | 15.3 |
| 10 to <15 mm, % | 23.1 | 13.5 | 23.6 | 25.0 | 26.5 | 27.1 | 24.0 | 25.0 | 26.4 | 31.2 |
| 15 to 20mm, % | 24.1 | 26.9 | 18.6 | 22.6 | 22.2 | 27.5 | 22.5 | 27.3 | 21.1 | 30.1 |
| >20 mm, % | 30.5 | 38.4 | 24.6 | 23.4 | 23.3 | 22.3 | 24.2 | 22.3 | 26.8 | 23.3 |
| Lymph node | ||||||||||
| No. of invasive cancers | 1,130 | 355 | 3,882 | 1,088 | 2,066 | 556 | 479 | 224 | 511 | 180 |
| Positive, % | 31.3 | 36.6 | 21.3 | 20.7 | 26.5 | 28.4 | 25.5 | 25.0 | 26.4 | 29.4 |
| Negative, % | 68.7 | 63.4 | 78.7 | 79.3 | 73.5 | 71.6 | 74.5 | 75.0 | 73.6 | 70.6 |
| Composite measure of any less-favorable characteristicd | ||||||||||
| No. of invasive cancers | 1,105 | 349 | 3,735 | 1,062 | 1,995 | 547 | 462 | 222 | 483 | 176 |
| Any, % | 59.7 | 65.6 | 46.5 | 46.5 | 50.4 | 50.8 | 49.1 | 47.7 | 51.6 | 54 |
| None, % | 40.3 | 34.4 | 53.5 | 53.5 | 49.6 | 49.2 | 50.9 | 52.3 | 48.4 | 46 |
Abbreviation: DCIS, ductal carcinoma in situ; AJCC, American Joint Committee on Cancer.
Analysis restricted to women with known hysterectomy status. Women with a uterus were assumed to be using estrogen plus progestogen. Women without a uterus were assumed to be using estrogen only.
Annual includes cancers diagnosed within 12 months of screening exam performed 11-14 months after prior mammogram; Biennial includes cancers diagnosed within 24 months of screening exam performed 23-26 months after prior mammogram.
AJCC stage IIB or higher was imputed based on tumor size or extension, nodal status, metastasis, or SEER summary stage, when available, for women missing AJCC stage.
Stage IIB or higher, tumor size >15 mm, or positive nodes.
We calculated the RRs of less-favorable tumor characteristics for women with invasive breast cancer following a biennial versus annual screen, adjusting for race/ethnicity, family history of breast cancer, and BCSC registry (Table 4). Within age groups, RR estimates were close to one with no significant differences between biennial versus annual screeners. However, among premenopausal women, compared to annual screeners, biannual screeners were at increased risk of stage IIB or higher tumors (RR=1.28, 95% CI=1.01-1.63, p=0.040), tumors >15 mm (RR=1.21, 95% CI=1.07-1.37, p=0.002), and tumors with any less-favorable prognostic characteristic (RR=1.11, 95% CI=1.00-1.22, p=0.047). Among postmenopausal women not using HT at the time of the mammogram, RR estimates were close to one with no significant differences between biennial versus annual screeners except for a modest increased risk of tumors >15 mm (RR=1.11, 95% CI=1.00-1.22, p=0.045). Among postmenopausal women using HT at the time of the mammogram, RR estimates for biennial versus annual screeners were consistently above one, with borderline-significant increases in risk of tumors >15 mm (RR=1.13, 95% CI = 0.98-1.31, p=0.087), positive lymph nodes (RR=1.18, 95% CI = 0.98-1.42, p=0.089), and tumors with less-favorable prognosis (RR=1.12, 95% CI = 1.00-1.25, p=0.053). Subdividing HT users with known hysterectomy status by the likely type of HT used did not change most results, which remained statistically nonsignificant except for increased risk of tumors >15 mm among biennial versus annual screeners using estrogen plus progestogen (RR=1.38, 95% CI=1.04-1.82, p=0.024).
Table 4.
Relative Risk (95% confidence interval) of Less-favorable Invasive Cancer Characteristics for Biennial versus Annual Screeners, by Age, Menopausal Status, and Current Hormone Therapy Use, Adjusted for Race/Ethnicity, First-Degree Family History of Breast Cancer, and Breast Cancer Surveillance Consortium Registry Using Log-Binomial Regression Unless Otherwise Specified
| Tumor Prognostic Characteristic |
||||
|---|---|---|---|---|
| Stage IIB, III, or IV vs. I or IIAa | Tumor size >15 mm vs. <=15 mma | Lymph node positive vs. negative | Less- vs. more-favorable prognostic characteristicsa,b | |
| Age | ||||
| 40-49 y | 1.17 (0.93, 1.46) | 1.10 (0.98, 1.25) | 1.09 (0.92, 1.29) | 1.04 (0.94, 1.14) |
| 50-59 y | 0.98 (0.80, 1.21) | 1.09 (0.97, 1.21) | 1.05 (0.90, 1.22) | 1.03 (0.94, 1.12) |
| 60-69 y | 0.99 (0.79, 1.24) | 1.13 (1.00, 1.27) | 0.93 (0.78, 1.12) | 1.07 (0.97, 1.19) |
| 70-85 y | 0.98 (0.76, 1.27) | 1.13 (0.99, 1.29) | 0.91 (0.74, 1.12) | 1.05 (0.94, 1.18) |
| Menopausal status | ||||
| Premenopausal | 1.28 (1.01, 1.63) | 1.21 (1.07, 1.37) | 1.15 (0.96, 1.38) | 1.11 (1.00, 1.22) |
| Postmenopausal, without HT use | 0.95 (0.79, 1.15) | 1.11 (1.00, 1.22) | 0.89 (0.77, 1.04) | 1.03 (0.95, 1.12) |
| Postmenopausal, with HT use | 1.14 (0.89, 1.47) | 1.13 (0.98, 1.31) | 1.18 (0.98, 1.42) | 1.12 (1.00, 1.25) |
| Estrogen plus progestogen usedc | 1.01 (0.94, 1.08)d | 1.38 (1.04, 1.82) | 0.95 (0.64, 1.41) | 1.16 (0.91, 1.47) |
| Estrogen only usedc | 1.19 (0.78, 1.83) | 1.19 (0.95, 1.50) | 1.26 (0.90, 1.77) | 1.14 (0.94, 1.37) |
Abbreviation: HT, postmenopausal hormone therapy.
Bold, significantly different from one.
Less favorable = stage IIB or higher, size >15 mm, or node positive.
Analysis restricted to women with known hysterectomy status: with uterus assumed to be estrogen plus progestogen; without uterus assumed to be estrogen only.
Relative risk estimated by Poisson regression with robust error variance.
Sensitivity analyses that classified tumors as IIA or higher or size > 20 mm did not substantially change results (data not shown).
Discussion
Premenopausal women diagnosed with invasive breast cancer following a biennial screening mammogram were more likely to have tumors with less-favorable prognostic characteristics than women diagnosed following an annual screening mammogram. In contrast, postmenopausal women diagnosed with invasive breast cancer after biennial versus annual screening showed no statistically significant differences in the likelihood of less-favorable prognostic characteristics, with the exception of small differences of borderline significance among women taking postmenopausal HT. We found no statistically significant differences in breast tumor prognostic characteristics for biennial versus annual screeners within 10-year age groups.
Our findings suggest that menopausal status may be more important than age when considering breast cancer screening intervals, which is biologically plausible. Tumors exposed to estrogen may grow faster, decreasing the detectable preclinical phase and resulting in a higher proportion of interval cancers with poorer tumor characteristics.14,15,18 In addition, breast density decreases after menopause, making it easier to diagnose breast cancers when they are smaller.8,40,41 In our sample of premenopausal women with breast cancer, 70% were age 40-49 and 30% were age 50-54. In a study of all women in the BCSC, only 10% of women aged 40-49 were postmenopausal and 25% of women aged 50-54 were premenopausal.36 Thus, if screening guidelines were based on menopausal status rather than age, some women between ages 40-54 might be recommended for more frequent screening and others, less frequent screening.
Our study refines prior BCSC studies that used wider screening intervals to classify women as annual or biennial screeners.20-25 Similar to our study, these prior studies found no difference in the proportion of invasive cancers with less favorable prognostic characteristics with biennial versus annual screening for women aged 50 and older.20-22 In contrast to our study, White et al. found that women aged 40-49 were less likely to have late versus early stage invasive cancer if screened annually compared to biennially;20 however, an updated analysis with more recent data that included digital mammography found no difference by screening interval in the proportions of late-stage disease for women aged 40-49 years, consistent with our findings.21 Kerlikowske et al.22 found a significantly higher proportion of less favorable tumors with biennial versus annual screening in women aged 40-49 years but only among women with extremely dense breasts; however, CIs were wide within density groups. Buist et al. showed the higher interval cancer rates in women aged 40-49 compared with older women observed in randomized trials were still evident in modern (film-screen based) service screening, which they attributed to younger women having faster-growing tumors and greater mammographic breast density.18
In other prior BCSC analyses, O'Meara and colleagues25 compared intervals within racial and ethnic groups. Biennial versus annual screening was not associated with overall increased risk of less-favorable tumor characteristics among women who were white, black, or Hispanic and aged 40-49 years, or among Asian women aged 50-74; however, Hispanic women aged 50-74 years who were screened biennially versus annually had an increased risk of late-stage disease and larger tumors, and Asian women aged 40-49 who were screened biennially were at high risk of a node-positive diagnosis. Dittus et al.23 observed that premenopausal obese women undergoing biennial screening had a borderline significantly increased risk of diagnosis with a tumor >20 mm relative to annual screeners. In contrast, across all body mass index categories, postmenopausal women undergoing biennial screening versus annual screening did not present with more advanced stage or larger tumor sizes. Braithwaite et al.24 examined tumor characteristics among women aged 66-89 years and found no statistically significant difference in adverse tumor characteristics by screening interval within age-by-comorbidity subgroups. Kerlikowske et al.22 found women aged 50-74 years undergoing biennial screening mammography had similar risk of advanced stage disease as women undergoing annual mammography within subgroups defined by HT use and breast density.
These findings add to the body of evidence that is providing greater confidence in the potential for advising women and their providers about screening frequency based on personal risk factors. When considering recommendations regarding screening intervals, the potential benefit of diagnosing cancers at an earlier stage must be weighed against the increased potential for harms associated with more frequent screening such as false positive recalls and biopsies, which are 1.5 to 2 times higher in annual versus biennial screeners.2,21-25,29,30 Future studies should focus on strategies to reduce these harms. We also need are studies that improve our understanding of tumor growth rates and aggressiveness among pre and postmenopausal women, the duration of the transition from shorter to longer sojourn times, and the degree to which risk factors may change the association between menopausal status, screening interval, and tumor characteristics observed here.
Our study has several limitations. First, the potential for confounding is always a concern in observational studies. For example, women who know they have breast cancer risk factors might undergo more frequent screening than women without these factors. We adjusted for family history, race, and ethnicity in our analyses to minimize bias, but did not adjust for other risk factors such as benign breast disease or reproductive factors. Second, some of our comparisons might be significant by chance alone so the magnitude and consistency of differences and CI widths should be considered. Another limitation is that we maximized sample sizes within subgroups by including data back to 1996, which included film-screen mammograms. Overall, the sensitivities of digital and film-screen mammography are similar, but sensitivity may be higher for digital mammography in some subgroups, especially women with dense breasts and premenopausal women.8,42,43 We did not collect HT type, relying instead on a surrogate based on hysterectomy status, which was available for only 53% of HT users. Results within HT-type subgroups were inconsistent and had wide CIs, limiting our ability to make inferences in this group. Lastly, we did not measure breast cancer mortality. Thus, we do not know if the observed increases in the proportions of less favorable tumors with biennial versus annual screening would result in differences in breast cancer mortality.
Conclusions
Premenopausal women diagnosed with breast cancer following a biennial mammogram are more likely to have tumors with less-favorable prognostic characteristics than women with breast cancers diagnosed after annual screening. Postmenopausal women not using HT who are diagnosed with breast cancer following a biennial or annual screen have similar proportions of tumors with less-favorable prognostic characteristics. Results are less clear for women using postmenopausal HT. Our findings of a lower proportion of less favorable tumors with more frequent screening in premenopausal women, and no statistically significant difference in the proportion of less favorable tumors in postmenopausal women by screening interval, adds to evidence about the potential benefits and harms of screening that policymakers can use to set guidelines about screening intervals and women can use when making personal screening decisions with their health care providers.
ACKNOWLEDGEMENTS
Chris Tachibana from Group Health Research Institute provided scientific editing. This research was supported the American Cancer Society. Collection of mammography performance data was supported by the National Cancer Institute's Breast Cancer Surveillance Consortium (BCSC; P01CA154292 and HHSN261201100031C) and U54CA163303. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S.; For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. The American Cancer Society Inc. requested the study to inform their screening guidelines, but had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The National Cancer Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication. Weiwei Zhu, MS, from Group Health Research Institute had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/.
REFERENCES
- 1.U.S. Preventive Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 2.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013 Mar-Apr;63(2):88–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 4.Breast cancer screening. Obstet Gynecol. 2011 Aug;118(2 Pt 1):372–382. doi: 10.1097/AOG.0b013e31822c98e5. Practice bulletin no. 122. [DOI] [PubMed] [Google Scholar]
- 5.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. Journal of the National Comprehensive Cancer Network : JNCCN. 2009 Nov;7(10):1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010 Jan;7(1):18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa LE, Barlow WE, Anderson ML, et al. Time trends in radiologists’ interpretive performance at screening mammography from the community-based Breast Cancer Surveillance Consortium, 1996-2004. Radiology. 2010 Jul;256(1):74–82. doi: 10.1148/radiol.10091881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: A cohort study. Ann Intern Med. 2011;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brawley OW. Risk-based mammography screening: An effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156(9):662–663. doi: 10.7326/0003-4819-156-9-201205010-00012. [DOI] [PubMed] [Google Scholar]
- 10.Vilaprinyo E, Forne C, Carles M, et al. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PLoS One. 2014;9(2):e86858. doi: 10.1371/journal.pone.0086858. 20140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandelblatt JS, Stout N, Trentham-Dietz A. To screen or not to screen women in their 40s for breast cancer: is personalized risk-based screening the answer? Ann Intern Med. 2011 Jul 5;155(1):58–60. doi: 10.7326/0003-4819-155-1-201107050-00008. [DOI] [PubMed] [Google Scholar]
- 12.Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014 Oct 1;120(19):2955–2964. doi: 10.1002/cncr.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011 Jul 5;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabar L, Faberberg G, Day NE, Holmberg L. What is the optimum interval between mammographic screening examinations? An analysis based on the latest results of the Swedish two-county breast cancer screening trial. Br J Cancer. 1987 May;55(5):547–551. doi: 10.1038/bjc.1987.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabar L, Fagerberg G, Chen HH, Duffy SW, Gad A. Tumour development, histology and grade of breast cancers: prognosis and progression. Int J Cancer. 1996 May 16;66(4):413–419. doi: 10.1002/(SICI)1097-0215(19960516)66:4<413::AID-IJC1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Baker LH. Breast Cancer Detection Demonstration Project: five-year summary report. CA Cancer J Clin. 1982 Jul-Aug;32(4):194–225. doi: 10.3322/canjclin.32.4.194. [DOI] [PubMed] [Google Scholar]
- 17.Tabar L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985 Apr 13;1(8433):829–832. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- 18.Buist DS, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst. 2004;96(19):1432–1440. doi: 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- 19.Anderson TJ, Waller M, Ellis IO, Bobrow L, Moss S. Influence of annual mammography from age 40 on breast cancer pathology. Human pathology. 2004 Oct;35(10):1252–1259. doi: 10.1016/j.humpath.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 20.White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96(24):1832–1839. doi: 10.1093/jnci/djh337. [DOI] [PubMed] [Google Scholar]
- 21.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011 Oct 18;155(8):481–492. doi: 10.1059/0003-4819-155-8-201110180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerlikowske K, Zhu W, Hubbard RA, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Internal Medicine. 2013 May 13;173(9):807–816. doi: 10.1001/jamainternmed.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dittus K, Geller B, Weaver DL, et al. Impact of Mammography Screening Interval on Breast Cancer Diagnosis by Menopausal Status and BMI. J Gen Intern Med. 2013 Jun 13; doi: 10.1007/s11606-013-2507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013 Mar 6;105(5):334–341. doi: 10.1093/jnci/djs645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Meara ES, Zhu W, Hubbard RA, et al. Mammographic screening interval in relation to tumor characteristics and false-positive risk by race/ethnicity and age. Cancer. 2013 Nov 15;119(22):3959–3967. doi: 10.1002/cncr.28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coldman A, Phillips N, Warren L, Kan L. Breast cancer mortality after screening mammography in British Columbia women. Int J Cancer. 2007 Mar 1;120(5):1076–1080. doi: 10.1002/ijc.22249. [DOI] [PubMed] [Google Scholar]
- 27.Randall D, Morrell S, Taylor R, Hung W. Annual or biennial mammography screening for women at a higher risk with a family history of breast cancer: prognostic indicators of screen-detected cancers in New South Wales, Australia. Cancer Causes and Control. 2009;20(5):559–566. doi: 10.1007/s10552-008-9264-0. [DOI] [PubMed] [Google Scholar]
- 28.Goel A, Littenberg B, Burack RC. The association between the pre-diagnosis mammography screening interval and advanced breast cancer. Breast Cancer Res Treat. 2007 May;102(3):339–345. doi: 10.1007/s10549-006-9334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: A comparative modeling study of risk. Ann Intern Med. 2012;156(9):609–617. doi: 10.1059/0003-4819-156-9-201205010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stout NK, Lee SJ, Schechter CB, et al. Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. J Natl Cancer Inst. 2014 Jun;106(6):dju092. doi: 10.1093/jnci/dju092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donoghue C, Eklund M, Ozanne EM, Esserman LJ. Aggregate Cost of Mammography Screening in the United States: Comparison of Current Practice and Advocated Guidelines. Annals of Internal Medicine. 2014;160(3):145–153. doi: 10.7326/M13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;169(4):1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 33.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235(3):775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]
- 34.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 35.Breast Cancer Surveillance C. BCSC Glossary of Terms. 2009 http://breastscreening.cancer.gov/data/bcsc_data_definitions.pdf. Accessed 3/9/2015, 2015.
- 36.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010 May 20;67(1):60–66. doi: 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerlikowske K, Miglioretti DL, Ballard-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21(23):4314–4321. doi: 10.1200/JCO.2003.05.151. [DOI] [PubMed] [Google Scholar]
- 38.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; New York, NY: 2002. [Google Scholar]
- 39.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 40.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 41.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007 Mar 7;99(5):386–395. doi: 10.1093/jnci/djk066. [DOI] [PubMed] [Google Scholar]
- 42.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 43.Pisano ED, Hendrick RE, Yaffe MJ, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008 Feb;246(2):376–383. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]