Abstract
Inflammation plays a critical role in the pathogenesis of ischemic stroke. This process depends, in part, upon proinflammatory factors released by activated resident central nervous system (CNS) microglia (MG). Previous studies demonstrated that transfer of IL-10+ B-cells reduced infarct volumes in male C57BL/6J recipient mice when given 24 h prior to or therapeutically at 4 h or 24 h after experimental stroke induced by 60 min middle cerebral artery occlusion (MCAO). The present study assesses possible sex differences in immunoregulation by IL-10+ B-cells on primary male vs. female MG cultured from naïve and ischemic stroke-induced mice. Thus, MG cultures were treated with recombinant (r)IL-10, rIL-4 or IL-10+ B-cells after lipopolysaccharide (LPS) activation and evaluated by flow cytometry for production of proinflammatory and anti-inflammatory factors. We found that IL-10+ B-cells significantly reduced MG production of TNF-α, IL-1β and CCL3 post-MCAO and increased their expression of the anti-inflammatory M2 marker, CD206, by cell-cell interactions. Moreover, MG from female vs. male mice had higher expression of IL-4 and IL-10 receptors and increased production of IL-4, especially after treatment with IL-10+ B-cells. These findings indicate that IL-10-producing B-cells play a crucial role in regulating MG activation, proinflammatory cytokine release and M2 phenotype induction, post-MCAO, with heightened sensitivity of female MG to IL-4 and IL-10. This study, coupled with our previous demonstration of increased numbers of transferred IL-10+ B-cells in the ischemic hemisphere, provide a mechanistic basis for local regulation by secreted IL-10 and IL-4 as well as direct B-cell/MG interactions that promote M2+-MG.
Keywords: MCAO, IL-10-secreting B-cells, microglia, M1 and M2 MG states
Introduction
Stroke still remains the fourth leading cause of death and one of the major causes of disability in the United States, affecting 7 million people (Onwuekwe and Ezeala-Adikaibe 2012; Towfighi and Saver 2011). Ischemic stroke constitutes 87% of all strokes and is caused by the occlusion of a blood vessel. Inflammation plays a critical role in the pathogenesis of ischemic stroke and other forms of ischemic brain injuries. Key features of this neuroinflammatory reaction are the invasion of peripheral immune cells across the damaged blood–brain barrier (BBB), the activation of resident innate immune cells (microglia) and the production of inflammatory humoral mediators such as cytokines and chemokines (Iadecola and Anrather 2011). Although the cells of the innate immune system, especially neutrophils and monocytes, remain one focus, recent studies have demonstrated that the resident microglia are also one of the critical players in controlling the course of inflammation and/or generating ant-inflammatory/repair in the ischemic regions of the brain after injury (Taylor and Sansing 2013).
Microglia (MG) are the resident immune cells of the central nervous system (CNS). Microglia and activated macrophages recruited from the periphery are rapidly mobilized to the site of injury and represent the first line of defense against brain injuries such as stroke. These cells release effector molecules and recruit other immune cells (Kissela et al. 2009), but may have opposing functions with respect to neurological recovery (Patel et al. 2013). On one hand, activated MG and macrophages promote brain recovery by clearing cell debris, resolving local inflammation and releasing trophic factors (Hanisch and Kettenmann 2007; Kwon et al. 2013; Miron et al. 2013; Thored et al. 2009). On the other, these cells can hinder CNS repair and expand tissue damage (Ekdahl et al. 2003; Liu et al. 2007; Miron et al. 2013). The role of microglia/macrophages in ischemic brain injury, however, is still unclear. A number of studies contend that microglia/macrophages are highly plastic cells that can assume diverse phenotypes and engage different functional programs in response to specific microenvironmental signals (Chu et al. 2012; Hu et al. 2015; Sozmen et al. 2009). In particular, in vitro stimulation with lipopolysaccharide and interferon-γ (IFN-γ) promotes the differentiation of “classically activated” M1 microglia/macrophages that typically leads to release of destructive pro-inflammatory mediators (Rosenzweig and Carmichael 2013). In contrast, interleukin (IL)-4 (Pepe et al. 2014; Xiong et al. 2011) and IL-10 induce an “alternatively activated” M2 phenotype that possesses neuroprotective properties (Chu et al. 2012; Jalal et al. 2012; Wang et al. 2013). Typically, the damaged tissue environment in vivo immediately after stroke attracts neutrophils and promotes an M1 phenotype (Hu et al. 2012), including M1 microglia and recruited peripheral macrophages, that increase inflammation and cell death beyond the initial ischemic infarct (Denker et al. 2007; Schilling et al. 2005). However, anti-inflammatory signals such as IL-4 or IL-10 are also triggered that may ameliorate tissue damage (Hu et al. 2012), enhance M2 responses and reduce cognitive impairment following cerebral ischemia (Cherry et al. 2014; Perez-de Puig et al. 2013; Xiong et al. 2011). The dual roles of distinctly polarized macrophage populations have been reported in several CNS diseases, including multiple sclerosis (Miron et al. 2013) and spinal cord injury (Chu et al. 2012). The concept of microglial M1 and M2 phenotypes is also being addressed in the field of stroke research (Butovsky et al. 2006b). However, a comprehensive characterization of microglia/macrophage polarization after ischemic brain injury is still missing.
The severity of ischemic damage is influenced by sex, but few stroke laboratories study female animals or use cell models of ischemic brain injury that are sex-specific. In part, this is due to the historical assumption that cellular/molecular injury and repair mechanisms are the same in males vs. females. The persistent lack of pre-clinical animal data in both sexes poses a severe evidence gap for clinical trials that will test new therapies in males and females. It is now clear that males and females respond to stroke differently. Females have a lower incidence of stroke and are relatively protected from immediate responses to ischemia compared to males (Alkayed et al. 1998; Murphy et al. 2004; Sudlow and Warlow 1997). The underlying molecular and inflammatory mechanisms that lead to stroke-induced sex discrepancy have not been extensively studied.
Our laboratory’s long-term goal in designing stroke therapies has been to not only determine the critical detrimental factors but also to understand how the protective immunological cells act in acute stroke. Over the past few years we have convincingly demonstrated the protective role of a small sub-population of B cells, called regulatory B cells (Bregs), that have the capacity to produce the anti-inflammatory cytokine IL-10. The immunoregulatory role of IL-10+ B cells (Bregs) was clearly demonstrated by a significant reduction in the infarct size in not only B-cell-deficient (μMT−/−) mice (Bodhankar et al. 2013) but also in B-cell-sufficient mice (Bodhankar et al. 2014b). IL-10-rich B-cells were efficient in limiting infarct volumes when given prophylactically (24 h before) or therapeutically (4 h after and as late as 24 h after) MCAO-induction (Bodhankar et al. 2014a; Bodhankar et al. 2014b). The primary purpose of the present study was to determine whether IL-10-rich B-cells can elicit immunoregulation in both male and female mice and also whether there are any sex differences upon treating MG, obtained from ischemic stroke-induced mice. Our results clearly demonstrate that treatment with IL-10+ B-cells significantly ameliorated proinflammatory responses generated in microglia post-MCAO in both sexes. The dampening of proinflammatory responses by the Bregs was more evident in the female mice as compared to the males. Moreover, the IL-10+ B-cells could not only dampen the proinflammatory status of the microglia, but also elicit an anti-inflammatory M2 MG phenotype. Our studies are the first to demonstrate that while the proinflammatory milieu in the microglia could be dampened by soluble factors secreted by the Bregs, the induction of M2 phenotype was a result of cell-to-cell interaction with the Bregs.
Materials and methods
Animals
Ten to twelve week old wild type C57BL/6J (WT) male and female mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Naïve WT male mice were housed at the VA Portland Health Care System (VAPORHCS), and mice that were used for transient focal cerebral ischemia were housed at the Oregon Health & Science University Animal Resource Facility. Age and sex matched B6(Cg)-Il10tm1.1Karp/J (IL-10GFP) mice, used as donors of IL-10+ B cells, were obtained from the in-house breeding colony in the Animal Resource Facility at the VAPORHCS, consistent with institutional guidelines. This strain has been previously described (Bodhankar et al. 2014b; Madan et al. 2009). In brief, the sequence for enhanced green fluorescent fusion protein (eGFP) has been inserted into the non-coding region of Il10 on a C57BL/6J background, leading to co-expression of eGFP and IL-10. All animals were housed under pathogen-free conditions with ad libitum access to food and water. Procedures were performed in accordance with the National Institute of Health Guidelines for the Care & Use of Laboratory Animals and both VAPORHCS and Oregon Health & Science University Animal Care and Use Committees approved all protocols.
Primary CNS Culture and Purification of Microglia
Tissue culture and enrichment for microglia
Mice were deeply anesthetized using 4% isoflurane inhaled anesthetic and maintained at 2.5% isoflurane during intracardiac perfusion with cold phosphate buffered saline (PBS, Fisher Scientific, Fair Lawn, NJ, USA). Brains were removed aseptically, olfactory bulbs and brain stem were removed and the remaining tissue washed twice in cold Hank’s balanced salt solution lacking Mg2+, Ca2+ and phenol red (HBSS, Life Technologies, Grand Island, NY, USA).
A unicellular suspension of CNS tissue was obtained using the protocol and reagents supplied in the Neural Tissue Dissociation Kit P from Miltenyi Biotec (Auburn, CA, USA) with few modifications (Moussaud and Draheim 2010). Briefly, tissues were disrupted mechanically on a gentleMACS tissue dissociator (Miltenyi Biotec) and incubated with the enzyme cocktails included with the kit. Following trituration using a P1000 pipette, the homogenate was passed through a 100µm nylon mesh screen (Fisher Scientific) and washed in HBSS to quell enzymatic activity.
After disaggregation, total brain cells were prepared for culture based on a published method (Moussaud and Draheim 2010). Myelin was removed by density centrifugation in 20% Percoll (GE Healthcare, Pittsburg, PA, USA), overlayed with cold HBSS for 20 minutes at 200 × g with no brake and then washed once in cold HBSS. Pelleted cells were resuspended in warmed Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 with Glutamax supplement (DMEM, Life Technologies) culture medium completed with 10% heat inactivated fetal bovine serum (FBS, GE Healthcare), 1% antibiotic-antimycotic (anti-anti, Life Technologies), and 5ng/mL carrier-free recombinant mouse granulocyte macrophage colony-stimulating factor (GM-CSF, Life Technologies) and counted. 1.3 × 106 cells were seeded into tissue culture grade poly L-lysine (L-lysine, Sigma-Aldrich, St. Louis, MO, USA) coated T75 cell culture treated flasks (Corning Life Sciences, Tewksbury, MA, USA) and placed in a 37°C incubator with 5% CO2 and 85% relative humidity. Culture supernatant was replaced twice weekly with 10mL fresh completed medium until confluency of cells was observed, at approximately three weeks.
Harvest of microglia from enriched cultures
Upon confluence, loosely adherent cells were dislodged from culture flasks by agitation using an orbital shaker (Benchmark, Edison, NJ, USA). Flasks were shaken at 280 revolutions per minute for 4 hours and supernatants were harvested and centrifuged. Before the start of the in vitro co-culture experiments, cells were resuspended in culture medium free of GM-CSF, counted, and seeded into 24 well plates at a density of 2×105 cells per well and incubated at 37°C and 5% CO2 for 5 days prior to assay. Remaining cells were checked for purity and their phenotype was determined by flow cytometry. CD11b+CD45+ microglia comprised ≥96% of cells harvested in this manner.
B-cell isolation and IL-10 enrichment
Splenocytes from IL-10-GFP reporter male and female mice were collected as described in (Bodhankar et al. 2014a). Splenic CD19+ B-cells were purified using paramagnetic bead-conjugated antibodies (Abs) from the CD19 cell isolation kit and subsequently separated by AutoMACS (Miltenyi Biotec, Auburn, CA). The negative fraction of the cells thus separated were CD19+ B-cells with a purity of ≥ 95%. CD19+ B-cells were suspended in RPMI 1640 medium (Mediatech, Manassas, VA, USA) supplemented with 2% Fetal Bovine Serum (FBS), 57.2μM 2-mercaptoethanol (BME, Sigma-Aldrich), 1% L-glutamate (Life Technologies), 1% sodium pyruvate (Life Technologies), and cultured in the presence of 1 μg/mL lipopolysaccharide (LPS, E. coli strain K12). After 48 h of culture, B-cells were harvested from culture plates, washed free of LPS and counted in 0.2% trypan blue (Life Technologies) in a cell counter (Nexcelom Bioscience, Lawrence, MA, USA), washed, and resuspended in DMEM culture medium without GM-CSF for co-culture with microglia.
B Cell:Microglia Co-culture
Co-culture setup
On the day of co-culture, the medium in 24 well plates was replaced with 1mL fresh culture medium or medium containing 10ng/mL LPS (Sigma-Aldrich). After 4 hrs, supernatants were discarded and one of the following treatments was given in 1mL of fresh culture medium: no treatment, 20ng recombinant IL-10 (rIL-10, Peprotech, Rocky Hill, NJ, USA), 20ng recombinant IL-4 (rIL-4, Peprotech), B cells at a ratio of 1:1 with microglia, B cells at a ratio of 1:2 with microglia. The MG cells were incubated with mentioned treatment at 37°C and 5% CO2 for 24 hours.
Examination of contact independent interactions
Co-cultures were set up as above, but B cells (at a 1:1 ratio) were separated from microglia by polyethylene terephthalate hanging cell culture inserts with a pore size of 1μm (EMD Millipore, Billerica, MA, USA). This size of pore was chosen to preclude direct contact between these populations while allowing the diffusion of secreted factors.
Analysis of cell populations by fluorescence-activated cell sorting (FACS)
Antibodies
All antibodies were purchased from BD Biosciences unless otherwise noted. Clones are indicated in parentheses. To determine microglia phenotype: anti-CD11b FITC (M1/70), anti-CD11b PerCPCy5.5 (M1/70), CD11c PECy7 (HL3), anti-CD45 APC (30-F11), anti-CD45 FITC (30-F11), anti-CD124 AlexaFluor 647 (mIL4R-M1), anti-CD210 PE (1B1.3a), anti-F4/80 PE (T45-2342), anti-I-Ab PE (AF6-120.1), anti-Ly-6G PE (1A8) were used. For staining of intracellular cytokines/chemokines: anti-CCL3 PE (DNT3CC, eBioscience), anti-CD206 APC (C068C2, Biolegend, San Diego, CA, USA), anti-IL-1β APC (NJTEN3, eBioscience), anti-IL-4 PE (11B11), anti-IL-10 APC (JES5-16E3) and anti-TNF PE (MP6-XT22) were used.
Extracellular staining
Single cell suspensions were washed with staining buffer (PBS with 0.1% NaN3 (Sigma-Aldrich) and 0.5% bovine serum albumin (BSA, Sigma-Aldrich)). Fc receptors were blocked with anti-CD16/32 antibody (2.4G2, BD Biosciences) and cells were incubated with monoclonal antibodies (mAbs) listed above. Unbound mAbs were washed away with staining buffer prior to four-color fluorescence flow cytometry analysis using a BD Accuri C6 flow cytometer (BD Biosciences).
Intracellular staining
Cells to be analyzed for intracellular cytokine production by flow cytometry received 1μL GolgiPlug (BD Biosciences, San Jose, CA, USA) protein transport inhibitor 4hrs prior to immunostaining. Cells were fixed in 4% paraformaldehyde (PFA, eBioscience) after extracellular stains were applied as above, then permeabilized in Permabilization/Wash buffer (Perm/Wash, BD Biosciences). mAbs against intracellular targets were incubated with cells in Perm/Wash, unbound mAbs were eluted and cells were resuspended in staining buffer for acquisition. Isotype matched mAb served as negative controls to demonstrate specificity. All data were acquired and analyzed using the Accuri C6 software included with the instrument.
Middle cerebral artery occlusion (MCAO) model
Transient focal cerebral ischemia was induced in male and female WT mice for 1 h by reversible right MCAO under isoflurane anesthesia followed by 96 h of reperfusion as previously described (Bodhankar et al. 2014b). The individual performing all MCAO surgeries was blinded to treatment group. Head and body temperature were controlled at 36.5 ± 1.0°C during surgery, MCAO, and early reperfusion with warm water pads and a heating lamp. The common carotid artery was exposed and the external carotid artery was ligated and cauterized. Unilateral MCAO was accomplished by inserting a 6-0 nylon monofilament surgical suture (ETHICON, Inc., Somerville, NJ, USA) with a heat-rounded and silicone-coated (Xantopren comfort light, Heraeus, Germany) tip into the internal carotid artery via the external carotid artery stump. Adequacy of MCAO was confirmed by monitoring cortical blood flow at the onset of the occlusion with a Laser Doppler Flowmetry (LDF) probe (Model DRT4, Moor Instruments, Inc., Wilmington, DE, USA) affixed to the skull. Animals were excluded if mean intra-ischemic LDF was greater than 30% pre-ischemic baseline (Bodhankar S et al., 2013). At 1 hour of occlusion, the occluding filament was withdrawn to allow for reperfusion and the incision was closed with 6–0 surgical sutures (ETHICON, Inc., Somerville, NJ, USA). Each mouse was then awakened and recovered in a separate cage with a warm water pad.
RNA isolation and reverse transcription-polymerase chain reaction
Total RNA was isolated from MG subject to the treatments indicated above using the RNeasy mini kit protocol (Qiagen, Valencia, CA, USA) and converted into cDNA using oligo-dT primers and Superscript RT II (Life Technologies). Quantitative real time PCR was performed on a StepOnePlus Real Time PCR System (Applied Biosystems, Foster City, CA, USA) using the TaqMan Gene Expression Assays in Taqman Universal Master Mix (all Applied Biosystems) that included primers for Cd80 and Cx3xr1. ΔCt was calculated against the expression of the endogenous control GAPDH. Fold change in targeted transcript expression was determined using the formula 2−ΔΔCt.
Statistical Analysis
Statistical significance for data obtained by flow cytometry analysis for yields and purity of cultured microglia was analyzed by Student’s t-test. Statistical significance for differences in cytokine/chemokine production after MG treatments were analyzed by one-way analysis of variance (ANOVA) followed by a post hoc Dunnett’s Multiple comparison test where values were compared to the LPS treatment. Statistical significance for difference between sexes was determined by two-way ANOVA followed by the post-hoc Bonferroni multiple comparison test. The criterion for statistical significance was p≤0.05. All values are reported as mean ± SEM. Significant differences are denoted as *p≤0.05; **p≤0.01; ***p≤0.001 for differences in treatments within each sex and #p≤0.05; ##p≤0.01 for differences in treatments between the sexes.
Results
Yield and purity of primary microglia cultures isolated from adult naïve mice
Studies were initiated in naïve WT male mice to optimize the conditions of MG isolation from adult mice. MG were isolated from the brains of naïve male 11-12 week old adult mice. Prior to seeding for mixed neuron/glia culture (days in vitro (div) 0), the number of cells yielded was calculated and the CD11b+CD45+ fraction was determined by flow cytometry. The glial cell culture was incubated at 37°C at 5% CO2 and replenished with fresh medium containing GM-CSF every 3 days. On day 21 of culture (21 div), the MG phenotype was re-characterized by flow cytometry. At 21 div, a conspicuous and significant rise was observed in the number of cells yielded per flask compared to the number of cells initially retrieved per brain (Fig. 1A). These cells were overwhelmingly double-positive for the microglia/monocyte markers CD11b and CD45 (>96%), indicating substantial, statistically significant enrichment of the MG population (Fig. 1b). Further analyses indicated that the percent of CD11b+CD45lo (resting MG) from day of seeding to day 21 div, went from 20% to 93.2% with only 4.5% of the MG being the CD11b+CD45high (activated microglia/monocytes) population (data not shown). Upon further phenotype characterization, the purified MG expressed the classic MG marker, F4/80 (98.3%). The MG also expressed minimal MHCII molecules, suggesting that they had an antigen-presenting capacity. They also had minimal expression of Gr-1, an inflammatory marker on monocytes (1.3%) and CD11c (5.8%), an antigen-presenting cell-phenotype acquired when microglia become activated (data not shown). Thus, we could successfully establish primary MG cultures resulting in increased yields and purity from the day of their isolation up until the day of harvest (21 div) for further co-culture studies.
Fig. 1. Yield and purity of primary microglia cultures isolated from adult naïve mice.
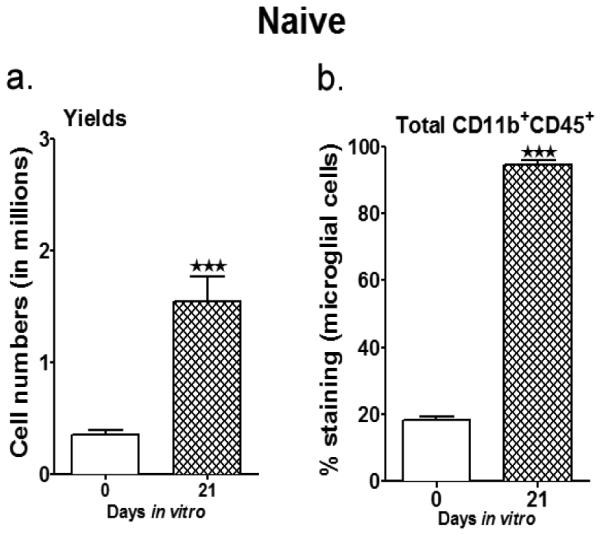
a. Summarizes the harvest yields on the day of MG isolation (mixed glial cells) and then after 21 days in vitro; with mixed glial cells cultured with 5 ng/ml GM-CSF through all the days. b. The purity of the MG was determined by staining for expression of CD11b and CD45. Values are given as mean ± S.E.M. Data presented are representative of n = 8 separate MG isolations and primary culture set-ups for naïve male WT mice. Statistical analysis was performed using Student’s t-test. Significant differences between sample means are indicated as ***P≤0.001 as compared to the values from day of MG isolation
Effects of rIL-10 and B cells on microglial activation
One of the best known consequences of MG activation is their release of toxic factors, including TNF-α, IL-1β, iNOS, a variety of reactive oxygen species and prostanoids (Ransohoff and Brown 2012). It is not fully known whether MG activation is detrimental to the adjoining injured neuron. However, it is well recognized that blocking MG activation confers neuroprotection in vivo and in vitro (Lalancette-Hebert et al. 2007). Our goal was to determine the concentration of recombinant interleukin-10 (rIL-10) needed to inhibit activation of MG and determine if secreted levels of IL-10 produced by the IL-10+ B cells was sufficient to inhibit MG activation. Studies were initiated in naïve WT male mice to optimize the conditions of MG isolation from adults. After phenotyping the primary MG cultures (demonstrated in Fig. 1), MG were activated such that they secreted proinflammatory factors. For activation, cultured primary adult MG (2 × 104 cells/well in a 24-well plate) were incubated with 10 ng/ml LPS for 4 h. After activation, LPS was washed off and MG were treated with 20 ng/ml IL-10 or IL-10+ GFP+ B-cells (in a 1:1 or 1:2 B:MG ratio) or vehicle. The frequencies of MG producing TNF-α were determined by flow cytometry 24 h later. Our results demonstrate that 20ng rIL-10 was able to significantly reduce the TNF-α levels (from ~14.7 % TNF-α+ LPS-treated MG to ~7.8%). Also, the TNF-α levels were significantly reduced when IL-10+ B-cells were added at a 1:1 ratio (6.8% TNF-α) (Fig. 2a). A ratio of 1:2 IL-10+ B cells:MG was also tested in an attempt to titrate the capacity of IL-10+ B cells to control inflammation and surprisingly could reduce the production of TNF-α levels to 7.8%.
Fig. 2. Effects of rIL-10 and B cells on microglial activation.
Primary MG, isolated and cultured from naïve WT male mice, were harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10ng/ml LPS for 4 hours. Supernatants were discarded after 4 hours and one of the following treatments was given in 1mL of fresh culture medium: no treatment, 20ng recombinant IL-10 (rIL-10), IL-10+ B cells at a 1:1 ratio or at a 1:2 ratio with MG. The MG cells were incubated with mentioned treatments at 37°C and 5% CO2 for 24 hours and proinflammatory cytokines A. TNF-α and B. IL-β and C. chemokine CCL3 (MIP-1α) were determined by flow cytometry. Values are given as mean ± S.E.M. Data presented are representative of n = 5, 3 and 2 separate co-culture set-ups, respectively for TNF-α, IL-1β and CCL3 with each condition done in duplicate for every experimental set-up. Statistical analysis was performed using One way ANOVA with post-hoc Dunnett test. Significant differences between sample means are indicated as * p≤0.05, ** p≤0.01, ***p≤0.001 as compared to the LPS-stimulated condition
Using the same experimental set-up, we also tested the ability of both rIL-10 and IL-10+ B cells to control the production of other pro-inflammatory parameters i.e. IL-1β and the chemokine CCL3 (MIP-1α). rIL-10 and B cells (in a 1:1 ratio) significantly reduced IL-1β production in MG isolated from naïve male mice (Fig. 2b) (from 13.1% to 7.45% with rIL-10 and 7.4% with B1:1 treatments). However this reduction was not significant when IL-10+ B cells were used at a 1:2 ratio (8.6% IL-1β production) relative to the MG. Similarly a remarkable reduction in CCL3 levels was observed when LPS-stimulated MG were cultured either with rIL-10 or B cells (both at 1:1 and 1:2 ratios) (Fig. 2c). CCL3 levels went from 14.12% to 7% for rIL-10, 4.8% for B cell (1:1) and 8.2% for B cell (1:2) treatments. Thus, primary MG obtained from naïve WT male mice responded to LPS stimulation to produce large quantities of pro-inflammatory mediators, which could be reduced to almost basal levels (Med), upon treatment with not only rIL-10 but more importantly also with IL-10-enriched B cells. These results provided a reliable approach for testing the efficacy of IL-10+ B cells in dampening the proinflammatory status of microglia, post-MCAO.
Enhanced dampening of proinflammatory status of microglia from female mice in response to treatment with IL-10+ B cells, post-MCAO
After having tested and optimized our co-culture system using naïve MG, we further extended our studies to test the effects of the IL-10-enriched B cells on MG obtained not only from MCAO male mice, but also to those isolated from female mice, in an attempt to discern sex differences in immunoregulation. Age-matched male and female WT mice were subjected to 60min MCAO followed by 96 h of reperfusion. MG were isolated from brains of these male and female mice after 96 h of reperfusion, seeded in tissue culture flasks and after 21 div, harvested to be co-cultured with rIL-10 and B cells as described above. Similar to naïve mice, the yield and purity of MG after 21 days in vitro significantly increased in both male and female mice (Fig. 3a and 3b). The MG from MCAO mice proliferated much better than the MG from naïve mice and the overall yields after 21 div were higher those obtained from naïve mice (~3.3 million for male and 3.1 million for female MG, post-MCAO as compared to 1.5 million for naïve male MG) (Fig. 3a), while the purity (CD11b+CD45+) went from 18.4% to 94.4% for male MG and 23.6% to 96.2% for female MG (Fig. 3b). Due to culture conditions and the presence of GM-CSF in the culture medium, the majority of the MG obtained from stroke-afflicted mice attained a resting MG phenotype (CD11b+CD45lo). However, there was still the presence of activated microglia/infiltrated monocytes in the culture at 21 div (Fig. 3c and 3d). Further analysis of the MG demonstrated that the overall activated state of the MG from the day of seeding (28.4% in male and 38.8% in females) was reduced by day 21 div (14% in males and 13.6% in females) (Fig. 3c and 3d).
Fig. 3. Yield and purity of primary microglia cultures isolated from adult MCAO-induced male and female mice.
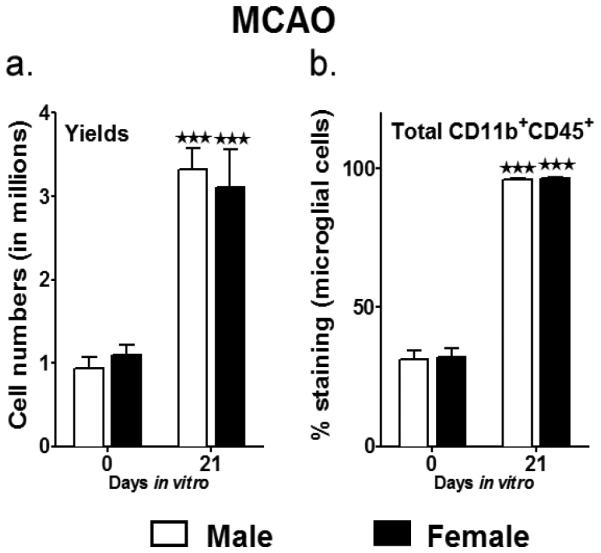
a. Summarizes the harvest yields on the day of MG isolation (mixed glial cells) and then after 21 days in vitro; with mixed glial cells cultured with 5 ng/ml GM-CSF through all the days. b. The purity of the MG was determined by staining for CD11b and CD45. Values are given as mean ± S.E.M. Data presented are representative of n = 5 separate MG isolations and primary culture set-ups each for male and female WT mice, post-MCAO. Representative dot plots indicating decrease in the CD11b+CD45hi (activated microglia/infiltrating macrophages) in c. male and d. female primary MG cultures. Statistical analysis was performed using Student’s t-test. Significant differences between sample means are indicated as ***P≤0.001 as compared to the values from day of MG isolation
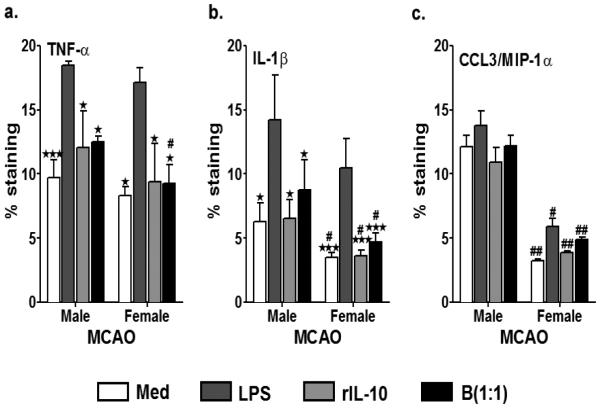
MG obtained from MCAO mice were further stimulated with LPS for 4 hours, with the no treatment group serving as the negative control. The MCAO-MG were treated with 20ng/ml rIL-10 and B cells at a 1:1 ratio (B cells:microglia) and pro-inflammatory cytokines, TNF-α, IL-1β and CCL3, were determined by flow cytometry after 24 hr of treatment. As indicated in Fig. 4a, 4b and 4c, treatment of LPS activated male and female MG after MCAO with rIL-10 and B cells (1:1) significantly inhibited TNF-α, IL-1β and CCL3/MIP-1α production to baseline levels, although baselines for IL-1β and CCL3 were lower for females than males. For TNF-α, levels went from 18.4% in the LPS-treated to 12% after rIL-10 and to 12.5% after B cell (1:1) treatment in male MG and from 17.2% in LPS-treated to 9.4% after rIL-10 and to 9.3% after B cell (1:1) treatment in female MG (Fig. 4A). There was a similar pattern of inhibition for IL-1β (Fig. 4B) and CCL3/MIP-1α (Fig. 4C). Overall, the cytokine levels produced by male MG after MCAO were higher than that produced by naïve male MG (naïve female MG were not tested).
Fig. 4. Enhanced dampening of proinflammatory status of microglia from female mice in response to treatment with IL-10+ B cells, post-MCAO.
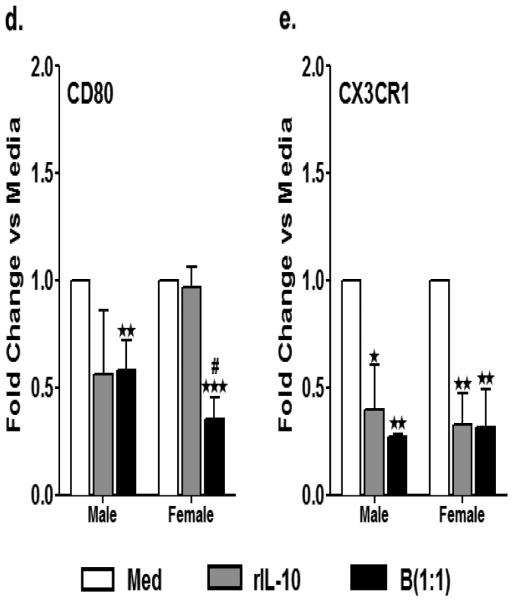
Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10ng/ml LPS for 4 hours. Supernatants were discarded after 4 hours and one of the following treatments was given in 1mL of fresh culture medium: no treatment, 20ng rIL-10 or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37°C and 5% CO2 for 24 hours and proinflammatory cytokines a. TNF-α and b. IL-β and c. chemokine CCL3 (MIP-1α) were determined by flow cytometry. Values are given as mean ± S.E.M. Data presented are representative of n = 3, 3 and 2 separate co-culture set-ups, respectively for TNF-α, IL-1β and CCL3 with each treatment condition done in duplicate for every experimental set-up. RNA was isolated from MG from each of the treatments and real time PCR analysis for d. cd80 and e. cx3cr1 were performed. Statistical analysis was performed using One way ANOVA with post-hoc Dunnett test. Significant differences between sample means are indicated as *p≤0.05, **p≤0.01, ***p≤0.001 as compared to the LPS-stimulated condition. Statistical differences between the two sexes were performed by two-way ANOVA followed by the post-hoc Bonferroni multiple comparison test #p≤0.05 and ##p≤0.01, as compared to the respective treatment in male microglia
To further assess additional M1 markers, mRNA was isolated from the MG treated with IL-10+ B cells (1:1) and the positive control, rIL-10, for 24 h of co-culture. Real time PCR was performed to determine the gene expression levels of the classic M1 markers, namely cd80 and cx3cr1. As expected, the MG treated with IL-10+ B cells demonstrated a significantly lower expression of the gene cd80 as compared to the medium control both in male and female mice (Fig. 4d). However, the expression of cd80 in female MG was much lower than in males (Fig. 4d). Similarly, the relative expression of another M1 marker, cx3cr1, was significantly down regulated in the MG treated with rIL-10 and IL-10-enriched B cells with no difference in the level of expression between the B cell-treated male and female MG (Fig. 4e). Thus, overall these results indicate that the increase in M1 phenotype, particularly cd80, attained by MG post-MCAO, could be efficiently ameliorated by the IL-10+ B cell treatment more efficiently in female than male MG.
Treatment with IL-10+ B cells promotes M2 phenotype induction in microglia, post-MCAO
CD206 is a M2 marker characterized as an anti-inflammatory mediator that is known to be induced by IL4. To determine whether the IL-10-enriched B cells can not only inhibit proinflammatory factors but also can induce an anti-inflammatory milieu in MG, we tested the expression of CD206 on MG obtained from MCAO-induced male and female mice after 24 hours of co-culture with Bregs. Since the production of CD206 in MG is induced in the presence of IL-4 in the milieu (Stein et al. 1992), rIL-4 (20ng/ml) was used as a positive control. As indicated in Fig. 5, there was a marked increase in expression of CD206 compared to baseline (4-7%) in the presence of rIL-4 (20.95% in male MG and 17.4% in female MG) and rIL-10 (13.7% in male MG and 20.8% in female MG). IL-10+ B cells (in a 1:1 ratio) could significantly upregulate CD206 expression both in the male (11.9%) and female (11%) MG, post-MCAO. While the B cells when used in half the amount (i.e. 1:2 ratio) were still capable of upregulating the CD206 vs. the medium control, this change was not significantly lower compared to the LPS-stimulated condition. To determine whether there existed a sex difference in the ability of B cells to induce CD206, male and female values for each treatment condition were compared. There was no difference in the expression of CD206 when treated with IL-10-enriched B cells, between male and female MG, post-MCAO, except for a significantly higher efficiency of rIL-4 to induce CD206 in the females as compared to their male counter-parts with similar treatment (Fig. 5). More importantly, treatment of MCAO-MG with Bregs resulted in a significantly higher induction of CD206 as compared to the no treatment condition (basal levels of expression). Thus, these results convincingly demonstrate that Bregs could induce an M2 phenotype in MCAO-affected MG.
Fig. 5. Treatment with IL-10+ B cells promotes M2 phenotype induction in microglia, post-MCAO.
Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10ng/ml LPS for 4 hours. Supernatants were discarded after 4 hours and one of the following treatments was given in 1mL of fresh culture medium: no treatment, 20ng recombinant IL-10 (rIL-10), 20ng/ml rIL-4 or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37°C and 5% CO2 for 24 hours and the M2 marker, CD0206 was determined by flow cytometry. Values are given as mean ± S.E.M. Data presented are representative of n = 2 separate co-culture set-ups, with each treatment condition done in duplicate for every experimental set-up. Statistical analysis was performed using One way ANOVA with post-hoc Dunnett test. Significant differences between sample means are indicated as *p≤0.05 and ***p≤0.001 as compared to the LPS-stimulated condition. Statistical differences between the two sexes were performed by two-way ANOVA followed by the post-hoc Bonferroni multiple comparison test, with #p≤0.05 as compared to the respective treatment in male MG
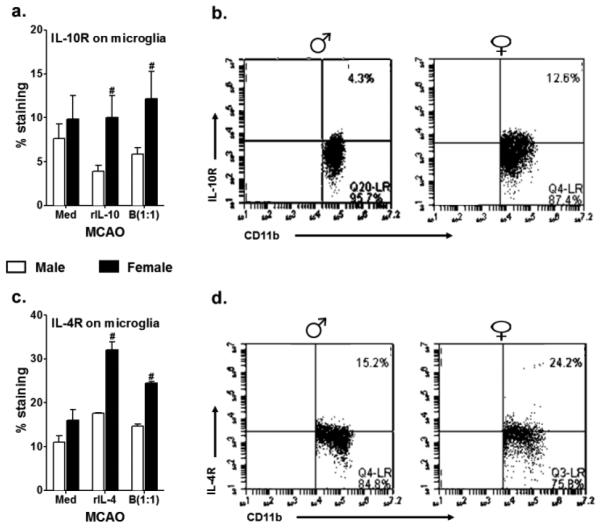
Increased microglial surface expression of IL-4Rα, and IL-10R by female microglia, post – MCAO
In lieu of the above results wherein Bregs could induce M2 phenotype in the MG, we assessed the presence of IL-4 and IL-10 receptors on the microglia, in the same culture condition settings as described in Fig. 5. CD206 was induced in presence of IL-4 and IL-10, hence the IL-4 and IL-10 receptors are pertinent to be present on MG in order to respond to the positive stimuli. We have consistently demonstrated over the past few studies that the Bregs can ameliorate the pro-inflammatory milieu in the ischemic hemispheres of male mice, and that this decrease in the proinflammatory status was attributed to the enhanced IL-10 producing capacity of the B cells. So, we first sought to assess the expression levels of IL-10R on the microglia in an attempt to answer whether the Bregs regulate MG via this receptor. Surprisingly, the MG obtained from male mice post-MCAO had lower levels of IL-10R expression in response to rIL-10 (3.9% from 7.6% at basal levels). This was also true but to a lesser extent in the presence of Bregs (5.8%). However, in the female MG, while the treatment with rIL-10 resulted in a minor increase (10.2% from 9.8% from basal levels), the Breg treatment could induce a much higher expression of IL-10R (12.1%). The female MG demonstrated a significant expression of IL-10R both in response to the positive control i.e. rIL-10 and also upon treatment with IL-10-enriched B cells as compared to its male counterparts (Fig. 6a & 6b). Similarly, while there was an upregulation in the expression of IL-4Rα on the MCAO-subjected MG of male mice in the presence of rIL-4 (17.6% from 11%) and Bregs (14.6%), it was the MG obtained from MCAO females that demonstrated the greatest increase in the expression of the IL-4R, not only in response to rIL-4 (32%) but more importantly in presence of Bregs (24.5%) (Fig. 6c & 6d) and this was significantly higher when compared to male MG. Thus, MG obtained from female mice demonstrated a higher expression of both IL-10 and IL-4 receptors, indicating a better ability to respond to the respective anti-inflammatory factors.
Fig. 6. Increased microglial surface expression of IL-4Rα, and IL-10R by female microglia, post-MCAO.
Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10ng/ml LPS for 4 hours. Supernatants were discarded after 4 hours and one of the following treatments was given in 1mL of fresh culture medium: no treatment, 20ng recombinant IL-10 (rIL-10), 20ng/ml rIL-4 or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37°C and 5% CO2 for 24 hours and A. IL-10 receptor (IL-10R) and C. IL-4Rα were determined by flow cytometry. Representative dot plots indicating increase in the B. IL-10R and D. IL-4Rα in female as compared to male primary MG cultures, post-MCAO. Values are given as mean ± S.E.M. Data presented are representative of n = 2 separate co-culture set-ups, with each treatment condition done in duplicate for every experimental set-up. Statistical differences between the two sexes were performed by two-way ANOVA followed by the post-hoc Bonferroni multiple comparison test, with #p≤0.05 as compared to the respective treatment in male MG
Treatment with IL-10+ B cells leads to increased IL-4 production by female microglia post-MCAO
MG are known to produce the anti-inflammatory cytokines when modulated by the right kind of precursors and are known to control their own polarization through autocrine and paracrine means (Cherry et al. 2014). Hence, we determined the inherent production of IL-4 and IL-10 by the microglia from male and female MG, post-MCAO in presence of the relevant positive controls and Bregs (Fig. 7a and 7b). Both male and female MG exhibited upregulated IL-4 and IL-10 production in the presence of the corresponding positive inducers, and to some extent also in the presence of Bregs. However, very interesting phenotypic characteristics were observed when the IL-4 and IL-10 production were compared between the two sexes. While the female MG produced robust amounts of IL-4 in presence of Bregs (22.9%) as compared to the male counterparts (8.6%) (Fig. 7a), the male MG could produce significantly higher levels of IL-10 (17.4%) as compared to the females (5.5%), when treated with Bregs (Fig. 7b). Interestingly, CD11b−CD45+ population (presumable the B cells) was also found to have a capacity to produce significantly higher levels of IL-4 when co-cultured with female post-MCAO MG (data not shown). These results suggest that very distinct differences in the autocrine production of anti-inflammatory factors exists between the two sexes.
Fig. 7. Treatment with IL-10+ B cells leads to increased IL-4 production by female microglia post-MCAO.
Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10ng/ml LPS for 4 hours. Supernatants were discarded after 4 hours and one of the following treatments was given in 1mL of fresh culture medium: no treatment, 20ng recombinant IL-10 (rIL-10), 20ng/ml rIL-4 or IL-10+ B cells at a 1:1 ratio with MG. The MG cells were incubated with mentioned treatments at 37°C and 5% CO2 for 24 hours and A. IL-4 production and B. IL-10 production were determined by flow cytometry. Values are given as mean ± S.E.M. Data presented are representative of n = 2 separate co-culture set-ups, with each treatment condition done in duplicate for every experimental set-up. Statistical differences between the two sexes were performed by two-way ANOVA followed by the post-hoc Bonferroni multiple comparison test, with #p≤0.05 and ###p≤0.001 as compared to the respective treatment in male MG
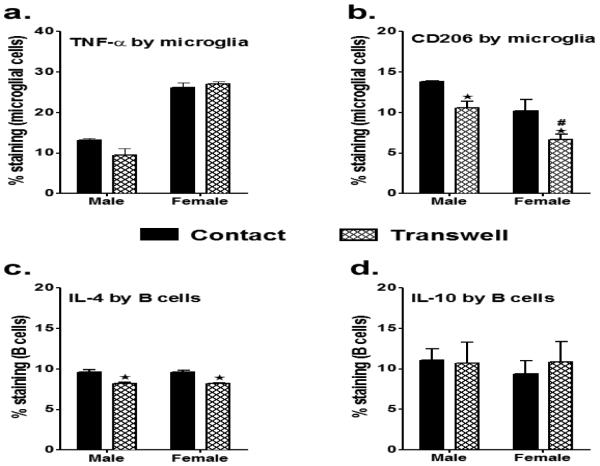
Induction of M2 phenotype requires cell-to-cell interactions with Bregs
To elucidate whether the MG M2 phenotype is induced by the Breg cells themselves and/or the cytokines produced by them, we made use of a Transwell system that prevented direct MG/B-cell contact. MG obtained from MCAO male and female mice were plated as described before and IL-10-enriched B cells were applied into the Transwell chamber. After 24 hours, the M1 cytokine (TNFα) and M2 marker (CD206) were determined in the MG. The presence of B cells in the Transwell resulted in a marked but insignificant reduction of TNFα production in the male MG (9.5% from 13.5%) and as well with the female MG, there was no difference in the TNFα production when the regulatory B cells were in the Transwell (17%) as opposed to direct contact (16.2%) with MG (Fig. 8a). Interestingly, the expression of the M2 marker, CD206, was significantly reduced both in the males (10.5% from 13.8%) and females (6.7% from 10.2%) when B cells were present in Transwells (Fig. 8b), indicating the need for cell-to-cell contact. Since our preliminary results had indicated that B cells (CD11b−CD45+ cells) co-cultured with the MG were able to produce IL-4, we assessed the IL-4 production in Bregs obtained from the IL-10GFP reporter donors. These B cells were not able to produce IL-4 prior to being co-cultured with the MG, but produced more IL-4 when in direct contact with MG (9.6%) than when present in Transwells (8.2%, Fig. 8C). In contrast, these Bregs produced equivalent amounts of IL-10 both when in contact and/or in Transwells with MG (Fig. 8D). Thus, the B cells acquired an enhanced ability to produce IL-4 only when in cell-to-cell contact with MG. These results suggest that in both male and female MG, the M1 phenotype in ischemic MG was inhibited via soluble factors, including IL-10 secreted by the regulatory B cells, while augmentation of the M2 phenotype involved cell-to-cell contact with the Bregs and IL-4 production.
Fig. 8. Induction of M2 phenotype requires cell-to-cell interactions with Bregs.
Primary MG, isolated and cultured from MCAO-treated WT male and female mice, were harvested after 21 days in vitro (at confluency) and cultured in GM-CSF-free medium for 5 days. MG were stimulated with 10ng/ml LPS for 4 hours. Supernatants were discarded after 4 hours and one of the following treatments was given in 1mL of fresh culture medium: IL-10+ B cells at a 1:1 ratio with MG, either in cell-to-cell contact or in Transwells. The MG cells were incubated with mentioned treatments at 37°C and 5% CO2 for 24 hours and A. TNF-α and B. CD206 expression in MG; C. IL-4 production and D. IL-10 production in the Bregs when in cell-to-cell contact vs. in Transwells were determined by flow cytometry. Values are given as mean ± S.E.M. Data presented are representative of n = 2 separate co-culture set-ups, with each treatment condition done in duplicate for every experimental set-up. Statistical analysis was performed using One way ANOVA with post-hoc Dunnett test. Significant differences between sample means are indicated, with #p≤0.05 and ###p≤0.001 as compared to the respective treatment in male MG
Discussion
Reperfusion of the brain after ischemic stroke is known to lead to infiltration of peripheral leukocytes into the area of brain injury that secrete proinflammatory cytokines, eventually causing secondary injury (Onwuekwe and Ezeala-Adikaibe 2012). As resident immune cells in the CNS, microglia are also known to both phagocytose debris and secrete proinflammatory cytokines under ischemic conditions, contributing to the tissue damage (Gregersen et al. 2000). However, the MG may also secrete anti-inflammatory cytokines such as IL-10 and TGF-β (de Bilbao et al. 2009; Lehrmann et al. 1998; Parada et al. 2013) that are known to quench inflammation. In studies where MG were ablated, mice had larger infarcts and a doubling of apoptotic neurons after ischemia, indicating that on balance the MG are needed to alleviate injury after ischemic stroke (Lalancette-Hebert et al. 2012). One would surmise that the deleterious vs. the beneficial processes might depend on the location of the microglia, the degree of ischemia and the timing after injury. Clearly, a better understanding of the role of MG in post-ischemic injury is needed to further our knowledge and to devise more effective therapies for stroke.
Our primary goal in the current study was to determine whether the regulatory B cells can influence post-stroke MG and to test their efficacy in dampening MG activation. Historically, MG cell lines or primary MG isolated from embryonic (Gingras et al. 2007) or neo-natal animals (Floden and Combs 2007; Giulian and Baker 1986) have been used as in vitro MG models. However, knowing that the MG cell lines are not a perfect model (Horvath et al. 2008), the embryonic and neo-natal primary MG are not completely mature (Draheim et al. 1999) and that they behave differently than adult MG (Sierra et al. 2007), we optimized the MG isolation and culture procedures from adult mouse brains with the eventual goal to culture MG from MCAO-treated mice. The primary MG isolation and culture procedures used in our study were as described in (Moussaud and Draheim 2010), albeit with a few modifications to enhance their ability to dampen inflammatory cytokines and chemokines.
Our results demonstrate that the MG from naïve mice, upon LPS-stimulation, produce high levels of pro-inflammatory cytokines, the production of which was dampened both in the presence of rIL-10 and Bregs. LPS stimulation of MG was used to mimic the cascade of inflammatory events after ischemic stroke. Similarly, when the MG isolated from ischemic mice were subjected to rIL-10 and Breg treatments, a significant reduction in proinflammatory factors was demonstrated. MG are known to recognize harmful stimuli and respond by producing inflammatory cytokines such as TNFα, IL-6, IL-1β, interferon-γ (IFNγ), and several chemokines (Boche et al. 2013). This constellation of cytokines is essential for the polarization of MG into what has been termed a classically activated, ‘M1’, state (Mills et al. 2000). Hence, some of the proinflammatory factors tested included TNF-α, IL-1β and the chemokine CCL3 (MIP-1α), all of which are well recognized as primary effector molecules in acute response to ischemic injury. The pleiotropic cytokine, tumor necrosis factor (TNFα), is known to play an important role in the regulation of cell survival and inflammation in the injured and diseased CNS (Hallenbeck 2002; Owens et al. 2001). In humans, TNFα is present at elevated levels and is believed to be implicated in the pathogenesis of stroke (Zaremba and Losy 2001). IL-1β is also a major neuroinflammatory molecule (Allan et al. 2005) that mediates several key physiological and behavioral changes associated with sickness (Kelley et al. 2003) and is known to be regulated by IL-10 (Heyen et al. 2000; Pousset et al. 1999). When the efficacy of the Bregs to dampen classical M1 markers was tested, we found that the mRNA levels of CD80 and the chemokine CX3CR1 produced by the MG were significantly reduced in the B cell-treated co-culture conditions. The chemokine receptor CX3CR1 is expressed at high levels on murine microglia under homeostatic conditions. Mice genetically deficient for CX3CR1 have smaller infarct volumes after MCAO (Denes et al. 2008; Fumagalli et al. 2013), hence suggesting its relevance as an inflammatory mediator. Thus, our results convincingly demonstrated that Bregs were efficient in alleviating the overall proinflammatory status of the activated MG, post-MCAO. This effect was more pronounced in the females as compared to the males (Fig. 4a-4e). Ex vivo, MG derived from male and female brains have shown divergent inflammatory signaling responses to LPS and estradiol treatment (Loram et al. 2012), which argues that sex differences in MG in the brain may be more extensive than just a difference in number or morphology, but may also be phenotypically distinct. With the emerging importance of immune system mediators in normal brain development, we now know that the role of MG is far more complex and at times subtle yet profoundly important (Lenz and McCarthy 2015). In general, our results demonstrated that the MG from the MCAO female mice had a lower inflammatory status than males, in accordance with our previous studies (Banerjee et al. 2013), but the fact that the regulatory B cells could further alleviate the proinflammatory milieu seems promising.
CD206 is a mannose receptor belonging to the ‘M2a’ family of classic M2 markers expressed by MG, with its main function being suppression of inflammation (Stein et al. 1992). It is now known that at 24 hours after MCO, CD206 is exclusively found in the ischemic core (Perego et al. 2011). In accordance, another study examining the location of M1 versus M2 microglia/macrophages confirmed the M2 microglia/macrophages infiltrating the ischemic core at 24 hours, peaking at 5 days, and declining in the striatum by 14 days (Hu et al. 2012). The expression of CD206+ cells in the cortex at the border zone of ischemia peaked at day 5 after ischemia before being outnumbered by M1 cells (Hu et al. 2012). Hence, the appearance of CD206 suggests that the microglia/macrophages in the ischemic core promote tissue repair. The importance of the M2 phenotype acquired by the microglia, post-injury, has also been emphasized in other CNS diseases like EAE, wherein M2 microglia and macrophages were demonstrated to drive oligodendrocyte differentiation during CNS remyelination (Miron et al. 2013). Our findings corroborate these studies in that the treatment with Bregs could significantly induce an M2 phenotype in the ischemic MG, both in males and females. Thus, we can speculate that treatment with Bregs not only dampens the proinflammatory milieu in the ischemic brain after stroke, but also promotes an M2 status leading to tissue repair.
The prototypical cytokine used to first induce the alternative M2 activation state in MG is IL-4 (Stein et al. 1992). Both IL-4 and the closely related cytokine IL-13 signal through IL-4Rα to induce a host of downstream processes that lead to potent anti-inflammatory functions (Gadani et al. 2012; Sica and Mantovani 2012; Taylor et al. 2005). Our studies demonstrate that MG derived from females are adept at expressing not only IL-10R but more importantly the IL-4R (Fig. 6a&b). The cellular source of the CNS-derived IL-4 could be from a number of cell types (Pepe et al. 2014), including MG cells, although the signals that drive IL-4 production by MG cells in the CNS are not known. Recently it was shown that IL-4-treated MG cells injected into the CSF of mice with EAE suppressed clinical symptoms and promoted oligodendrogenesis in the spinal cord (Butovsky et al. 2006a). Moreover, the addition of IL-4 to LPS-stimulated MG and motorneuron cocultures suppressed the release of NO and decreased motorneuron injury (Zhao et al. 2006). A recent study also demonstrated that the central administration of rIL-4 elicits a heterogeneous induction of the MG M2 phenotype (Pepe et al. 2014). These studies suggest that IL-4-induced MG cells are both anti-inflammatory and neuroprotective and lead us to speculate that the female MG are better able to control their proinflammatory state due to the enhanced ability to produce IL-4 (Fig. 7). The literature supports the contention that the Bregs are distinguished by their ability to secrete IL-10 and/or TGFβ-1, while effector B cell populations produce cytokines such as IL-2, IL-4, TNFα, IL-6 (Be-2 cells) or IFNγ, IL-12 and TNFα (Be-1 cells). B cells primed by Th2 cells and antigen (Be-2 cells) make IL-2, IL-13 and IL-4; Be-2 differentiation is dependent on the engagement of IL-4Rα on B cells (Lund 2008). Hence our results indicate that the ability of Bregs to produce IL-4 appears to be a response potentially amplified by an IL-4-driven feedback loop between the MG and the Bregs that is promoted by cell-to-cell contact with each other (Fig. 8).
Although tissue plasminogen activator (tPA) is available to aid in reperfusion for selected patients with ischemic strokes, there is a necessity for additional treatments to reduce injury and promote CNS tissue repair after stroke. MG activation is one potential target. Several studies have investigated the role of minocycline on inhibiting or altering MG activation. In a mouse model of Amyotrophic Lateral Sclerosis (ALS), minocycline attenuated MG activation and reduced the expression of M1, but not M2, microglia/macrophage markers, suggesting that minocycline might inhibit the proinflammatory microglia/macrophages (Kobayashi et al. 2013) (Taylor and Sansing 2013). In the current study, our results clearly demonstrate that rIL-10 was able to reduce the TNF-α, IL-1β and macrophage inflammatory protein-1a (MIP-1α or CCL3) levels in activated MG. More importantly, the levels of TNF-α, IL-1β and CCL3 levels were significantly reduced when IL-10+ B-cells were added at a 1:1 ratio and there were sex differences in this immunoregulation. Not only were the IL-10-enriched B-cells able to ameliorate pro-inflammatory cytokine production by the microglia, but they also could induce expression of CD206, an anti-inflammatory M2 phenotypic marker. MG from female mice had a higher expression of both IL-4 and IL-10 receptors and were better able to produce IL-4 compared to their male counterparts, especially after Breg treatment.
In summary, our results demonstrate that IL-10-producing B-cells indeed play a crucial role in regulating MG activation, post-MCAO. Our studies are the first to demonstrate that while the proinflammatory milieu in the MG could be dampened by soluble factors secreted by the Bregs, induction of the anti-inflammatory M2 phenotype was promoted by cell-to-cell interactions with the Bregs. Our novel observations also highlight potentially important sex differences in MG responses (e.g. increased IL-4 production and IL-4R and IL-10R expression in female MG) induced by treatment with IL-10+ regulatory B cells that could account for lower basal levels of pro-inflammatory factors and less severe infarcts after MCAO.
Acknowledgements
The authors wish to thank Gail Kent for assistance with manuscript submission. This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant 1RO1 NS075887. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- BBB
Blood Brain Barrier
- CNS
Central nervous system
- MCAO
Middle cerebral artery occlusion
- WT
wild-type
- TNF-α
Tumor necrosis factor α
- CD
Cluster of Differentiation
- MHC II
Major Histocompatibility Complex II
- RPMI
Roswell Park Memorial Institute
- IL
Interleukin
- FACS
Fluorescence Activated Cell Sorter
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Committee.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. doi:10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. doi:10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. doi:10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Lapato A, Vandenbark AA, Murphy SJ, Saugstad JA, Offner H. Regulatory CD8CD122 T-cells predominate in CNS after treatment of experimental stroke in male mice with IL-10-secreting B-cells. Metab Brain Dis. 2014a doi: 10.1007/s11011-014-9639-8. doi:10.1007/s11011-014-9639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke Metab. Brain Dis. 2013;28:375–386. doi: 10.1007/s11011-013-9413-3. doi:10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metab Brain Dis. 2014b;29:59–73. doi: 10.1007/s11011-013-9474-3. doi:10.1007/s11011-013-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006a;116:905–915. doi: 10.1172/JCI26836. doi:10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006b;31:149–160. doi: 10.1016/j.mcn.2005.10.006. doi:10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. doi:10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, et al. Focal cerebral ischemia activates neurovascular restorative dynamics in mouse brain. Front Biosci (Elite Ed) 2012;4:1926–1936. doi: 10.2741/513. [DOI] [PubMed] [Google Scholar]
- de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, Giannakopoulos P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem. 2009;110:12–22. doi: 10.1111/j.1471-4159.2009.06098.x. doi:10.1111/j.1471-4159.2009.06098.x. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. doi:10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. doi:10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim HJ, Prinz M, Weber JR, Weiser T, Kettenmann H, Hanisch UK. Induction of potassium channels in mouse brain microglia: cells acquire responsiveness to pneumococcal cell wall components during late development. Neuroscience. 1999;89:1379–1390. doi: 10.1016/s0306-4522(98)00407-2. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. doi:10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. Journal of neuroscience methods. 2007;164:218–224. doi: 10.1016/j.jneumeth.2007.04.018. doi:10.1016/j.jneumeth.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S, Perego C, Ortolano F, De Simoni MG. CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia. 2013;61:827–842. doi: 10.1002/glia.22474. doi:10.1002/glia.22474. [DOI] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. Journal of immunology. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. doi:10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras M, Gagnon V, Minotti S, Durham HD, Berthod F. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. Journal of neuroscience methods. 2007;163:111–118. doi: 10.1016/j.jneumeth.2007.02.024. doi:10.1016/j.jneumeth.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. doi:10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. doi:10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. doi:10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Heyen JR, Ye S, Finck BN, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res Mol Brain Res. 2000;77:138–147. doi: 10.1016/s0169-328x(00)00042-5. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, Nutile-McMenemy N, Alkaitis MS, Deleo JA. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J Neurochem. 2008;107:557–569. doi: 10.1111/j.1471-4159.2008.05633.x. doi:10.1111/j.1471-4159.2008.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. doi:10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. doi:10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. doi:10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke. 2012;43:1115–1122. doi: 10.1161/STROKEAHA.111.643080. doi:10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kissela B, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40:530–536. doi: 10.1161/STROKEAHA.108.521906. doi:10.1161/STROKEAHA.108.521906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, et al. Minocycline selectively inhibits M1 polarization of microglia Cell. Death Dis. 2013;4:e525. doi: 10.1038/cddis.2013.54. doi:10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, et al. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33:15095–15108. doi: 10.1523/JNEUROSCI.0278-13.2013. doi:10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. doi:10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, et al. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci. 2012;32:10383–10395. doi: 10.1523/JNEUROSCI.1498-12.2012. doi:10.1523/JNEUROSCI.1498-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrmann E, et al. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24:437–448. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21:306–321. doi: 10.1177/1073858414536468. doi:10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. doi:10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Loram LC, et al. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. doi:10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. doi:10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. doi:jimmunol.0900185 [pii] 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. Journal of immunology. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. doi:10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaud S, Draheim HJ. A new method to isolate microglia from adult mice and culture them for an extended period of time. Journal of neuroscience methods. 2010;187:243–253. doi: 10.1016/j.jneumeth.2010.01.017. doi:10.1016/j.jneumeth.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. Ilar J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- Onwuekwe I, Ezeala-Adikaibe B. Ischemic stroke and neuroprotection. Ann Med Health Sci Res. 2012;2:186–190. doi: 10.4103/2141-9248.105669. doi:10.4103/2141-9248.105669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T, Wekerle H, Antel J. Genetic models for CNS inflammation. Nat Med. 2001;7:161–166. doi: 10.1038/84603. doi:10.1038/84603. [DOI] [PubMed] [Google Scholar]
- Parada E, et al. The microglial alpha7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. 2013;19:1135–1148. doi: 10.1089/ars.2012.4671. doi:10.1089/ars.2012.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol. 2013;5:73–90. [PMC free article] [PubMed] [Google Scholar]
- Pepe G, Calderazzi G, De Maglie M, Villa A, Vegeto E. Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4. J Neuroinflammation. 2014;11:1031. doi: 10.1186/s12974-014-0211-6. doi:10.1186/s12974-014-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. doi:10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-de Puig I, Miro F, Salas-Perdomo A, Bonfill-Teixidor E, Ferrer-Ferrer M, Marquez-Kisinousky L, Planas AM. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J Cereb Blood Flow Metab. 2013;33:1955–1966. doi: 10.1038/jcbfm.2013.155. doi:10.1038/jcbfm.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousset F, Cremona S, Dantzer R, Kelley K, Parnet P. Interleukin-4 and interleukin-10 regulate IL1-beta induced mouse primary astrocyte activation: a comparative study. Glia. 1999;26:12–21. doi: 10.1002/(sici)1098-1136(199903)26:1<12::aid-glia2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. doi:10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke. 2013;44:2579–2586. doi: 10.1161/STROKEAHA.113.001796. doi:10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. doi:10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. doi:10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. doi:10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods. 2009;180:261–272. doi: 10.1016/j.jneumeth.2009.03.017. doi:10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. doi:10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 20132013:746068. doi: 10.1155/2013/746068. doi:10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. doi:10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. doi:10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- Wang G, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. doi:10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42:2026–2032. doi: 10.1161/STROKEAHA.110.593772. doi:10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba J, Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. doi:10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]