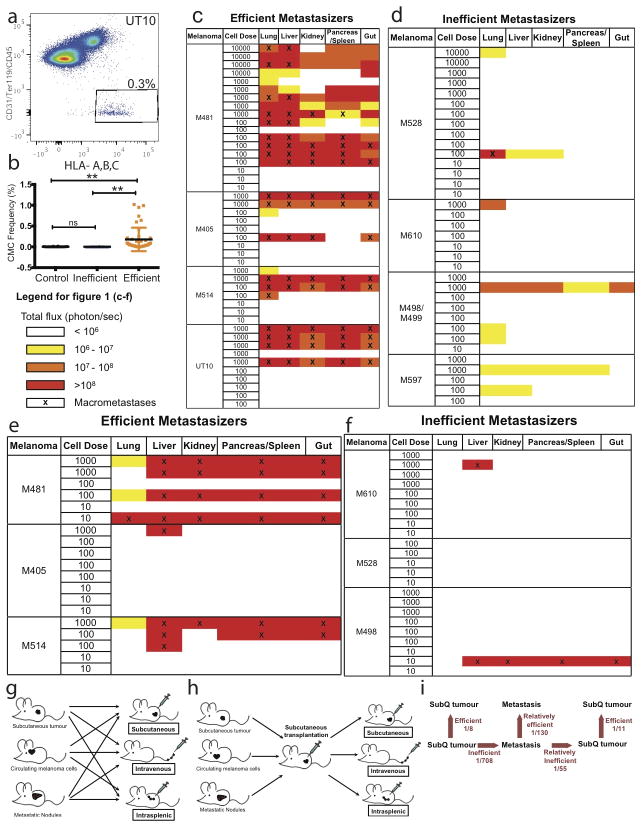
Extended data figure 3. Barriers to distant metastasis in vivo.
a) Live human melanoma cells were identified by flow cytometry based on the expression of DsRed (all melanomas in this study stably expressed DsRed) and human HLA and the lack of expression of mouse CD45, CD31 and Ter119 (to exclude mouse hematopoietic and endothelial cells). Human melanoma cells were observed in the blood of NSG mice bearing efficiently metastasizing melanomas. b) Mice xenografted with efficiently metastasizing melanomas (n=43 mice with tumours derived from 4 patients) had significantly higher frequencies of circulating melanoma cells (CMCs) in their blood than mice xenografted with inefficiently metastasizing melanomas (n=13 mice with tumours derived from 4 patients) or control mice that had not been xenografted (n=18 mice). Blood was collected by cardiac puncture. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons (**, p< 0.005). c–f) Bioluminescence analysis of total photon flux (photons/second) from mouse organs after intravenous injection (c, d) or intrasplenic injection (e, f) of luciferase-tagged melanoma cells derived from efficiently metastasizing (c, e) or inefficiently metastasizing (d, f) melanomas. Each melanoma was derived from a different patient and was studied in an independent experiment. g) Schematic of the experiment shown in Table 2a. h) Schematic of the experiment shown in Table 2b. i) Summary of mean limiting dilution frequencies of tumour-forming cells after subcutaneous, intravenous, or intrasplenic transplantation into NSG mice.