Abstract
Background
Reticulocyte hemoglobin content (RET‐He)—an established indicator of iron status in children and adults—was determined in very low birth weight (VLBW) infants.
Methods
Longitudinal retrospective RET‐He data in 26 VLBW neonates during the first month of age were compared with: (a) concurrent complete blood counts (CBCs), including hemoglobin (Hb) concentration, reticulocyte count, and immature reticulocyte fraction (IRF), and erythropoietin (EPO) levels; (b) clinical variables; and (c) RET‐He data from the literature for term infants, children, and adults.
Results
RET‐He within 24 hr following birth was 31.8 ± 1.1 pg (mean ± SEM). This was followed by an abrupt, significant decline to 28.3 ± 1.1 pg at 2–4 days, and to steady state levels of 28.4 ± 0.5 pg thereafter. The changes in RET‐He were mirrored by changes in plasma EPO, reticulocyte count, and IRF, but not Hb. Steady state RET‐He values after 4 days were significantly lower than RET‐He values for term infants, children, and adults (31.6 ± 0.11, 32.0 ± 0.12, and 33.0 ± 0.13 pg, respectively).
Conclusion
Although RET‐He values in VLBW infant were lower than term infants, children, and adults, the significance and mechanism(s) responsible are unknown. The present VLBW infant data are relevant to investigations assessing hemoglobinization following treatment with recombinant human EPO (r‐HuEPO) and/or iron.
Keywords: reticulocyte hemoglobin content, reticulocyte indices, RET‐He, CHr, erythrocyte
INTRODUCTION
Premature critically ill infants, particularly very low birth weight (VLBW) infants weighing <1,500 g at birth, routinely develop clinically significant anemia during the first few weeks of age. While their anemia is largely attributable to the frequent clinical blood sampling needed for laboratory testing 1, anemia of prematurity is also a significant contributor 2. The prevention and treatment of neonatal anemia with recombinant human erythropoietin (r‐HuEPO) to augment erythropoiesis has demonstrated modest effectiveness in reducing RBC transfusions provided that iron supplementation is concomitantly administered for adequate hemoglobinization 3. Iron supplementation is also administered during infancy to prevent the development of nutritional iron deficiency (ID) and its more severe manifestation as iron deficiency anemia (IDA).
It is well established that nutritional ID and IDA developing during infancy can lead to significant subsequent detrimental neurodevelopmental and behavioral effects 4. This makes it imperative that ID and IDA be promptly diagnosed and treated. The diagnosis of ID is based on laboratory parameters indicative of the body's iron status. These include plasma iron, ferritin, soluble transferrin receptor (sTfR), transferrin saturation, hepcidin, and erythrocyte zinc protoporphyrin/heme ratio 5, 6. With the recent availability of sophisticated flow cytometry based hematology analyzers, RBC and reticulocyte indices—particularly reticulocyte hemoglobin content—have also served as indicators of iron status for diagnosing ID and IDA 6, 7. An advantage of reticulocyte indices relative to other indicators of iron status is that they provide a direct real time measure of hemoglobinization in newly produced erythrocytes, without perturbation by inflammation. In addition, because of their brief 1‐ to 2‐day lifespan in the circulation, the determination of reticulocyte indices has advantage over the determination of similar indices in mature RBCs, that is, this provides a real‐time assessment of iron status unperturbed by RBC transfusion 8. When reticulocyte indices are measured in individuals without hemoglobinopathies, the amount of Hb present within individual reticulocytes (in picogram) has been demonstrated to be a reliable indicator of iron availability for Hb synthesis in children and adults 8, 9, 10, 11. In infants there has been only one recent publication suggesting this may also be true in VLBW infants 12. Two manufacturers of clinical hematology analyzers have developed separate proprietary approaches for deriving reticulocyte indices 13. These indices include the reticulocyte hemoglobin content “CHr” parameter of the Bayer H٭3 and ADVIA 120 systems (Bayer Diagnostics, Tarrytown, NY), and “RET‐He” parameter of the Sysmex (Kobe, Japan; 13, 14). While normal values for RET‐He have been published for term infants 15, children 10, and adults 6, 7, RET‐He values for premature newborn infants have not.
Thus, the aims of the present study were to determine RET‐He values in critically ill, ventilated VLBW neonates during the first month of life and to compare these with: (a) concurrent longitudinal complete blood count (CBC) measurements, including hemoglobin concentration (Hb), reticulocyte count, and immature reticulocyte fraction (IRF); (b) clinical variables, including gestational age at birth, birth weight, birth weight z‐score, gender, postnatal age, and plasma ferritin as a primary indicator of iron status; and (c) RET‐He data from the literature for different developmental and patient groups.
MATERIALS AND METHODS
Subjects and Study Design
The University of Iowa Committee on Research on Human Subjects approved this study. Informed parental written consent was obtained. These same subjects have been previously reported, but with different primary objectives 16, 17, 18.
Study subjects included VLBW infants born between February 2007 and August 2009. The 26 subjects eligible for enrollment included inborn and outborn neonates delivered at <29 weeks gestation, who were intubated and ventilated during the first day of life. Gestational age was determined by obstetrical criteria unless physical and neurodevelopmental examination findings were inconsistent by >2 weeks, in which case gestational age based on the physical examination was used 19. Infants excluded were those presenting with hematological disease at birth, those receiving RBC transfusions prior to enrollment, and those receiving erythropoiesis‐stimulating agents (ESA). Infants were studied for approximately the first month of life during which all subjects received one or more clinically indicated RBC transfusions.
Clinical Data
Clinical data were gathered from the subject's electronic health record and combined with research laboratory data. Clinical data included relevant perinatal demographic data. These data were used to derive birth weight z‐score with individualized adjustment for gestational age with gender, and multiple birth 20.
Laboratory Methods
C‐reactive protein
Clinically ordered C‐reactive protein (CRP) analyses were performed within the first day of age for all infants by the central hospital laboratory utilizing the immunoturbidimetric assay on the Roche/Hitachi Cobas® systems analyzer (Indianapolis, IN). CRP was measured as an indicator of acute inflammation to aid in the interpretation of plasma ferritin with increased CRP levels indicative of unreliable ferritin results.
Research testing was performed on anticoagulated leftover blood from clinical sampling. Samples were refrigerated at 4°C for <72 hr prior to analysis or centrifugation to separate plasma that was stored at −80°C for analysis later.
Hematological testing
Analyses were performed on EDTA whole blood samples in our research laboratory using the Sysmex XE 2100. Analyses included Hb, reticulocyte count, and reticulocyte indices. These were performed in manual mode on 135 μl whole blood samples. The manufacturer's recommended quality control procedures were performed daily.
Erythropoietin
Plasma EPO was measured in duplicate on heparinized or EDTA samples using a specific double antibody radioimmunoassay 21.
Ferritin
Plasma ferritin was measured in duplicate using 20 μl heparinized or EDTA plasma samples in an enzyme immunoassay kit (Ramco Laboratories, Inc., Stafford, TX). Plasma ferritin data included the first value following birth for which there was sufficient leftover plasma. For all but three infants, plasma ferritin was measured once in the first 24 hr of age; two were measured on the second day and one on the third day.
Literature Review
To identify publications, including original RET‐He data, a PubMed literature search was performed. Only human studies of infants, children, and adults with “reticulocyte hemoglobin content,” “RET‐He,” “CHr,” “reticulocyte indices,” and/or “hematological indices” were included. Ten publications were identified 6, 7, 10, 11, 15, 22, 23, 24, 25, 26. To locate additional publications with original RET‐He data, the reference section of these ten papers were reviewed by three of the coauthors (RA, DN, and JW). No additional references were found. Of the ten publications, seven contained RET‐He variance data allowing comparison with the present study's VLBW infants 6, 7, 10, 11, 15, 25, 26.
Statistical Analysis
Data are presented expressed as the mean ± SEM or the mean ± SD as appropriate. Change with advancing postnatal age for the laboratory variables during the first month of age was evaluated using linear mixed model analysis using the following postnatal age intervals: <1 day, 2–4 days, 5–7 days, and weekly thereafter. For laboratory variables demonstrating a statistically significant change over time, post hoc comparison of <1 day mean with each of the other time intervals was performed using Dunnett's test 27. Pearson correlation analysis was performed to examine the association of plasma ferritin at birth and infant clinical variables. In addition, Pearson partial correlation coefficient was computed to assess the relationship between plasma ferritin at birth and the CBC variables after 4 days once steady state erythropoiesis was achieved. Since for this analysis the first available CBC data after 4 days varied by infant, the Pearson partial correlation of ferritin with the CBC variable was assessed after controlling for the effect of age on the CBC variable.
The mean VLBW infant values for the primary RET‐He study variable after 4 days was compared with mean RET‐He reported in the literature for normal, iron‐sufficient term infants (2 days of age; 15, 28), children (6 months to 18 years; 10), and adults 6, 7. For these analyses, the first RET‐He value for each infant after day 4 of age was used in computing the mean RET‐He. The adult studies were combined by computing a weighted average of the means and the pooled SD. The two‐sample t‐test was then used to test for difference in RET‐He mean between VLBW infants and the mean reported in literature for the other age groups. P values were adjusted for multiple testing using Bonferroni's method 27.
RESULTS
The mean ± SD for gestational age, birth weight, and birth weight z‐score for the 26 infants were 26.6 ± 1.28 weeks, 872 ± 245 g, and −0.61 ± 1.10, respectively (Table 1). There were 11 males and 15 females; four infants were twins and one was a triplet. During the first month of age, a total of 1,498 sequential plasma EPO and 175 CBC measurements were made. Infants received 3.9 ± 2.1 RBC transfusions, while daily laboratory phlebotomy loss of individual infants during the first month averaged 1.9 ± 0.9 ml/kg. Thus, during the 1‐month study period, individual infants had approximately the same volume of blood removed for laboratory testing as was transfused, that is, 57.0 and 58.5 ml/kg, respectively.
Table 1.
Clinical Features of Study Subjects During the First Month of Age
| Subject no. | Gestation age (weeks) | Birth weight (g) | Birth weight (z‐score) | Gender | RBC transfusions (no.) | Laboratory phlebotomies (ml/kg/day) |
|---|---|---|---|---|---|---|
| 1 | 27.4 | 1487 | 1.97 | M | 2 | 0.6 |
| 2 | 26.4 | 697 | −1.39 | F | 3 | 1.6 |
| 3 | 25.6 | 571 | −1.80 | F | 8 | 2.8 |
| 4 | 27.6 | 1069 | −0.10 | F | 3 | 1.4 |
| 5 | 28.1 | 863 | −1.37 | F | 2 | 0.8 |
| 6 | 27.0 | 694 | −2.00 | M | 4 | 2.8 |
| 7 | 27.4 | 762 | −1.52 | F | 4 | 2.1 |
| 8 | 25.0 | 689 | −0.02 | F | 6 | 2.8 |
| 9 | 26.3 | 572 | −2.41 | M | 5 | 2.0 |
| 10 | 28.6 | 1121 | −0.51 | F | 1 | 0.6 |
| 11 | 26.4 | 652 | −1.97 | M | 8 | 3.8 |
| 12 | 27.7 | 548 | −2.70 | F | 7 | 2.9 |
| 13 | 26.3 | 829 | −0.55 | F | 4 | 1.9 |
| 14 | 24.7 | 693 | −0.70 | M | 6 | 3.1 |
| 15 | 25.0 | 730 | −0.35 | F | 5 | 3.0 |
| 16 | 26.7 | 963 | −0.04 | F | 3 | 1.8 |
| 17 | 28.3 | 1089 | −0.57 | M | 2 | 0.9 |
| 18 | 26.6 | 758 | −1.44 | M | 6 | 2.4 |
| 19 | 27.7 | 1317 | 0.82 | M | 1 | 1.0 |
| 20 | 27.0 | 1029 | 0.11 | F | 1 | 0.7 |
| 21 | 24.3 | 734 | 0.19 | F | 4 | 2.8 |
| 22 | 26.0 | 942 | 0.09 | M | 4 | 1.3 |
| 23 | 25.3 | 906 | 0.68 | F | 3 | 1.6 |
| 24 | 27.9 | 1262 | 0.62 | M | 2 | 1.0 |
| 25 | 24.1 | 678 | −0.37 | M | 5 | 2.6 |
| 26 | 28.0 | 1014 | −0.62 | F | 2 | 1.1 |
Parallel changes occurred during the 1‐month study period for all the erythropoietic variables except Hb concentration when analyzed using linear mixed modeling (Fig. 1 and Table 2). Plasma EPO (P = 0.03), reticulocyte count (P < 0.0001), IRF (P < 0.0001), and RET‐He (P = 0.05) all demonstrated significant decline after 4 days of age. Subsequently, these four erythropoietic variables reached steady‐state levels with no further change observed thereafter. The slow progressive decline in infant Hb levels did not become significant until 4 weeks of age, most certainly because the heavy phlebotomy loss experienced by these VLBW infants was blunted by the resultant RBC transfusions administered (Dunnett's test, P = 0.02). None of these laboratory variables demonstrated a significant association with gestational age, birth weight, birth weight z‐score, or gender.
Figure 1.
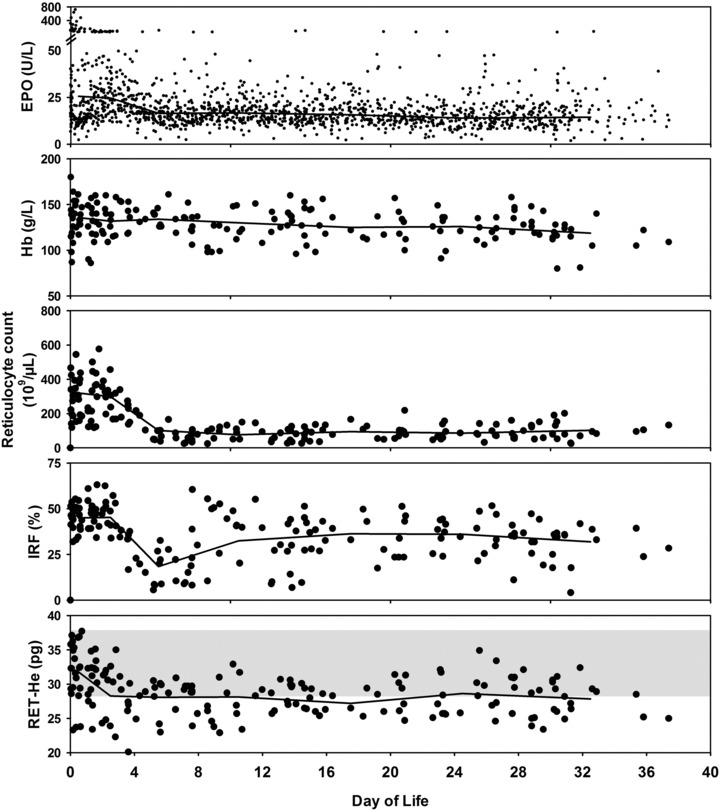
Change in individual erythropoietic laboratory parameters over the first postnatal month of age. Individual data points for EPO, Hb, reticulocyte count, IRF, and RET‐He data are shown for all 26 VLBW infant study subjects. Linear mixed model analysis showed significantly lower values after 4 days of age were observed for EPO, reticulocyte count, IRF, and RET‐He (all P < 0.05). Hb did not demonstrate a significant decline until 4 weeks of age (P < 0.05). Shaded area within RET‐He figure represents the mean ±2 SD for iron‐sufficient healthy adults 6, 7.
Table 2.
Mean (SEM) RBC and Reticulocyte Indices Over Timea
| RBC/reticulocyte index | <1 Day | 2–4 Days | 5–7 Days | 8–14 Days | 15–21 Days | 22–28 Days | >28 Days |
|---|---|---|---|---|---|---|---|
| EPO (U/l) | 25.5 (4.1) | 24.6 (1.8) | 16.4 (1.1) | 16.7 (0.7) | 15.7 (0.9) | 14.0 (1.0) | 14.4 (1.1) |
| Hb (g/l) | 13.6 (0.4) | 13.2 (0.4) | 13.4 (0.6) | 13.0 (0.4) | 12.5 (0.4) | 12.6 (0.4) | 11.9 (0.4) |
| Reticulocyte count (109/μl) | 320 (25) | 300 (28) | 101 (19) | 76 (9) | 95 (10) | 86 (9) | 102 (11) |
| IRF (%) | 45.0 (1.5) | 45.3 (2.0) | 18.4 (3.1) | 32.4 (3.5) | 36.4 (2.4) | 36.0 (2.1) | 31.9 (2.4) |
| RET‐He (pg) | 31.8 (1.1) | 28.3 (1.1) | 28.1 (0.9) | 28.1 (0.5) | 27.2 (0.6) | 28.6 (0.7) | 27.8 (0.6) |
Analysis by mixed linear modeling.
None of the 26 infants met plasma ferritin criteria for ID for term infants, that is, <15 μg/l 29. Mean plasma ferritin levels following birth was 143 ± 126 μg/l. CRP postdelivery levels were all normal, that is, ≤5 mg/l, making all plasma ferritin interpretable.
When plasma ferritin was examined for its association with steady state laboratory indices of erythropoiesis and hemoglobinization, no significant association was found (Table 3). There was no association of plasma ferritin with any of the clinical features included in Table 1 (data not shown).
Table 3.
Correlation of Plasma Ferritin Levels With Clinical and Laboratory Variables
| Correlation | 95% Confidence | ||
|---|---|---|---|
| coefficient | intervals | P‐value | |
| Gestational age at birth | 0.128 | (−0.273, 0.491) | 0.530 |
| Birth weight | 0.149 | (−0.381, 0.394) | 0.462 |
| Birth weight z‐score | 0.071 | (−0.326, 0.446) | 0.729 |
| EPO (U/l)a | 0.113b | (−0.304, 0.494) | 0.595 |
| Hb (g/l)c | 0.359b | (−0.052, 0.666) | 0.079 |
| Reticulocyte count (109/μl)c | 0.157b | (−0.263, 0.527) | 0.459 |
| IRF (%)c | 0.037b | (−0.372, 0.434) | 0.861 |
| RET‐He (pg)c | 0.205b | (−0.216, 0.561) | 0.330 |
Includes EPO value closest to the matching RET‐He value after 4 days of age.
Pearson partial correlation coefficient.
First value after 4 days of age.
Infant steady‐state RET‐He values after 4 days of age were compared to RET‐He values for iron‐sufficient groups from the literature that included 2 days old term infants 15, 28, 6 months to 18 years old children 10, and adults (6, 7; Fig. 2). VLBW study infants had significantly lower RET‐He values than the three other developmental groups (Bonferroni adjusted P < 0.0001).
Figure 2.
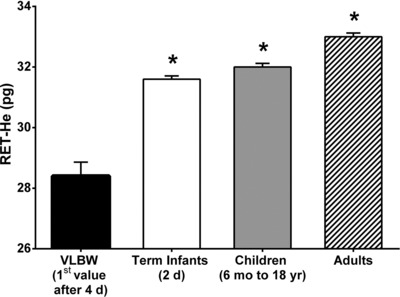
Developmental comparison between RET‐He of VLBW infants at age (4–26 days) with other iron‐sufficient developmental groups. Developmental comparison groups included iron‐sufficient term infants at 2 days of age, children ages 6 months to 18 years, and adults. Data are shown for mean ± SEM.
Additional comparisons of RET‐He values in VLBW infants were performed with the same three developmental groups along with other groups from the literature with disease states impacting erythropoiesis and potentially perturbing RET‐He results (Fig. 3). In both VLBW and iron‐sufficient term infants studied longitudinally 15, 28, RET‐He was highest at birth and subsequently declined. As expected, published reports of children and adults with IDA had significantly lower RET‐He values relative to iron‐sufficient individuals 6, 10, 11. Anemic pregnant women (Hb ≤110 g/L) treated with iron for 4 weeks demonstrated a significant acute increase in RET‐He 26. Iron‐sufficient adults with chronic kidney disease, including those receiving and not receiving hemodialysis, and those being treated with r‐HuEPO and parenteral iron, had RET‐He values that were not different from healthy, iron‐sufficient adults 6, 11. Twelve adults with chronic kidney disease and IDA who were then treated with IV iron responded with a significant increase in RET‐He within 2 weeks 25. For unselected hospitalized children with diverse medical conditions, RET‐He values were slightly, but not significantly, lower than iron‐sufficient healthy children 7. Adults with β‐thalassemia had the lowest RET‐He values 11.
Figure 3.
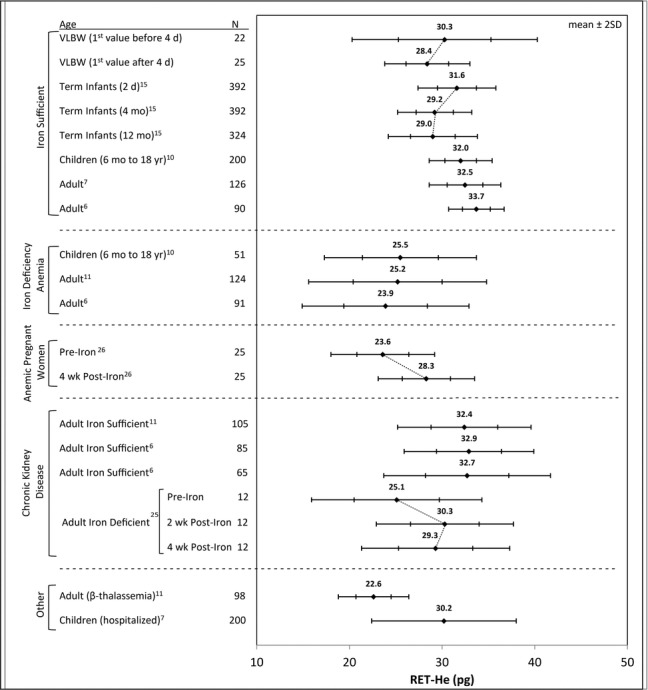
RET‐He ranges for different patient groups. The filled diamond indicates the mean with the ±1 and ±2 SD range indicated horizontally. The diagonal lines connecting different age groups indicate paired longitudinal data for the same study groups. The different subject groups include iron‐sufficient subjects at different developmental periods, IDA, pregnant women, chronic kidney disease subjects receiving r‐HuEPO, and iron treatment, and two other groups, that is, those with β‐thalassemia and hospitalized children with diverse medical conditions.
DISCUSSION
This is the first report of RET‐He data in critically ill, premature VLBW infants during the first month of life. Prior RET‐He studies in children and adults have demonstrated its utility—and that of its counterpart, CHr—in the diagnosis and treatment of ID and IDA and in the management of patients with chronic renal disease being treated with r‐HuEPO and iron 7, 30. Because of their relevance in evaluating iron status, laboratory hematological variables included Hb concentration and reticulocyte indices. The present study of VLBW infants yielded two unexpected findings. First, an abrupt decline in RET‐He was observed over the first 4 days of age. This fall mirrored that observed for plasma EPO, reticulocyte count, and IRF, and suggests that these changes may in some way be related. Second, VLBW infants had significantly lower steady state RET‐He values after the first 4 days of age compared to RET‐He data in 2 days old term infants, children, and adults. These findings have implications in the diagnosis and management of iron disorders in premature infants, particularly those being treated with r‐HuEPO.
The explanation for the rapid decline in RET‐He values in VLBW study subjects from the day of birth (30.3 ± 1.1 pg) to 4 days (28.4 ± 0.5 pg) is unknown. The normal concurrent parallel changes in plasma EPO concentration and reticulocyte count, which is caused by the sudden transition from placental to pulmonary respiratory dependency resulting in improved oxygenation may somehow be related 1, 16, 31. While prior studies have shown an association of plasma EPO with indicators of iron status 32, they did not include temporal data consistent with the relatively short duration observed in the present study. In this regard, it would be of interest to know if an abrupt increase in erythropoiesis, such as that immediately following r‐HuEPO administration, would result in an initial abrupt increase in RET‐He of a similar magnitude to that following birth. Another possibility is that the sudden decrease in RET‐He following birth is a response to the rapid decline in neonatal plasma iron and transferrin iron saturation (from 90% to 25%) with the cessation of active placental iron transport 33. A third possible explanation is that there is a sudden developmental decrease in the intracellular volume of individual circulating reticulocytes initiated at birth, while intracellular Hb concentration remains unchanged 34. Since mean reticulocyte volume measurements are not available for Sysmex instruments, this possibility cannot be assessed. Hence, in the absence of a more evidence‐based understanding for the abrupt fall in RET‐He levels following birth, the use of RET‐He data as an indicator of the adequacy of the supply of iron for erythropoiesis in the 4 days following birth is tenuous.
An explanation for the study's second unexpected finding—steady state RET‐He values beginning at 4 days of age that are significantly lower relative to other patient groups—is also uncertain. A measurement error seems unlikely because the studies were performed when quality control standards were all within the expected range. Because plasma CRP levels of the VLBW study infants were all normal immediately following birth, concurrently measured plasma ferritin levels obtained should reflect infant perinatal iron status 35. When ferritin levels for the 26 study subjects around the time of birth were examined relative to the clinical and laboratory variables no significant associations were identified. This suggests that RET‐He does not indicate functional iron insufficiency at birth—a finding consistent with increased fetal plasma ferritin levels at birth as a result of active iron transport by the placenta. Furthermore, the observation that the erythropoietic study variables (with the exception of Hb) did not change over the first month of age suggests iron status did not change during this period. It is possible that unidentified, developmentally mediated mechanisms are responsible for low RET‐He values in VLBW infants compared to more mature groups.
The ability to measure reticulocyte indices, including RET‐He and CHr, is relatively recent. This first became possible with the availability of new sophisticated, laser‐based, flow cytometry based automated hematology analyzers that currently include Bayer–Siemens analyzers (in 1995), and Sysmex analyzers (in 2005). Both utilize proprietary software to derive reticulocyte index parameters. Reticulocyte hemoglobin content provides clinically relevant information regarding iron status with respect to iron‐restricted erythropoiesis 8, 9, 10, 11, 12, 36. Unlike ferritin, RET‐He and CHr are not falsely increased during acute inflammation. Because of the utility of reticulocyte hemoglobin content in assessing real‐time iron status, it has been designated by the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guidelines as an established parameter in assessing iron status in hemodialysis patients with chronic kidney disease receiving ESA therapy.
In comparing the present study's RET‐He data in VLBW infants to that of other groups, important features regarding the interpretation of RET‐He become apparent. First, the significant postdelivery decline in RET‐He values in the present study has also been noted for full‐term infants studied sequentially at 2 days of age and at 4 and 12 months of age 15. Based on the 2 days of age when the full‐term infant group was studied in the context of the rapid decline observed in RET‐He at 4 days following birth among VLBW infants, it is uncertain whether sufficient time had passed to allow term infant RET‐He levels to decline to steady state levels. RET‐He data from ID children 10 and adults 6, 11 are clearly lower than data for their iron‐sufficient counterparts 10. These RET‐He data support similar reports for CHr demonstrating that both these reticulocyte indices are indicative of the adequacy of functional iron for ongoing erythropoiesis. Data for anemic pregnant women with low RET‐He levels treated for 4 weeks with oral iron that resulted in a rapid increase in RET‐He support the utility of reticulocyte hemoglobin content in identifying states of inadequate iron supply for normal erythropoiesis and for assessing the efficacy of iron treatment 26. Among adults with chronic kidney disease, including some on dialysis, RET‐He levels are within the normal range when these patients are treated with adequate iron and ESAs 6, 11. In one group of such patients who were found to have ID, RET‐He levels increased within 2 weeks of instituting iron treatment from iron deficient levels to iron‐sufficient levels 25. In another study, the lowest RET‐He values observed among all patient groups were individuals with β‐thalassemia 11. β‐Thalassemic patients have a surplus of iron due to frequent RBC transfusions. This along with the potential of RBC transfusions to repress hepcidin production could lead to an increase in iron absorption 23. The exceedingly low RET‐He values in β‐thalassemic patients are likely the consequence of ineffective erythropoiesis, which like ID results in pronounced RBC hypochromia.
Limitations of the present study are that it is a retrospective study performed at a single NICU not designed to identify the mechanism(s) responsible for the changes observed in RET‐He. Second, the number of study subjects was small, with sampling not performed at specific, predetermined intervals. Third, study subjects would not be considered healthy infants because all were mechanically ventilated for respiratory failure, primarily due to respiratory distress syndrome, with the sicker infants more likely to have had multiple hematology laboratory testing.
In conclusion, the reticulocyte parameter, RET‐He, measured in VLBW infants unexpectedly demonstrated an abrupt decrease after the first 4 days following birth. During the remainder of their first month of life significantly lower, steady state RET‐He levels—and other indicators of erythropoiesis—were observed. Equally unexpected was the finding that steady state RET‐He values in VLBW infants were significantly lower than those of more mature iron‐sufficient patient groups. Although the present study was not designed to identify the mechanism(s) responsible for these findings, the RET‐He data themselves must be taken into consideration in future studies in premature infants. We speculate that RET‐He measured in VLBW infants can provide previously unavailable information clarifying the pathogenesis of neonatal anemia and providing real‐time assessment of the role of iron in the prevention and treatment of ID and IDA, and the treatment and prevention of anemia with r‐HuEPO therapy. Additional RET‐He studies in premature infants will be needed before this speculation can be definitely assessed.
CONFLICT OF INTEREST
The XE‐2100 hematology automated analyzer used in these experiments was on loan to J.A.W. from Sysmex, Inc., Lincolnshire, IL.
ACKNOWLEDGMENTS
The authors appreciate the outstanding contributions of the entire satellite clinical laboratory staff led by Mitchell J. Owen with oversight from Matthew D. Krasowski, M.D., Ph.D. We also appreciate the substantial contributions from our laboratory research team (Earl L. Gingerich and Jessica A. Goehring) and our research nursing team (Karen J. Johnson, Laura Knosp, Nancy E. Krutzfield, Sara K. B. Scott, and Ruthann Schrock). Manuscript review, critique, and suggestions by Robert D. Christensen, M.D., were insightful and appreciated. Mark Hart provided expert editorial and secretarial help. This publication was supported in part by US Public Health Service National Institutes of Health (NIH) grant P01 HL046925 (to J. A. W.), by the Thrasher Research Fund 0285–3, and by grant UL1RR024979 from the National Center for Research Resources (to J. A. W.), a part of the NIH. The paper's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the Thrasher Research Fund, or the Clinical and Translational Science Award (CTSA) Program.
REFERENCES
- 1. Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. NeoReviews 2008;9:e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dallman PR. Anemia of prematurity. Annu Rev Med 1981;32:143–160. [DOI] [PubMed] [Google Scholar]
- 3. Juul S. Erythropoietin in anemia of prematurity. J Matern Fetal Neonatal Med 2012;25:80–84. [DOI] [PubMed] [Google Scholar]
- 4. Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long‐lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64:S34–S43; discussion S72–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorenz L, Peter A, Poets CF, Franz AR. A Review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology 2013;104:194–202. [DOI] [PubMed] [Google Scholar]
- 6. Urrechaga E, Borque L, Escanero JF. Erythrocyte and reticulocyte indices in the assessment of erythropoiesis activity and iron availability. Int J Lab Hematol 2013;35:144–149. [DOI] [PubMed] [Google Scholar]
- 7. Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron‐deficient states. Clin Lab Haematol 2006;28:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brugnara C. Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci 2000;37:93–130. [DOI] [PubMed] [Google Scholar]
- 9. Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes's original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–230. [DOI] [PubMed] [Google Scholar]
- 10. Osta V, Caldirola MS, Fernandez M, et al. Utility of new mature erythrocyte and reticulocyte indices in screening for iron‐deficiency anemia in a pediatric population. Int J Lab Hematol 2013;35:400–405. [DOI] [PubMed] [Google Scholar]
- 11. Urrechaga E, Borque L, Escanero JF. Analysis of reticulocyte parameters on the Sysmex XE 5000 and LH 750 analyzers in the diagnosis of inefficient erythropoiesis. Int J Lab Hematol 2011;33:37–44. [DOI] [PubMed] [Google Scholar]
- 12. Muller KF, Lorenz L, Poets CF, Westerman M, Franz AR. Hepcidin concentrations in serum and urine correlate with iron homeostasis in preterm infants. J Pediatr 2012;160:949–53. e2. [DOI] [PubMed] [Google Scholar]
- 13. Canals C, Remacha AF, Sarda MP, Piazuelo JM, Royo MT, Romero MA. Clinical utility of the new Sysmex XE 2100 parameter—reticulocyte hemoglobin equivalent—in the diagnosis of anemia. Haematologica 2005;90:1133–1134. [PubMed] [Google Scholar]
- 14. Urrechaga E, Borque L, Escanero JF. Biomarkers of hypochromia: the contemporary assessment of iron status and erythropoiesis. Biomed Res Int 2013;2013:603786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Löfving A, Andersson O, Hellström‐Westas L, Domellöf M. Reference limits for reticulocyte haemoglobin content in healthy infants. Pediatr Res 2011;70:812. [DOI] [PubMed] [Google Scholar]
- 16. Freise KJ, Widness JA, Veng‐Pedersen P. Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol Exp Ther 2010;332:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosebraugh MR, Widness JA, Nalbant D, Veng‐Pedersen P. A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia. Transfusion 2013;53:1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosebraugh MR, Widness JA, Veng‐Pedersen P. Multidose optimization simulation of erythropoietin treatment in preterm infants. Pediatr Res 2012;71:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ACOG Practice Bulletin . Clinical management guidelines for obstetricians‐gynecologists. Number 55, September 2004 (replaces practice pattern number 6, October 1997). Management of postterm pregnancy. Obstet Gynecol 2004;104:639–646. [DOI] [PubMed] [Google Scholar]
- 20. Arbuckle TE, Wilkins R, Sherman GJ. Birth weight percentiles by gestational age in Canada. Obstet Gynecol 1993;81:39–48. [PubMed] [Google Scholar]
- 21. Widness JA, Susa JB, Garcia JF et al. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest 1981;67:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maconi M, Cavalca L, Danise P, Cardarelli F, Brini M. Erythrocyte and reticulocyte indices in iron deficiency in chronic kidney disease: comparison of two methods. Scand J Clin Lab Invest 2009;69:365–370. [DOI] [PubMed] [Google Scholar]
- 23. Sudmann AA, Piehler A, Urdal P. Reticulocyte hemoglobin equivalent to detect thalassemia and thalassemic hemoglobin variants. Int J Lab Hematol 2012;34:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buttarello M, Pajola R, Novello E, et al. Diagnosis of iron deficiency in patients undergoing hemodialysis. Am J Clin Pathol 2010;133:949–954. [DOI] [PubMed] [Google Scholar]
- 25. Miwa N, Akiba T, Kimata N, et al. Usefulness of measuring reticulocyte hemoglobin equivalent in the management of haemodialysis patients with iron deficiency. Int J Lab Hematol 2010;32:248–255. [DOI] [PubMed] [Google Scholar]
- 26. Schoorl M, Schoorl M, van der Gaag D, Bartels PC. Effects of iron supplementation on red blood cell hemoglobin content in pregnancy. Hematol Rep 2012;4:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winer BJ. 1971. Design and Analysis of Single‐Factor Experiments, second edition, New York, NY: McGraw‐Hill Book Company. [Google Scholar]
- 28. Andersson O, Hellstrom‐Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ 2011;343:d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fomon SJ. 1993. Nutrition of Normal Infantsed. St. Louis: Mosby. [Google Scholar]
- 30. Kasper DC, Widness JA, Haiden N, et al. Characterization and differentiation of iron status in anemic very low birth weight infants using a diagnostic nomogram. Neonatology 2009;95:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kling PJ, Schmidt RL, Roberts RA, Widness JA. Serum erythropoietin levels during infancy: associations with erythropoiesis. J Pediatr 1996;128:791–796. [DOI] [PubMed] [Google Scholar]
- 32. Erdem A, Erdem M, Arslan M, Yazici G, Eskandari R, Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Matern Fetal Neonatal Med 2002;11:329–332. [DOI] [PubMed] [Google Scholar]
- 33. McArdle HJ, Lang C, Hayes H, Gambling L. Role of the placenta in regulation of fetal iron status. Nutr Rev 2011;69(Suppl 1):S17–S22. [DOI] [PubMed] [Google Scholar]
- 34. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987;262:9412–9420. [PubMed] [Google Scholar]
- 35. Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol 2009;36:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ullrich C, Wu A, Armsby C, et al. Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. J Am Med Assoc 2005;294:924–930. [DOI] [PubMed] [Google Scholar]


