Abstract
Background
Massive exchange-transfusion of 42-day-old red blood cells (RBCs) in a canine model of S. aureus pneumonia resulted in in vivo hemolysis with increases in cell-free hemoglobin (CFH), transferrin bound iron (TBI), non-transferrin bound iron (NTBI), and mortality. We have previously shown that washing 42-day-old RBCs before transfusion significantly decreased NTBI levels and mortality, but washing 7-day-old RBCs increased mortality and CFH levels. We now report the results of altering volume, washing, and age of RBCs.
Study Design and Methods
Two-year-old purpose-bred infected beagles were transfused with increasing volumes (5-10, 20-40, or 60-80 mL/kg) of either 42- or 7-day-old RBCs (n=36) or 80 mL/kg of either unwashed or washed RBCs with increasing storage age (14, 21, 28, or 35 days) (n=40).
Results
All volumes transfused (5-80 mL/kg) of 42-day-old RBCs, resulted in alike (i.e., not significantly different) increases in TBI during transfusion as well as in CFH, lung injury, and mortality rates after transfusion. Transfusion of 80 mL/kg of RBCs stored for 14, 21, 28 and 35 days resulted in increased CFH and NTBI in between levels found at 7 and 42 days of storage. However, washing RBCs of intermediate ages (14-35 days) does not alter NTBI and CFH levels or mortality rates.
Conclusions
Preclinical data suggest that any volume of 42-day-old blood potentially increases risks during established infection. In contrast, even massive volumes of 7-day-old blood result in minimal CFH and NTBI levels and risks. In contrast to the extremes of storage, washing blood stored for intermediate ages does not alter risks of transfusion or NTBI and CFH clearance.
Introduction
Blood transfusion is the recognized treatment of severe hemorrhage with shock. To optimize inventory management, the oldest compatible units are selected for transfusion (“first in, first out”).1,2 During refrigerated storage of red cells (RBCs), a series of structural, metabolic and functional changes occur that are collectively referred to as the “storage lesion.” 3 Although RBCs stored up to 42 days meet current Food and Drug Administration (FDA) criteria for licensure;1,4 laboratory,3,5-8 preclinical9-14 and observational clinical studies15-21 have raised concerns regarding the safety and efficacy of older stored RBCs. Several large multi-center randomized controlled trials (RCTs) have been completed or are ongoing to compare the use of “fresh” RBCs with the current transfusion practice.22-26 However, these trials are not designed to evaluate RBCs at the end of their shelf life. The mean age of transfused RBCs in the United States is only 17.9 days2 and, for ethical reasons, the oldest stored units cannot be selected intentionally for a clinical trial. The RCTs22-26 published so far have acknowledged they were not powered to evaluate RBCs transfused at 35 days of storage or older, and further indicate they can not address the possibility that subpopulations of their patients may be particularly vulnerable to the adverse consequences of prolonged red-cell storage.
To study the effects of the oldest versus freshest blood in an experimental setting, human volunteers,27-29 murine10,11 and canine9,30,31 models of transfusion have been employed. The canine model is particularly useful since veterinary blood bank practices are similar to clinical practices, are US government regulated, and permit RBC storage for up to 42 days.32,33 In previous studies, commercially available canine universal donor RBCs at the end of the storage period were associated with in vitro and in vivo hemolysis, resulting in increased release of cell-free hemoglobin (CFH), transferrin bound iron (TBI), and non-transferrin bound iron (NTBI).9,30,31 During experimental S. aureus pneumonia causing septic shock, massive exchange-transfusion with 42-day-old stored RBCs profoundly increased lung injury and mortality in comparison to 7-day-old stored RBCs. In contrast, older RBCs did not increase these risks during hemorrhagic shock or in normal uninfected control animals.30 During septic shock, washing older RBCs prior to transfusion significantly decreased NTBI levels and improved lung injury and survival rates.31 Surprisingly, washing very fresh RBCs resulted in worsened outcomes associated with increased in vivo release of CFH.31 Taken together these findings suggest that older transfused RBCs increase risks specifically during established infection.
Several important concerns regarding the effects of older blood transfusion during infection remain unresolved. It is unclear whether a critical volume or specific age of stored blood is necessary to increase transfusion risks or at what age of storage washing RBCs prior to transfusion changes from harmful, as seen at week one of storage, to beneficial, as seen at the sixth storage week. Here, we present a series of experiments designed to answer these questions.
Methods
The two experiments described below were conducted under two different protocols approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health.
In these experiments numerous combinations of age and volume of stored blood were possible to study the effects of increasing volume and/or age of blood on outcomes. To minimize animal use and still find effects, we studied the extremes of combinations of age and volume. In the first set of experiments, employing the oldest and potentially most toxic RBCs, we examined smaller transfused volumes to determine whether or not the increased risks of older RBCs during infection required massive transfusion. In the second set of experiments we transfused only massive volumes of different ages of younger stored RBCs, potentially less toxic, to evaluate if the harmful effects during massive transfusion of older RBCs would disappear with RBCs stored for shorter durations. A detailed description of the experimental procedures employed and statistical methods can be found in the online SUPPLEMENTAL METHODS and in our previous published studies using the canine S. aureus pneumonia-induced sepsis model.9,31,34
Protocol one: Altering volume of transfused older versus fresher stored RBCs during infection
Thirty-six purpose-bred beagles (12 to 28 months old, 9 to 12.5 kg) with experimental S. aureus pneumonia were exchange-transfused either 7- or 42-day-old canine universal donor stored blood (DEA 1.1 ABRINT, Dixon, CA) at increasing volumes: 5-10 (n=12), 20-40 (n=12), or 60-80 mL/kg (n=12). For nine consecutive weeks, four of the 36 animals were randomized to receive one of two different volumes from 5-80 ml/kg of 7- or 42-day-old blood and followed for 96h or until death. All animals, similar to subjects with pneumonia in an intensive care unit, received antibiotics, fluids, vasopressors, mechanical ventilation, and sedation titrated to physiologic endpoints. All animals were treated identically except for the age and the volume of the stored blood transfused.
Protocol two: Altering storage age of transfused washed and unwashed RBCs during infection
Using the same S. aureus pneumonia model with intensive care unit support, 40 animals were exchange-transfused washed or unwashed blood (80 mL/kg divided in four 20 mL/kg exchange-transfusions) stored for either 14 (n=14), 21(n=12), 28 (n=8) or 35 days (n=6). We hypothesized that the harmful effects of washing seen in previous experiments with 7-day-old blood would likely disappear with very little added storage time. Therefore, in order to minimize animal use, we designed the study so that a smaller number of animals were allocated to study as blood storage age increased from 14 to 35 days. For ten consecutive weeks, four of the 40 animals were studied. During each study week two of the four study animals were randomized to receive washed or unwashed blood at one of the four storage ages studied (14, 21, 28 or 35 days) while the other two animals received washed and unwashed blood at another of the four storage ages studied.
Results
Altering the volume of transfused older versus fresher stored blood
Survival
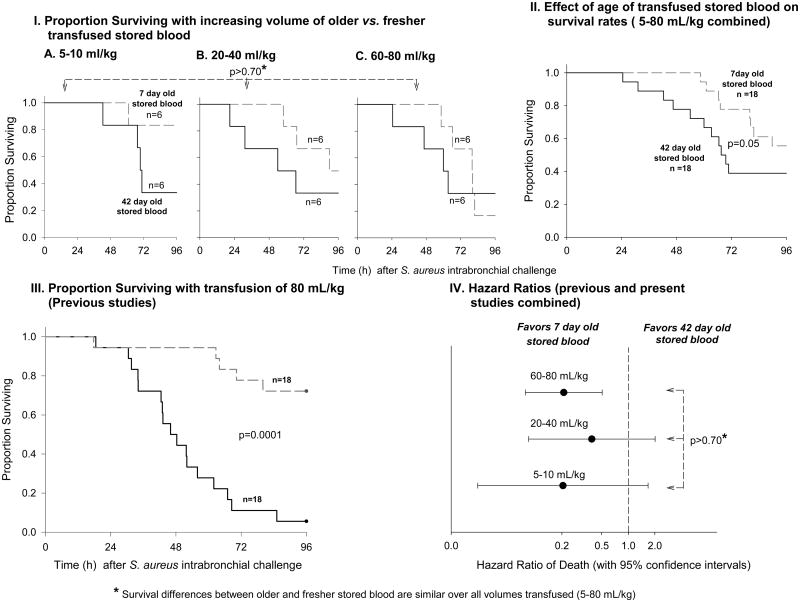
Mortality increased in transfusions with older RBCs compared to fresher RBCs similarly for all volumes of blood studied (5-80 mL/kg) (p>0.70 for similar survival effect of older versus fresher RBCs across all volumes transfused), though the trends did not reach statistical significance for the individual volumes. Combined across all volumes studied, there was a significant increase in mortality with older compared to fresher RBC transfusion (Figure 1, panel II, p=0.05). This increase in mortality with older RBCs is consistent with previous studies using the same sepsis model of exchange-transfusion with large volumes of transfused RBCs (80 mL/kg) (Figure 1, panel III, p=0.0001, previously published studies).9,31 The animals from previous studies receiving 80 mL/kg were combined with those in the present study receiving larger volumes (60-80 mL/kg) to calculate hazard ratios of the total experience using this model at different transfusion volumes. As shown in Figure 1, panel IV; based on all animals studied to date, there were no significant differences in the hazard ratio of death for animals receiving a small (5-10 mL/kg), medium (20-40 mL/kg) or large volume of RBC transfusion (60-80 mL/kg) (p>0.70 for similar increase in mortality of older versus fresher blood across volumes transfused).
Figure 1. Survival rates with increasing volume of older versus fresher transfused blood.
Panel I: Kaplan-Meier plots over the 96-hour study comparing 42- (solid line) and 7-day old blood (dashed line) at increasing volumes of transfusion (5-10, 20-40 and 60-80 mL/kg, Panel A, B and C respectively). The p-value above the plots indicates there was no significantly different effect of older versus fresher blood across all volumes of blood transfused. Panel II: Kaplan-Meier plot combining all volumes transfused shown in panel I (5-80 mL/kg) of 42- and 7-day old blood. Panel III: Kaplan-Meier plot showing our complete previous experience comparing 42- and 7-day old blood transfusion with massive volumes transfused (80 mL/kg). Panel IV: Hazard ratios of death (with 95% confidence intervals) for 42- versus 7-day old blood at different volumes of transfusion. Animals from previous experiments transfused with 80 mL/kg of older and fresher blood are included in the 60-80 mL/kg group. The p-value to the right of the hazard ratios indicates there was no significantly different effect of older versus fresher blood across all volumes of blood transfused.
Lung injury
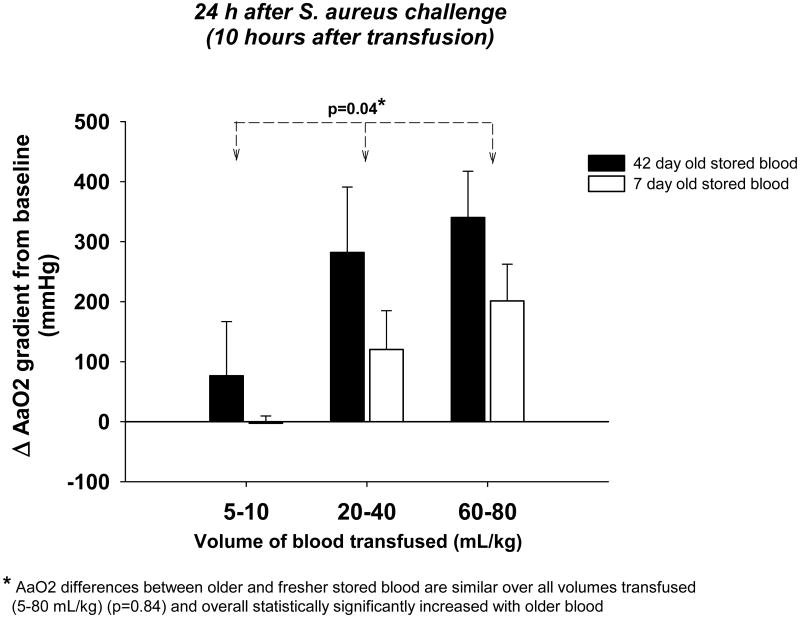
We evaluated the effect of 42- versus 7-day-old RBC transfusion on the alveolo-arterial oxygen (AaO2) gradient 24 h after bacterial challenge (Figure 2). The AaO2 gradient is a measure of lung injury; higher values correspond to worse gas exchange and therefore a higher degree of injury. At all volumes of blood studied, the increase in AaO2 with older versus fresher RBCs was statistically significant and did not differ significantly across different transfused volumes (p=0.84 for similar difference between older versus fresher RBC across all volumes studied). Overall, older blood transfusion significantly increased the AaO2 compared to fresher blood (p=0.04) (Figure 2).
Figure 2. Alveolo-arterial oxygen (AaO2) gradient 24 hours after bacterial challenge with increasing volumes of 42- versus 7-day old blood transfused.
Bar graphs comparing the mean (±SE) change from baseline of AaO2 gradient 24 hours after bacterial challenge (10 hours after transfusion) with 42- (black bars) and 7-day old (open bars) blood at increasing volumes of transfusion (5-10, 20-40 and 60-80 mL/kg). The differences between older and fresher blood are similar over all volumes (p=0.84 for interaction). The p-value above the bars denoted by an asterisk indicates a significant overall effect of older versus fresher blood across all volumes of blood transfused.
Cell-free hemoglobin (CFH), transferrin bound iron (TBI) and non-transferrin bound iron (NTBI) plasma levels
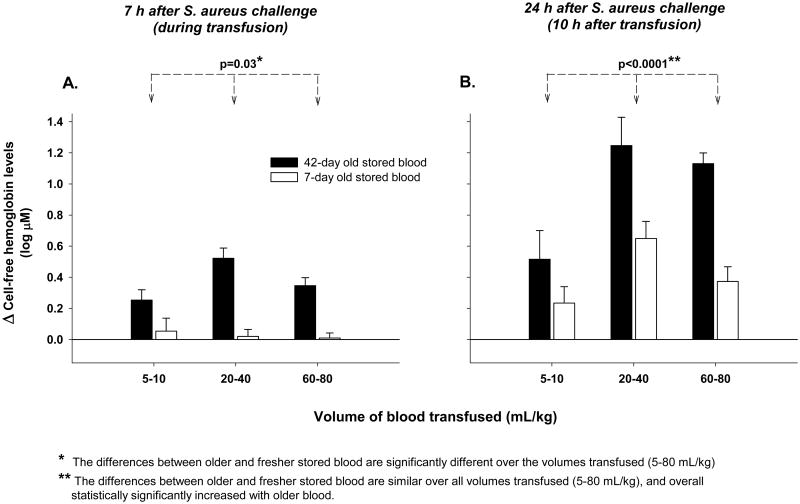
We determined plasma levels of CFH (Figure 3), TBI, and NTBI (Figure 4) during transfusion (7 h after bacterial inoculation) and 10 h after transfusion (24 h after bacterial inoculation). During transfusion at 7 h there was a significantly smaller increase in CFH levels between older and fresher RBCs at lower (5-10 mL/kg) compared to higher volumes (20-80 mL/kg) of blood transfused (p=0.03 for interaction) (Figure 3A). However, even after transfusing only 5-10 mL/kg, there was a greater increase in CFH levels with older compared to fresher RBCs at 7 h (p=0.054). Ten hours after transfusion (24 h after bacterial challenge), CFH levels continued to increase regardless of the age of transfused RBCs (Figure 3B). However, by this time, the differences in CFH levels between older and fresher RBCs transfused were only marginally greater as volumes transfused increased (5-80 mL/kg, p=0.10 for interaction). Older blood resulted in higher CFH levels over all volumes transfused at this time point (p<0.0001) (Figure 3D).
Figure 3. Changes from baseline in Cell-free hemoglobin (CFH) plasma levels (4h after bacterial challenge, immediately pre transfusion) to during (7 h after bacterial challenge) and 10 h after transfusion (24 h after bacterial challenge) with increasing volumes of 42- versus 7-day old blood transfused.
Bar graphs comparing the mean (±SE) log 10 based change from baseline of CFH plasma levels with 42- (black bars) and 7-day old (open bars) blood at increasing volumes of transfusion (5-10, 20-40 and 60-80 mL/kg) at 7 hours (during transfusion, panel A) and 24 hours (10 h after transfusion, panel B) after bacterial challenge. P-values are denoted by asterisks and represent (*) the differences between transfused older and fresher stored blood are significantly different over the volumes of transfused blood (5-80 mL/kg) and (**) the differences between transfused older and fresher stored blood are similar over all volumes of transfused blood (5-80 mL/kg), and overall statistically significantly increased with older blood. Baseline mean (±SE) values in μM are provided in Supplemental Table 1 to evaluate the origins of the above log base 10 changes plotted.
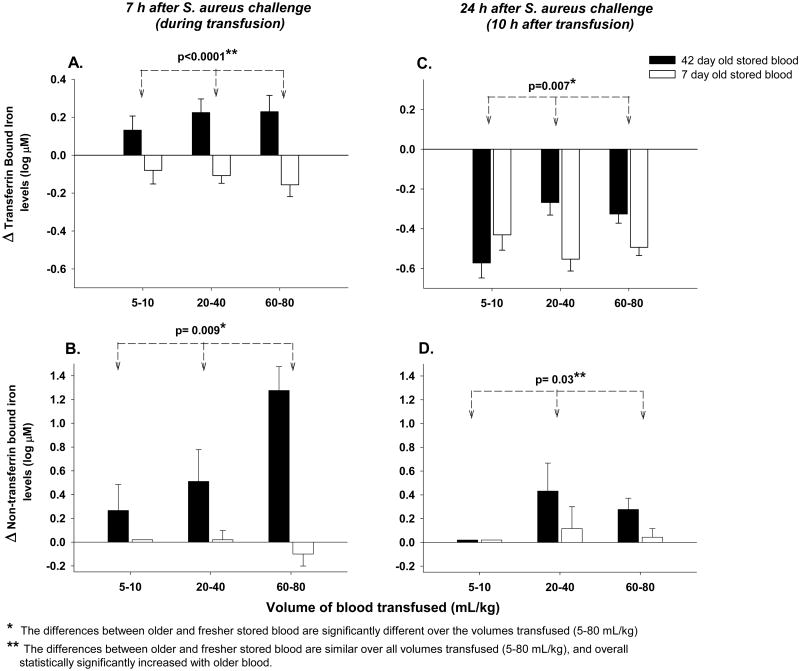
Figure 4. Changes from baseline in Transferrin bound iron (TBI) and non-transferrin bound iron (NTBI) plasma levels to during (7 h after bacterial challenge) and 10 after transfusion (24 h after bacterial challenge) with increasing volumes of 42- versus 7-day old blood transfused.
Bar graphs comparing the mean (±SE) log 10 based change from baseline of TBI plasma levels with 42- (black bars) and 7-day old (open bars) blood at increasing volumes of transfusion (5-10, 20-40 and 60-80 mL/kg) at 7 h after infection (during transfusion, panel A) and 24 hours after bacterial challenge (10 h after transfusion, panel C). NTBI plasma levels are represented in a similar fashion in panels B and D. P-values are denoted by asterisks and represent (*) the differences between transfused older and fresher stored blood are significantly different over the volumes of transfused blood (5-80 mL/kg) and (**) the differences between transfused older and fresher stored blood are similar over all volumes of transfused blood (5-80 mL/kg), and overall statistically significantly increased with older blood. Baseline mean (±SE) values in μM are provided in Supplemental Table 1 to evaluate the origins of the above log base 10 changes plotted.
During transfusion at 7 h, in contrast to CFH, TBI levels in fresh and old blood increased to a similar degree at all volumes studied (p=0.42 for similar effect over volumes transfused) and overall, levels were significantly increased from baseline (p<0.0001) (Figure 4A). Even after the transfusion of only 5-10 mL/kg of blood at 7h, older RBCs produced increased TBI levels in comparison to fresher RBCs (p=0.055). In contrast, differences in NTBI levels during transfusion between older and fresher transfused RBCs were significantly increased more as the volume of transfused blood increased (p= 0.009) (Figure 4B).
At 10 h after transfusion, compared to during transfusion, there was also a significantly different effect of older versus fresher RBCs on TBI levels across the different volumes of transfused blood (5-80 mL/kg) (p=0.007 for interaction, Figure 4C). At the lower volumes of older versus fresher transfused blood (5-10 mL/kg), TBI levels had decreased significantly more than after transfusion of higher volumes (20-80 mL/kg) (Figure 4C). NTBI levels at 10 h after transfusion in comparison to during transfusion were very low regardless of age of stored blood across all volumes, but at all volumes older versus fresher RBCs produced a small but significant increase in NTBI levels (p=0.03) (Figure 4D).
Altering the storage age of washed blood before transfusion
Survival
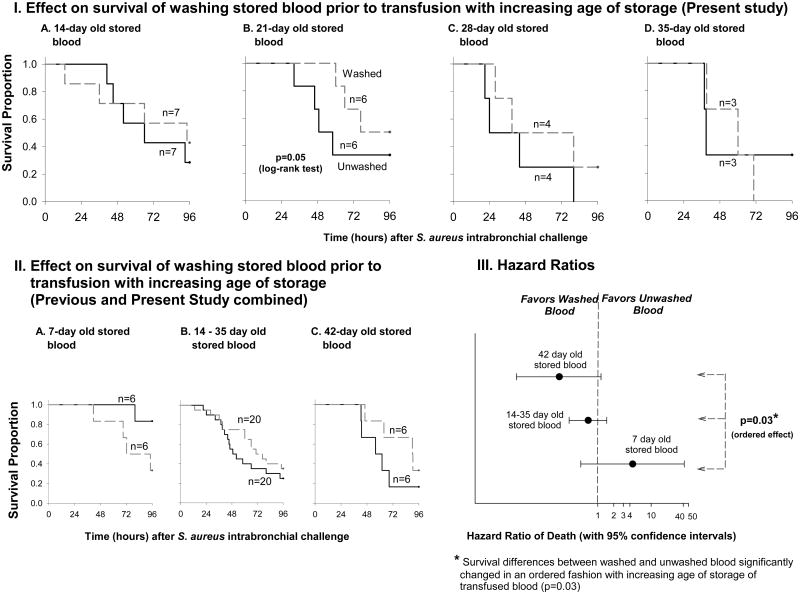
We previously found that washing units just prior to transfusion had significantly different and opposite effects if the washed RBCs were 42- versus 7-day-old.31 Canine survival from septic shock was improved when the oldest RBCs were washed, but worsened after washing the freshest units. Here, we studied the effect of washing stored RBCs between these two extremes (14, 21, 28, and 35 days). By 14 days of blood storage, the harmful effect of washing blood is no longer apparent; survival rates in animals receiving washed and unwashed blood are similar. The Kaplan–Meier survival curves overlap multiple times (Figure 5, panel IA). At 21 days of storage, separation begins to appear between washed and unwashed transfused RBCs on the Kaplan-Meier plots showing that washing improves survival compared to unwashed blood (Figure 5, panel IB). With increasing ages of blood (by 28 and 35 days of storage), we did not find statistically significant differences in survival between washed and unwashed units (Figure 5, panel IC and ID). Similarly we did not find statistically significant survival differences between washed and unwashed RBCs across the four intermediate storage times.
Figure 5. Survival rates with increasing age of unwashed versus washed transfused blood.
Panel I: Kaplan-Meier plots over the 96-hour study comparing unwashed (solid line) and washed red blood cells (dashed line) at increasing ages of blood storage (14, 21, 28, and 35 days, A, B, C and D respectively). The volume of blood transfused was 80 mL/kg for all animals. Panel II: Kaplan-Meier plots comparing unwashed and washed blood stored for 7 and 42 days (A and C respectively, previously published) and for the combination of all the intermediate ages represented in panel I (14 to 35 days. Panel III: Hazard ratios of death (with 95% confidence intervals) for washed versus unwashed blood at different volumes of transfusion.
We combined all animals receiving washed or unwashed stored blood across all storage ages employed in this study in order to compare the effect of washing in these intermediate ages (14, 21, 28 and 35 days) of storage to the effects previously seen with 7- and 42-day-old blood (Figure 5, panel II).31 The hazard ratio of death for animals receiving washed versus unwashed RBCs significantly decreased more with older ages of stored of blood as follows: mortality improved with washed compared to unwashed RBCs at 42-days of storage more than with 14- to 35-day-old stored transfused RBCs, and with 14- to 35-day-old stored RBCs mortality improved more with washing than with 7-day-old stored transfused RBCs (p=0.03, for ordered effect) (Figure 5, panel III).
Cell-free hemoglobin (CFH) and non-transferrin bound iron (NTBI) plasma levels
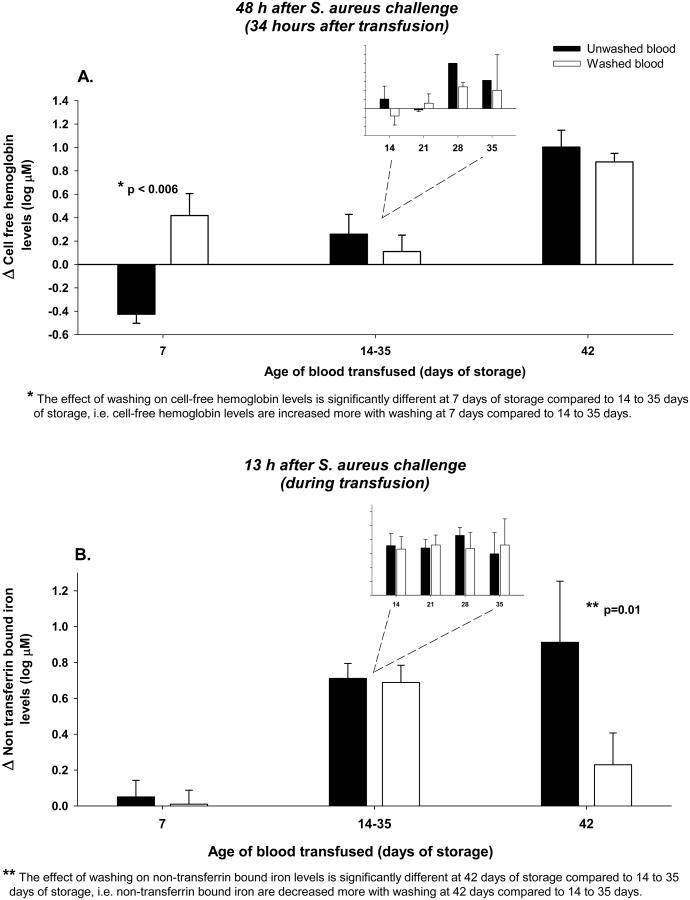
We evaluated the effects on CFH and NTBI levels (Figure 6) of washing RBCs prior to transfusion at these time points in the storage period from 14-35 days and then compared them to levels at 7 and 42 days (previously published).31
Figure 6. Changes from baseline in Cell-free hemoglobin (CFH) (4h after bacterial challenge, immediately pre transfusion) to 48h after bacterial challenge 34 h after transfusion and non-transferrin bound iron (NTBI) plasma levels 13h after bacterial challenge during infection with increasing ages of unwashed versus washed blood transfused.
Panel A: Bar graphs comparing the mean (±SE) log based 10 change from baseline of CFH plasma levels after transfusion of 80 mL/kg of unwashed (black bars) and washed (open bars) blood of increasing storage age (7, 14-35 and 42 days). In the insert, the log based 10 changes in CFH levels at the individual storage ages of 14, 21, 28 and 35 days are plotted. The differences between washed and unwashed blood were not significantly different across all this intermediate storage ages and thus, were combined for comparison to 7 and 42 days of storage. Panel B uses the same format as that of panel B, except now NTBI levels during transfusion are plotted in the y-axis. P-values are denoted by asterisks and represent (*) the effect of washing on cell-free hemoglobin levels is significantly different at 7 days of storage compared to 14 to 35 days of storage and (**) the effect of washing on non-transferrin bound iron levels is significantly different at 42 days of storage compared to 14 to 35 days of storage. Baseline mean (±SE) values in μM are provided in Supplemental Table 2 to evaluate the origins of the above log base 10 changes plotted.
Washing RBCs stored for 14, 21, 28, or 35 days prior to transfusion had no significant effect on the levels of CFH produced after transfusion (48 h after S. aureus challenge, p=0.30, 0.70, 0.45, 0.75) (Figure 6A). Moreover, after combining levels across all these storage ages, there was still no significant difference in plasma CFH levels produced comparing washed versus unwashed stored transfused RBCs (p=0.43). However, the effect of washing RBCs stored for only 7 days significantly increased CFH levels not only in comparison to washing 42-day-old stored RBCs as previously reported, but also after washing RBCs stored for 14-35 days, as found in this study (p<0.006) (Figure 6A). Only with the freshest 7-day-old stored RBCs did washing markedly increase hemolysis and CFH plasma levels.
Similarly, washing RBCs stored for 14, 21, 28 or 35 days had no significant effect on NTBI levels, and there were no significant differences in plasma NTBI levels during transfusion of washed versus unwashed RBCs after combining all these intermediate storage ages (Figure 6B, p=0.78, 0.82, 0.43, 0.64). However, during transfusion, washing decreased NTBI levels significantly not only at 42 days of storage in comparison to 7-day-old blood, as previously reported, but also in comparison to 14-35 days of storage (p=0.01) (Figure 6B). A significant decrease in NTBI plasma levels was observed only after washing the oldest units of RBCs.
Washing RBCs prior to transfusion appears to be increasingly beneficial as storage age and NTBI levels increase, in an ordered fashion (i.e. oldest RBC storage times and highest NTBI levels > intermediate RBC storage times and intermediate NTBI levels > freshest RBCs with very low NTBI levels, p=0.03 for ordered beneficial survival effect). However, the benefit of washing may only be clinically significant at the end of the storage period when NTBI levels are markedly elevated and washing has been shown to significantly lower these toxic levels.31
Discussion
In our canine septic shock model, we previously evaluated the effect of exchange-transfusion of 80 ml/kg of 42- or 7-day-old blood, equivalent to more than 10 units of packed RBCs in humans. Here, we sought to determine whether smaller volumes of older stored RBCs would produce similar results. We found that volumes clinically equivalent to the commonly transfused one to two units of older RBCs, led to increases in iron and CFH levels; these were associated with increases in lung injury and mortality comparable to those seen with the larger transfused volumes. Five to 10 ml/kg of older blood delivered sufficient iron to saturate the physiologic iron chaperone, transferrin. This was evidenced by increases in TBI with detectable levels of NTBI after transfusing these smaller volumes of RBCs.
With volumes of RBCs comparable to 5-10 units of packed RBCs in humans, older blood induced increases in TBI levels after transfusion similar to those seen after transfusing the equivalent to only 1-2 units of blood. In contrast, NTBI levels increased in a dose-dependent fashion with increasing volumes of older blood transfused. Our findings are most consistent with the notion that, in situations of intravascular hemolysis, iron will be bound initially by transferrin until saturation of the binding capacity. Further release of iron will be detectable as free NTBI.35 In our study, older blood in volumes commonly used clinically was sufficient to saturate transferrin and produce detectable mean increases in NTBI. Further, CFH levels after transfusion of the smaller volumes of older versus fresher RBCs, were increased but significantly lower than levels observed with the larger volumes. However, by 20h after transfusion, these increases in CFH levels were not significantly different over all volumes transfused. Thus, similar increases across all volumes in TBI levels during transfusion and in CFH levels after transfusion can account for increased lung injury and mortality regardless of the volume of older RBCs transfused.
Our results are consistent with human volunteers data in which transfusion of a single unit of older stored RBCs led to a significant increase in serum NTBI and transferrin saturation.27 These increases in iron levels with older blood were detectable for 24 hours but were not associated with clinical adverse effects. Similarly, in healthy canines transfused with massive amounts of 42-day-old RBCs, we found markedly increased NTBI levels greater than those seen in infected animals, but these increases were not associated with clinical injury.30 In the presence of established infection in canines, the increases in plasma iron levels were cleared significantly faster from plasma and this was associated with increased lung injury and septic shock mortality, potentially reflecting increased bacterial growth and infection severity.30 These pathogens have evolved mechanisms to acquire this necessary nutrient for growth not only as free NTBI, but also from transferrin and other iron binding proteins36,37 and by releasing siderophores.38,39
Taken together, our results suggest that, during established infection, there is no safe volume of 42-day-old blood because even the modest volumes administered clinically can increase iron and CFH levels. Murine models have shown that excess iron, delivered in comparable quantities by either older blood transfusion or in the form of iron dextran, can exacerbate established bacterial infections, significantly increasing lethality.14 Similarly, in a murine model of S. aureus pyelonephritis intramuscular iron markedly aggravated the infection.40 Moreover, data from human observational studies suggest that older blood may not only worsen established infection but also, in larger volumes, increase the risk of infection. In populations at risk of infection, increasing volumes of older blood transfusion appear to be associated with an increased risk of complicated sepsis41 and mortality.20 Similar findings derive from studies evaluating exclusively the use of iron. In a study of newborns, routine administration of intramuscular iron dextran was associated with a significantly higher incidence of neonatal sepsis that subsequently dropped after eliminating iron supplementation practices.42 A recent meta-analysis suggests that liberal transfusion practices increase the incidence of serious health-care associated infections in comparison to more restrictive strategies, in which less volume of blood (and likely less old blood and possibly less iron) is transfused.43 Increased CFH levels alone are also associated with a worse outcome in patients with septic shock44 and increased CFH levels which may occur with transfusion of older blood during established infection are another source of iron as well as a recognized cause of NO scavenging7,45 and oxidative reactions.46
Although transfusion of the equivalent of only 1-2 units of 42-day-old blood led to transferrin saturation and release of NTBI as well as substantial increases in CFH, massive amounts of fresh 7-day-old RBCs (equivalent to >10 units of blood) produced no increase in NTBI as well as very low levels of CFH. Additionally, massive amounts of 7-day-old RBCs appeared to increase the risks of transfusion less than any of the other combinations of ages and volumes of transfused RBCs studied (Figures 5-7). During massive transfusion, blood stored for 14-35 days, led to similar more substantial increases in NTBI levels. However, it was only after transfusion of 42-day-old stored blood that a significant lowering of NTBI levels with washing was observed. We previously found that washing these oldest units of stored blood prior to transfusion could improve outcomes during infection by reducing the amount of iron delivered after transfusion.31 Here, we found that washing RBCs units of intermediate storage ages (14-35 days old) had no substantial effect on NTBI or CFH levels. Consistent with this observation, we did not find a significant effect on survival even when all intermediate storage ages were combined. In contrast, washing 7-day-old stored blood, where no excess in NTBI is produced, increases in vivo hemolysis and release of CFH, causing worse survival and lung injury.
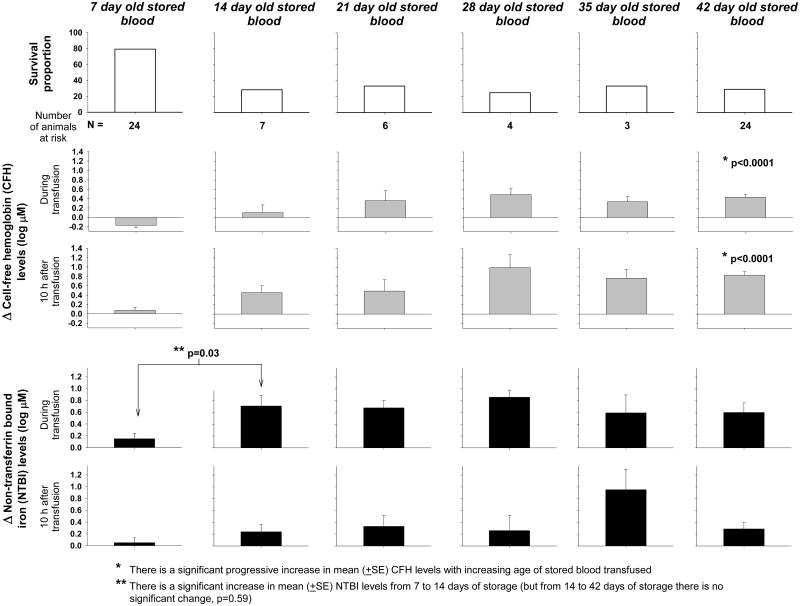
Figure 7. Survival proportion, and changes from baseline in cell-free hemoglobin (CFH) and non-transferrin bound iron (NTBI) plasma levels to during and 10 hours after transfusion of unwashed blood with increasing storage time.
To evaluate the total experience with massive exchange-transfusion of unwashed blood with increasing storage ages, we included all infected animals studied so far receiving 80 ml/kg. In the top panels, survival proportion is plotted as open bars for each storage age of the blood transfused (7, 14, 21, 28, 35 and 42 days). The total number of animals studied at each storage age (animals at risk) is denoted below each panel. In the middle panels, the mean (±SE) log based 10 changes from baseline (4h after bacterial challenge, immediately pre transfusion) of CFH plasma levels to during and 10 hours after transfusion are plotted as grey bars for each storage age of the blood transfused. In the bottom panels, the mean (±SE) log based 10 changes from baseline of NTBI plasma levels to during and 10 hours after transfusion are plotted as black bars for each storage age of the blood transfused. CFH plasma levels increase progressively with age but both survival and NTBI plasma levels appear to have a step up between 1 and 2 weeks of storage. During infection Transfusion of RBCs stored for only 1week results in the best survival associated with the lowest CFH and iron plasma levels.
It is difficult to determine from these data exactly when during the 6 weeks of RBC storage transfusion becomes associated with markedly increased risks during infection. However, with 7-day-old stored blood, even after transfusing massive quantities, there were very low NTBI and CFH levels as well as low mortality rates (18%, 1 of 6 animals died) (Figure 7) compared to RBCs at all other storage times studied. After transfusion of massive amounts of RBCs stored for longer times (14, 21, 28, 35, or 42 days), NTBI levels were always much greater and mortality rates were always nominally higher than with only 7 days of storage (i.e., mortality rate at or above 66% at each storage time >7 days, with a total of 44 animals studied). Thus, potential risks of blood as it ages appear to occur in situations of severe established infection, in which iron and CFH may be critically important and an excess can tip the balance in favor of bacterial growth. Taken together, these preclinical data suggest the safest blood for transfusion in severe bacterial infections is 7-day-old blood, which releases minimal levels of iron and lowest CFH levels even after transfusing massive amounts. Consistent with these results, data from human observational studies in which patients were divided according to weeks or quartiles of storage age of the units transfused, show a trend to progressive worsening of outcomes with increasing storage age.47-49 Moreover, significant harmful effects were only documented with the oldest units in these studies.
We recognize our findings have limitations. We do not have direct evidence that bacterial growth is enhanced with the excess free iron delivered by any volume of transfused older blood. Further, it is possible that these findings would change if a different type of bacterial challenge or even a different strain of S. aureus were employed. Our experimental model of exchange-transfusion is designed as a toxicity study and potentially pathophysiologically different to transfusion during anemia. Canine RBCs are also not identical to human. Canine RBCs may experience proportionally more damage during storage than do human RBC and, thus, extrapolation of our results to human transfusion practices must be done with caution. We previously showed that 42-day-old canine RBCs had less than 1% hemolysis in the bag and 60% survival 24 hours after transfusion. These values are within the low end of the range expected of stored human RBCs in clinical practice.50 However, even if canine 42-day-old blood is slightly less fit, in these experiments we studied all the storage ages between 7 and 42 days and we found similar levels of plasma iron after transfusion of 42-day-old compared to 14- to 35-day-old blood. Moreover, we studied the extremes of age and volume transfused and did not study all combinations possible and any of the combinations not studied could produce unexpected results different from the findings reported here. Animals transfused with washed and unwashed 7- and 42-day-old were not studied concurrently with the intermediate ages of blood. However, while small variations in the experimental conditions over time may potentially affect the comparisons across transfused blood of increasing ages, they should not affect the comparisons of washed verses versus unwashed blood at a given storage age, as these were reported as differences from a contemporaneous control. In addition, we cannot rule out that this released iron is worsening outcomes through other mechanisms such as generation of reactive oxygen species or impairing the host's immune response. However, the potential of NTBI to convert non-lethal bacterial infections into lethal ones has already been proven.14
In conclusion, these studies indicate that, during established infection, any volume of 42-day-old blood may enhance bacterial growth, organ injury, and mortality. Even a volume equivalent clinically to 1-2 units of old blood produces increases in CFH and substantial iron release that saturates normal physiologic transport mechanisms leading to increases in NTBI and worsened outcomes. During infection, the safest blood for transfusion appears to be 7-day-old RBCs. Transfusion of fresh blood, even in massive volumes, does not result in increases in levels of iron and results in very low CFH levels associated with substantially lower mortality rates during infection than transfused RBCs stored for 2-6 weeks. Whereas previous studies suggested that washing 7-day-old RBCs may be deleterious, washing RBC units stored for 14-35 days does not decrease iron or CFH levels and does not affect outcomes. The effect of washing RBC units prior to transfusion is beneficial when applied to blood at the end of the storage period, when the oldest RBCs are present and washing increases clearance of NTBI. These preclinical data suggest that RBCs at the end of their storage life-span should not be used for transfusion of infected subjects or, if no other option exists, they should be washed prior to transfusion.
Supplementary Material
Acknowledgments
Haemonetics (Braintree, MA) funded 10% of the study costs and provided the ACP215 (automated cell processing system) as well as the disposable kits (RBC De-Glycerol Set 325 mL BMB Ref.236) used throughout the experiments.
Sources of support: Intramural NIH funds and NIH external grants HL058091 and HL098032 were used to support this study.
Footnotes
Conflicts of Interest: D.K.-S. is listed as a coauthor on a patent application on methods of treating hemolysis and on patent applications related to development of blood substitutes using recombinant human hemoglobin. The other authors declare no conflicts of interest.
Disclaimer: The work by the authors was done as part of U.S. government-funded research; however, the opinions expressed are not necessarily those of the National Institutes of Health.
References
- 1.AABB. Requirements for Storage, Transportation, and Expiration Vol Reference Standard 5 1 8A. 27th. Bethesda, MD: AABB; 2011. Standards for Blood Banks and Transfusion Services. [Google Scholar]
- 2.Department of Health and Human Services. The 2011 National Blood Collection and Utilization Survey Report. 2011 http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf.
- 3.Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia. 2015;70 Suppl 1:29–37. e9–12. doi: 10.1111/anae.12891. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR Biomedical Excellence for Safer Transfusion C. Scientific problems in the regulation of red blood cell products. Transfusion. 2012;52:1827–35. doi: 10.1111/j.1537-2995.2011.03511.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank SM, Abazyan B, Ono M, et al. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;116:975–81. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–72. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrickson JE, Hod EA, Hudson KE, et al. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:2695–702. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baek JH, D'Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–58. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron DM, Beloiartsev A, Nakagawa A, et al. Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs*. Crit Care Med. 2013;41:2492–501. doi: 10.1097/CCM.0b013e31828cf456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prestia K, Bandyopadhyay S, Slate A, et al. Transfusion of stored blood impairs host defenses against Gram-negative pathogens in mice. Transfusion. 2014;54:2842–51. doi: 10.1111/trf.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg JA, McGwin G, Jr, Marques MB, et al. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–8. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 17.Pettila V, Westbrook AJ, Nichol AD, et al. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15:R116. doi: 10.1186/cc10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaukonen KM, Vaara ST, Pettila V, et al. Age of red blood cells and outcome in acute kidney injury. Crit Care. 2013;17:R222. doi: 10.1186/cc13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cywinski JB, You J, Argalious M, et al. Transfusion of older red blood cells is associated with decreased graft survival after orthotopic liver transplantation. Liver Transpl. 2013;19:1181–8. doi: 10.1002/lt.23695. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg JA, McGwin G, Jr, Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 31-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacroix J, Hebert PC, Fergusson DA, et al. Age of Transfused Blood in Critically Ill Adults. N Engl J Med. 2015 doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 24.Heddle NM, Cook RJ, Arnold DM, et al. The effect of blood storage duration on in-hospital mortality: a randomized controlled pilot feasibility trial. Transfusion. 2012;52:1203–12. doi: 10.1111/j.1537-2995.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaukonen KM, Bailey M, Ady B, et al. A randomised controlled trial of standard transfusion versus fresher red blood cell use in intensive care (TRANSFUSE): protocol and statistical analysis plan. Crit Care Resusc. 2014;16:255–61. [PubMed] [Google Scholar]
- 26.Age of Blood in Children in Pediatric Intensive Care Units (ABC PICU) doi: 10.1186/s13063-018-2809-y. https://clinicaltrials.gov/ct2/show/NCT01977547?term=age+of+blood+storage+red+cell&rank=1. [DOI] [PMC free article] [PubMed]
- 27.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiskopf RB, Feiner J, Toy P, et al. Fresh and stored red blood cell transfusion equivalently induce subclinical pulmonary gas exchange deficit in normal humans. Anesth Analg. 2012;114:511–9. doi: 10.1213/ANE.0b013e318241fcd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–20. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Cortes-Puch I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion. 2014;54:1712–24. doi: 10.1111/trf.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes-Puch I, Wang D, Sun J, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–11. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brownlee L, Wardrop KJ, Sellon RK, et al. Use of a prestorage leukoreduction filter effectively removes leukocytes from canine whole blood while preserving red blood cell viability. J Vet Intern Med. 2000;14:412–7. doi: 10.1892/0891-6640(2000)014<0412:uoaplf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Wardrop KJ. Selection of anticoagulant-preservatives for canine and feline blood storage. Vet Clin North Am Small Anim Pract. 1995;25:1263–76. doi: 10.1016/s0195-5616(95)50153-6. [DOI] [PubMed] [Google Scholar]
- 34.Minneci PC, Deans KJ, Hansen B, et al. A canine model of septic shock: balancing animal welfare and scientific relevance. Am J Physiol Heart Circ Physiol. 2007;293:H2487–500. doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- 35.Brissot P, Ropert M, Le Lan C, et al. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820:403–10. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Taylor JM, Heinrichs DE. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol Microbiol. 2002;43:1603–14. doi: 10.1046/j.1365-2958.2002.02850.x. [DOI] [PubMed] [Google Scholar]
- 37.Modun B, Morrissey J, Williams P. The staphylococcal transferrin receptor: a glycolytic enzyme with novel functions. Trends Microbiol. 2000;8:231–7. doi: 10.1016/s0966-842x(00)01728-5. [DOI] [PubMed] [Google Scholar]
- 38.Courcol RJ, Trivier D, Bissinger MC, et al. Siderophore production by Staphylococcus aureus and identification of iron-regulated proteins. Infect Immun. 1997;65:1944–8. doi: 10.1128/iai.65.5.1944-1948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dale SE, Doherty-Kirby A, Lajoie G, et al. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect Immun. 2004;72:29–37. doi: 10.1128/IAI.72.1.29-37.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ang O, Gungor M, Aricioglu F, et al. The effect of parenteral iron administration on the development of Staphylococcus aureus-induced experimental pyelonephritis in rats. Int J Exp Pathol. 1990;71:507–11. [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan M, Pham TN, Cuschieri J, et al. The association between the transfusion of older blood and outcomes after trauma. Shock. 2011;35:3–8. doi: 10.1097/SHK.0b013e3181e76274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barry DM, Reeve AW. Increased incidence of gram-negative neonatal sepsis with intramuscula iron administration. Pediatrics. 1977;60:908–12. [PubMed] [Google Scholar]
- 43.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41:784–90. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Liu X, Janes J, et al. Mechanism of faster NO scavenging by older stored red blood cells. Redox Biol. 2014;2:211–9. doi: 10.1016/j.redox.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallelian F, Pimenova T, Pereira CP, et al. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic Biol Med. 2008;45:1150–8. doi: 10.1016/j.freeradbiomed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Edgren G, Kamper-Jorgensen M, Eloranta S, et al. Duration of red blood cell storage and survival of transfused patients (CME) Transfusion. 2010;50:1185–95. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eikelboom JW, Cook RJ, Liu Y, et al. Duration of red cell storage before transfusion and in-hospital mortality. Am Heart J. 2010;159:737–43 e1. doi: 10.1016/j.ahj.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 49.Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–13. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 50.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.