Abstract
Background:
DBS are an increasingly common clinical matrix.
Methods & results:
Sensitive and specific methods for DBS and venous blood cocaine and metabolite detection by LC–HRMS and 2D GC–MS, respectively, were validated to examine correlation between concentrations following controlled intravenous cocaine administration. Linear ranges from 1 to 200 µg/l were achieved, with acceptable bias and imprecision. Authentic matched specimens’ (392 DBS, 97 venous blood) cocaine and benzoylecgonine concentrations were qualitatively similar, but DBS had much greater variability (21.4–105.9 %CV) and were lower than in blood.
Conclusion:
DBS offer advantages for monitoring cocaine intake; however, differences between capillary and venous blood and DBS concentration variability must be addressed.
Background
Cocaine is a widely abused CNS stimulant worldwide [1–3], with approximately two million cocaine users in the USA and Europe [3,4]. It is rapidly inactivated by plasma pseudocholinesterases and liver esterases via ester hydrolysis into ecgonine methyl ester (EME) and ecgonine (EG), and in the liver by carboxylesterases producing benzoylecgonine (BE). Additionally, cocaine is metabolized by N-demethylation to norcocaine, an active although minor metabolite in humans [5]. Cocaethylene is another metabolite of cocaine that can be produced when used in combination with ethyl alcohol by the transesterification of cocaine with ethyl alcohol via liver methylesterases. Cocaethylene also may be present in street and pharmaceutical cocaine [6]. Cocaine is unstable in biological matrices, hydrolyzing in vitro with conversion to BE, EME and EG spontaneously (without enzymes) at physiological temperature and pH [7].
DBS sampling refers to a microsampling technique of increasing interest in pharmaceutical, clinical and forensic settings [8]. DBS sampling is not a novel concept, as it was utilized over a century ago to monitor glucose concentrations in rabbits [8] and also proved useful in newborn infant screening programs [9,10]. Recently, the use of alternative matrices like DBS for detecting recent drug consumption is increasing due to easy, less invasive collection (via finger puncture) and increased stability compared with blood [11,12]. The increased analyte stability is especially important when rapid degradation of compounds in blood was documented [13,14]. Several studies demonstrated minimal analyte loss in DBS as compared with other biological matrices, like whole blood, for unstable compounds including benzodiazepines, zopiclone, 6-monoacetylmorphine (6-MAM) and cocaine [12,15,16]. Additionally, DBS concentrations, unlike urine or hair, should reflect current concentrations in blood [17].
There are several published DBS applications for therapeutic drug monitoring (TDM) [18–22]. DBS also was successfully applied to doping control [23] and drugs of abuse analysis including cannabinoids [24], opiates [14,25], 3,4-methylenedioxymethamphetamine (MDMA) [26], γ-hydroxybutyric acid (GHB) [27] and cocaine [10–12,15,17,23,25,28–30].
Cocaine PK is well studied in blood, plasma and urine after various routes of administration, but less is known about cocaine disposition in DBS following controlled drug administration. Henderson et al. described the first qualitative report of drugs of abuse in DBS to determine prenatal cocaine use in mothers utilizing a modified BE urine radioimmunoassay and found results correlated well with GC–MS determinations in venous blood [15]. Since then, multiple LC–MS-based methods were published for the detection of cocaine and/or cocaine metabolites (including BE, EME or cocaethylene) in DBS [8]; however, limited studies examined DBS results with corresponding venous blood concentrations from authentic specimens [10,11,17,25,29,30].
Mercolini et al. compared DBS and plasma cocaine, BE and cocaethylene concentrations from one cocaine user utilizing LC coupled to spectrofluorimetric detection with a 20 µg/l LOQ [11]. DBS were collected via finger puncture; 10 µl blood was collected with a micropipette and dispersed onto the DBS card. The authors found that capillary DBS correlated well with plasma concentrations after an arbitrary hematocrit factor (1.62 for women and 1.92 for men) was applied. Others also reported good correlations between DBS and venous blood cocaine and metabolite concentrations from limited specimens obtained from polydrug users [30], driving under the influence of drug (DUID) cases [25] and postmortem specimens [17]. A limitation of these studies is that the authors did not investigate capillary DBS, as Mercolini et al. performed [11]; rather, they dispersed whole blood onto DBS cards utilizing authentic venous blood. More studies are needed to demonstrate the equivalence between capillary DBS and venous blood before this alternative matrix can be utilized to determine recent drug consumption.
The present study compared cocaine and BE concentrations in DBS and venous blood after controlled intravenous cocaine administration. We developed and validated separate methods for quantifying cocaine and metabolites in DBS and in venous blood by LC–HRMS and 2D GC–MS to determine if capillary DBS concentrations correlated well with blood concentrations.
Materials & methods
Chemicals & materials
Cocaine, norcocaine (1 g/l) and IS d3-cocaine and d3-norcocaine (100 mg/l) in acetonitrile, and BE (1 g/l) and d8-BE (100 mg/l) in methanol were obtained from Cerilliant (TX, USA). SPE was performed with SOLA CX 10 mg 1 ml cartridges (Thermo Scientific, CA, USA) for DBS analyses and UCT Clean Screen DAU 200 mg 10 ml cartridges (United Chemical Technologies, Inc., PA, USA) for blood analyses. N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide (MTBSTFA) + 1% t-butyl-dimethylchlorosilane (t-BDMCS) utilized for derivatization was from Regis® (IL, USA). Formic acid, methanol, acetonitrile, dichloromethane, isopropanol, ethyl acetate and water were acquired from Fisher Scientific (NJ, USA). Ammonium hydroxide 28–30%, hydrochloric acid 37%, ammonium formate, potassium phosphate and sodium phosphate were from JT Baker (NJ, USA). All solvents employed in the extraction were HPLC grade, and LC–MS grade for the chromatographic system. Water for buffer preparation was purified-in-house by an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, MA, USA). Whatman 903 Protein Saver Cards™ and desiccant were obtained from Whatman GE Healthcare Bio-Sciences Corporation (NJ, USA) and Tenderlett® lancets (incision depth: 1.75 mm, length: 0.94 mm) were acquired from ICT (NJ, USA).
Preparation of standard solutions
DBS and blood calibrator working solutions at 10, 50, 100, 500, 1000 and 2000 µg/l were prepared by appropriate dilution in acetonitrile. QC working solutions were prepared in acetonitrile at low (30 µg/l), medium (750 µg/l) and high (1500 µg/l) concentrations from different lots than those employed for calibrators. IS containing d3-cocaine, d8-BE and d3-norcocaine at 250 ng/l was prepared in acetonitrile for DBS. For blood analyses, an IS solution of 100 µg/l d3-cocaine and 250 µg/l d8-BE was prepared. A lower d3-cocaine concentration was necessary due to presence of d0-cocaine ions at higher concentrations (250 µg/l) interfering with linearity and potential increasing of LOQ.
Preparation of DBS cards
Thirty microliters of calibrator or QC working solutions were added to 0.3 ml human blood aliquots to yield final concentration range (1–200 µg/l). Fifty microliters of blank and prepared fortified blood were spotted onto 903 Whatman DBS paper cards (GE Healthcare Bio-Sciences, PA, USA), and dried at room temperature for a minimum of 3 h before extraction.
DBS analysis
For sample extraction, a 3 mm diameter disc was punched manually and placed in a 10 ml polypropylene Sarstedt tube. A 1 ml aliquot 1% formic acid in water and 30-μl IS (250 ng/l d3-cocaine, d8-BE and d3-norcocaine) was added. Tubes were capped, gently vortexed for 30 s, sonicated for 15 min and centrifuged 4000× g at 4°C for 5 min. SPE was performed onto SOLA CX cartridges preconditioned with methanol (0.5 ml) and ultrapure water (1 ml). Columns were washed with 0.5 ml 2% formic acid in ultrapure water and 0.5 ml 2% formic acid in methanol. Cartridges were dried at 10 psi for 5 min and elution performed with 0.5 ml 5% ammonium hydroxide in methanol. After drying under compressed air at 40°C, samples were reconstituted in 150 μl mobile phase A (1 mmol/l ammonium formate + 0.01% formic acid), vortexed briefly and centrifuged at 4000× g at 4°C for 5 min. Twenty microliters were injected onto the LC–HRMS system.
LC–HRMS was performed on a Thermo Scientific NCS-3500RS Ultimate 3000 Binary Rapid system coupled to a QExactive mass spectrometer (Thermo Scientific). Chromatographic separation was achieved with a Phenomenex Synergi Polar-RP 100A (100 × 2.0 mm, 2.5 μm) column and identically packed guard cartridges (10 × 2.1 mm, 2.5 μm). Gradient elution was performed with mobile phase A and B (methanol) at 0.3 ml/min flow rate and 30°C. The initial composition (5% B) was maintained for 1.5 min, increased from 5 to 95% B over 5.5 min, held at 95% for 1.5 min and returned to initial conditions over 0.5 min. A 1.5 min equilibration followed yielding a total run time of 10.5 min.
The QExactive mass spectrometer was equipped with heated electrospray ionization source (HESI-II) and operated in positive ionization mode. The spray voltage was 3 kV, capillary temperature 350°C, heater temperature 425°C, S-lens RF level 50, sheath gas flow rate 50, auxiliary gas flow rate 13 and sweep gas 3 (manufacturer's units). The instrument was calibrated in the positive and negative mode every 25 h. The mass spectrometer acquired a targeted–MSMS scan at a resolution of 35,000 (full width at half maximum at m/z 200), automatic gain control (AGC) target 5 × 105 and maximum injection time 100 ms. Precursor ions are selected in the quadrupole with a 3 m/z window and subsequently fragmented in the HCD cell. A full scan of all fragmented ions originating from the precursor ion was performed, and two specific product ions utilized for data analysis with a mass tolerance of 5 ppm (Table 1). Thermo TraceFinder Clinical Research 3.1 software was used for data collection and processing.
Table 1. . LC–HRMS parameters and retention times for cocaine, metabolites and IS in DBS.
| Analyte | Precursor ion (m/z) | Quantitative product ion (m/z) | Qualitative product ion (m/z) | NCE (%) | RT (min) |
|---|---|---|---|---|---|
| BE |
290.1 |
168.1017 |
105.0338 |
50 |
6.42 |
| d8-BE |
298.2 |
171.1205 |
110.0651 |
50 |
6.39 |
| Cocaine |
304.1 |
182.1174 |
82.0656 |
50 |
7.18 |
| d3-cocaine |
307.2 |
185.1363 |
85.0844 |
50 |
7.17 |
| Norcocaine |
290.1 |
136.0756 |
168.1017 |
45 |
7.33 |
| d3-norcocaine | 293.1 | 171.1205 | 136.0756 | 20 | 7.30 |
BE: Benzoylecgonine; NCE: Normalized collision energy; RT: Retention time.
Blood analysis
Blank blood (0.25 ml) was fortified with 25 µl IS (100 µg/l d3-cocaine, 250 µg/l d8-BE) and their respective calibrator and QC concentrations yielding a final concentration range between 1 and 200 µg/l. Four milliliters of phosphate buffer pH 6 (prepared with KH2PO4 and Na2HPO4) was added and Sarstedt polypropylene tubes were vortexed and centrifuged at 4000× g, 4°C for 10 min. Supernatants were loaded onto UCT Clean Screen cartridges preconditioned with methanol (3 ml) and phosphate buffer pH 6 (3 ml). Columns were washed with water (6 ml), 0.1 mol/l HCl (3 ml) and methanol (3 ml) before drying under vacuum at 10 psi for 20 min. Analytes were eluted with 3 ml dichloromethane:isopropanol (80:20 v/v) with 2% ammonium hydroxide into conical glass centrifuge tubes. Eluates were evaporated to dryness under nitrogen at 40°C and reconstituted with 20 µl ethyl acetate:MTBSTFA + 1% t-BDMS (50:50 v/v). Samples were incubated for 40 min at 70°C and centrifuged at 1800× g, 20°C for 3 min before transferring to autosampler vials. The derivatized extracts (2 µl) were analyzed by an electron ionization 2D GC–MS method with modifications to the front inlet, back inlet and oven temperature programs [31].
Splitless injections (2 µl) were made onto a GC–MS system configured with a Deans switch, flame ionization detector (FID), 7683 autosampler and 6890N GC interfaced to a 5973 mass selective detector (MSD) (Agilent Technologies, DE, USA). The system also was equipped with a cryogenic focusing trap, mounted inside the GC oven (Joint Analytical Systems, NJ, USA) at the head of the second GC column and cooled with compressed air. The Deans switch connected two capillary chromatographic columns with a pneumatic valve directing output of the primary column (DBS-1MS, 15 m × 0.25 mm, 0.25 µm; Agilent Technologies) to either the FID or the inlet of the secondary column (ZB-50, 30 m × 0.32 mm, 0.25 µm; Phenomenex, CA, USA). The inlet end of the secondary column was inserted through the cryogenic trap and the outlet directed to the MSD. Operating parameters are outlined in Supplementary Table 1.
Cocaine and BE were initially separated on the primary column, with analyte elution times on the primary column determined by injection of high concentration standards with the Deans switch regulator directing effluent to the FID at the beginning of the analytical sequence. For authentic specimens, the cryogenic trap was maintained at 100°C to contain cocaine and BE based on elution times from the primary column. Immediately, after the last analyte was cold-trapped, the oven temperature was lowered to 180°C, the cryogenic trap ramped at 800°C/min and analytes revaporized for separation on the secondary column. The total run time was 18.63 min. The MSD was operated in electron impact (EI) -selected ion monitoring mode for cocaine and BE. A minimum of three ions for each analyte and IS were acquired. Target and qualifier ions are presented in Table 2.
Table 2. . GC mass selective detector parameters for cocaine, benzoylecgonine and their respective deuterated analogs in human venous blood.
| Analyte | Target ion | Qualifier ions | Dwell time (ms) | Deans switch cuts (min) |
|---|---|---|---|---|
| Cocaine |
82 |
94, 182, 303 |
30 |
3.70–4.10 |
| d3-cocaine |
85 |
185, 306 |
10 |
3.70–4.10 |
| Benzoylecgonine |
282 |
346, 403 |
30 |
4.64–5.04 |
| d8-benzoylecgonine | 285 | 354, 411 | 15 | 4.64–5.04 |
Participants
Eligibility criteria included healthy adults aged 18–50 years who smoked or used intravenous cocaine for at least 6 months and at least three-times per month during the 3 months prior to screening and who were currently healthy, based on a comprehensive medical and psychological evaluation. Exclusion criteria included pregnant or nursing women; current physical dependence on any drug other than cocaine, caffeine or nicotine; current clinically significant medical or psychiatric disorder; hemoglobin less than 12.5 g/dl and blood donation within 8 weeks; current hypertension or blood pressure readings consistently above 140 mm Hg systolic or 90 mm Hg diastolic while at rest; heart rate consistently above 90 or below 50 bpm while at rest; abnormal 12-lead electrocardiogram (ECG); history of clinically significant adverse reaction to cocaine, acetazolamide or quinine; or interest in drug abuse treatment within 3 months of study screening. Participants provided written informed consent to participate in this NIDA Institutional Review Board and the US FDA-approved study. Participants resided on a secure research unit for 13 days and 12 nights. The study's primary aims were to evaluate potential PD and PK interactions between cocaine and acetazolamide and quinine. Acetazolamide and quinine are being considered as compliance markers for cocaine dependence treatment pharmacotherapies.
Authentic specimens
Participants were administered a single 25 mg intravenous (iv.) cocaine dose through a peripheral venous catheter on three separate days during the study (days 1, 5 and 10). Cocaine was administered alone on day 1, with oral acetazolamide on day 5 and with oral quinine on day 10. Venous blood and DBS were collected before and 30, 60 and 90 min after intravenous cocaine administration. Venous blood was collected in gray-top tubes. For DBS, capillary blood was collected by finger puncture onto a Whatman Protein Saver Card 903, with five spots per card. Collection cards were dried for a minimum of 3 h at room temperature and stored in a dry plastic bag with desiccants at -20°C until analysis. Blood specimens also were stored at -20°C until analysis. Only central punches from the DBS and specimens greater than 3 mm (punch diameter disc) were included in the comparison between capillary DBS and venous blood.
Statistical analyses
Visual inspection of data and evaluation by D'Agostino-Pearson normality test (omnibus K2) indicated non-normal data distribution. Therefore, statistical comparisons between blood and DBS concentrations were conducted with nonparametric tests. Correlations of cocaine and BE DBS to blood concentrations were performed with Spearman's rho correlation (r s) and least-squares regression analysis in Prism Version 5.02 (GraphPad Software Inc, CA, USA). In addition, Bland-Altman analysis plots were conducted to further analyze these data to examine difference between two measurements as a function of the mean of the two measurements for each individual sample, resulting in a mean difference and standard deviation that is utilized to calculate 95% limits of agreement [32], which can be interpreted for clinical significance.
Results
Participants
Thirteen subjects (12 males, 1 female, 8 black, 4 white, 1 mixed race) aged 35–50 (Supplementary Table 2) participated in the study, with nine completing all dosing sessions. Two participants were medically discharged prior to the second cocaine dose on day 5, one after the second cocaine dose on day 5 and one prior to the third cocaine dose (day 10). These participants were medically discharged for change in postdosing ECG compared with predosing ECG, abnormal predosing ECG or elevated heart rate and/or blood pressure prior to cocaine administration. Frequency of use among participants (smoked or intravenous) ranged from daily to once per week, with a median length of cocaine use of 16.6 years (range: 6–32 years) (Supplementary Table 2).
DBS method validation results
DBS method was validated following Scientific Working Group for Forensic Toxicology (SWGTOX) recommendations [33]. Linearity of peak area ratios versus theoretical concentrations was verified from 1 to 200 µg/l with 1/x2 weighted linear regression (Table 3). Five calibration curves yielded determination coefficients (R2) above 0.9930 ± 0.0027, with residuals within ±15%. LODs were 0.5, 1 and 1 µg/l for BE, cocaine and norcocaine, respectively, with a 1 µg/l LOQ for all analytes. Supplementary Figure 1A illustrates a chromatogram of a DBS sample at LOQ. Bias and imprecision were evaluated in triplicate over 5 days (n = 15) at each QC concentration with a One-way Analysis of Variation (ANOVA) approach to calculate combined within- and between-run imprecision. Within-run imprecision (<7.1%), between-run imprecision (<10.47%) and % bias (-4.6–7.4%) were satisfactory at all three QC concentrations (Table 3). Extraction efficiency for each analyte was measured at each QC concentration. Blank venous blood samples (n = 5) were fortified with QC solution before spotting on DBS cards and extracting. These were compared with blank venous blood samples (n = 5) spotted on DBS cards that were extracted and fortified with corresponding QC solution after sample extraction. Extraction efficiencies were 43.3–52.1% for cocaine, 48.6–64.5% for BE and 35.2–48.6% for norcocaine, with process efficiencies of 47.4–49.1, 29.3–33.0 and 28.0–29.4%, respectively. Ion suppression (n = 10) was less than 43.9, 39.5 and 23.9% for cocaine, norcocaine and BE, respectively (%CV < 24.2%). When corrected for IS, observed matrix effects were -13.8–16.9, -15.1–10.0 and -11.9–14.3% for cocaine, BE and norcocaine, respectively (%CV < 20.5%). Interferences from endogenous DBS compounds were not observed (n = 10). Method selectivity was demonstrated by adding high concentrations (500 µg/l) of potentially interfering drugs and metabolites (Supplementary Table 3) to negative samples. No analyte was detected greater than LOD, indicating no interferences with analytes of interest. No carryover was detected after the injection of a sample at 1000 μg/l (five-times ULOQ). Analytes were stable in the autosampler for 48 h at low and high QC concentrations with percent differences between -7.4 and -0.1% (n = 3). Short-term stability was evaluated at room temperature for 24 h, at 4 and -20°C for 72 h and after three freeze–thaw cycles. Analytes were considered stable under evaluated conditions (% difference < 12.4%). Stability data are shown in Table 4.
Table 3. . Within-run imprecision, between-run imprecision and %bias for cocaine, benzoylecgonine and norcocaine in DBS and cocaine and benzoylecgonine in blood at low (3 µg/l), medium (75 µg/l) and high (150 µg/l) concentrations.
| Matrix | Analyte |
Within-run imprecision (n = 15, %CV) |
Between-run imprecision (n = 15, %CV) |
%bias (n = 15) |
Extraction efficiency (n = 5, % [%CV]) |
Process efficiency (n = 5, %) |
Matrix effect (n = 10, % [%CV]) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Med | High | Low | Med | High | Low | Med | High | Low | High | Low | High | Low | High | ||
| DBS | Cocaine | 7.0 | 5.2 | 4.1 | 8.9 | 6.5 | 5.7 | –2.5 | 7.4 | 4.3 | 43.3 (9.0) | 52.1 (18.8) | 47.4 | 49.1 | –23.7 (10.0) | –43.9 (24.2) |
| BE | 7.1 | 4.9 | 3.1 | 7.5 | 5.5 | 5.7 | –4.6 | –1.6 | –2.9 | 48.6 (1.7) | 64.5 (10.0) | 33.0 | 29.3 | –2.4 (7.0) | –23.9 (20.9) | |
| |
Norcocaine |
5.3 |
5.2 |
4.3 |
10.7 |
8.0 |
7.2 |
–2.6 |
4.4 |
2.3 |
35.2 (5.1) |
48.6 (14.1) |
28.0 |
29.4 |
–20.4 (9.6) |
–39.5 (22.8) |
| Blood | Cocaine | 3.3 | 4.6 | 1.6 | 3.4 | 4.2 | 2.5 | 6.7 | –7.8 | 2.6 | 105.8 (12.6) | 98.6 (6.8) | – | – | – | – |
| BE | 1.9 | 3.9 | 1.8 | 2.8 | 3.9 | 2.0 | 2.3 | –3.2 | 9.1 | 87.6 (10.0) | 87.3 (6.9) | – | – | – | – | |
Extraction efficiency, process efficiency and matrix effects for analytes at low (3 µg/l) and high (150 µg/l) concentrations.
BE: Benzoylecgonine.
Table 4. . Short-term stability data (%difference) of cocaine, benzoylecgonine and norcocaine in DBS and cocaine and benzoylecgonine in blood at low (3 µg/l) and high (150 µg/l) concentrations after storage at 4°C for 48 h in autosampler (DBS), room temperature for 72 h in autosampler (blood), at room temperature for 24 h, at 4 and -20°C for 72 h, and after three freeze–thaw cycles (over 72 h).
| Matrix | Analyte |
48 h 4°C/72 RT autosampler (n = 3) |
RT 24 h (n = 3) |
4°C 72 h (n = 3) |
-20°C 72 h (n = 3) |
Three freeze–thaw cycles (n = 3) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | ||
| DBS | Cocaine | –3.4 | –4.3 | –5.4 | –9.3 | –7.6 | –8.9 | –1.5 | –6.7 | –5.9 | –6.0 |
| BE | –0.1 | –7.1 | –0.9 | –6.5 | –5.3 | –8.0 | –5.3 | –4.0 | –1.1 | –3.2 | |
| |
Norcocaine |
–2.8 |
–0.2 |
–5.3 |
–12.4 |
–7.6 |
–6.7 |
–5.0 |
–6.2 |
–6.2 |
–4.5 |
| Blood | Cocaine | –1.1 | –8.4 | –2.0 | –3.8 | –25.0 | –23.4 | –10.9 | –13.2 | –8.9 | –13.2 |
| BE | –3.3 | –11.0 | 1.3 | –1.3 | –6.4 | –2.9 | 1.8 | –1.4 | 3.2 | –1.6 | |
BE: Benzoylecgonine; RT: Room temperature.
Blood method validation results
Linearity from 1 to 200 µg/l with 1/x2 weighting was achieved for cocaine and BE in whole blood. Calibration curves (n = 5) yielded determination coefficients (R2) above 0.9937 ± 0.0029 with residuals less than 15%. LODs were 1 and 0.5 µg/l for cocaine and BE, respectively, with 1 µg/l LOQs (Supplementary Figure 1B). Within-run imprecision (<4.6%), between-run imprecision (<4.2%) and % bias (-7.8–9.1%) were acceptable for both analytes, at all three QC concentrations. Extraction efficiencies for cocaine were 98.6–106% and BE 87.3–87.6%. Table 3 highlights these data. Dilution integrity was acceptable (±20%) at 1:2, 1:5 and 1:10 dilutions. Carryover was not detected for either analyte above their respective LODs after injection of blank specimens fortified five-times the ULOQ (1000 µg/l). No exogenous interferences were observed from over 75 different compounds (500 µg/l) with the exception of norcocaine (Supplementary Table 3); however, norcocaine concentrations are generally less than 30 µg/l in clinical samples [34–36]. At the upper limit of linearity (200 µg/l), norcocaine did not produce an increase in cocaine concentrations at the low QC concentration (3 µg/l) [34–36]. Compounds were stable (within ±25% of freshly prepared QCs) on the autosampler (room temperature) for 72 h, for 24 h at room temperature, 72 h at -20°C and after three freeze–thaw cycles (Table 4).
Authentic specimens
In total, 392 DBS (up to five spots per collection) and 97 venous blood specimens were analyzed after 25 mg/kg controlled intravenous cocaine administration on days 1, 5 and 10. DBS and venous blood were collected 30, 60 and 90 min postcocaine dosing. Twenty-seven cocaine DBS and 19 BE DBS concentrations quantified above the ULOQ, and one cocaine DBS concentration was below the LOQ. All blood concentrations were within the method's limits of linearity. Norcocaine was not detected in any DBS specimens greater than the LOQ. Figure 1 illustrates chromatograms of cocaine and BE concentrations in DBS and matched blood samples from one participant 60 min after cocaine administration.
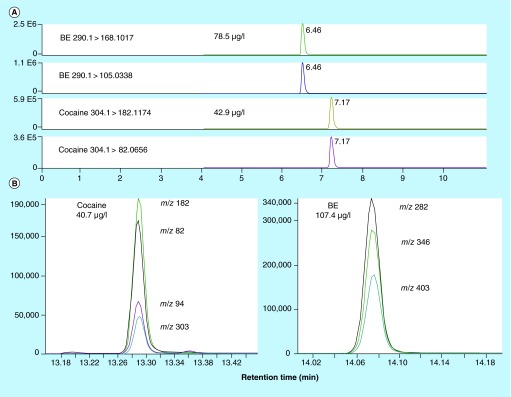
Figure 1. . Cocaine and benzoylecgonine in DBS and matched blood samples from participant L, 60 min after 25 mg intravenous cocaine.
(A) LC–MS/MS chromatogram of DBS specimen with 42.9 µg/l cocaine and 78.5 µg/l BE. Norcocaine was not detected. (B) Corresponding venous blood 2D GC–MS chromatogram with 40.7 µg/l cocaine and 107.4 µg/l BE.
BE: Benzoylecgonine.
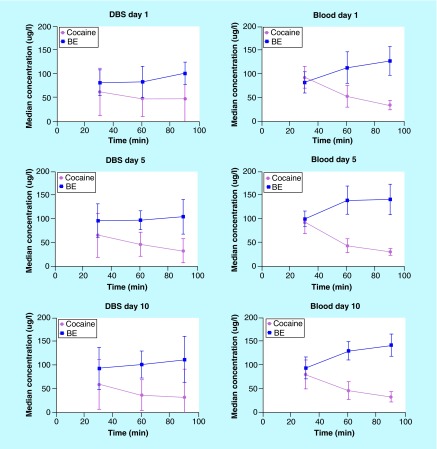
Table 5 summarizes cocaine and BE concentrations in DBS and venous blood on days 1, 5 and 10 at 30, 60 and 90 min postdosing. Cocaine DBS and venous blood concentrations ranged between 2.9–232.6 and 18.3–137.6 µg/l, respectively, with BE concentrations of 2.9–240.4 µg/l for DBS and 57.2–193.4 µg/l for blood. In general, median cocaine DBS concentrations were lower than their respective blood concentrations (Table 5); although DBS concentration ranges were wider. The same trends also were observed for BE (Table 5). Supplementary Figure 2 illustrates the above-mentioned results graphically as box and whisker plots. In addition, large intersubject-variability was observed with DBS collections at each time point (up to five spots per collection) as compared with blood specimens; %CV for cocaine ranged between 50.7–105.9% (DBS) and 22.5–43.0% (blood), with BE %CV from 21.4–50.8% (DBS) and 14.9–27.3% (blood). Similarly, DBS %CVs indicate high intra-DBS variability among the five spots per card, with medians (range) of 22.3% (4.9–122%) for cocaine and 9.8% (0.1–143%) for BE. Figure 2 illustrates median cocaine and BE concentrations in authentic DBS and venous blood samples following controlled intravenous cocaine administrations on days 1, 5 and 10, 30, 60 and 90 min postdosing, although capillary kinetics may be different from venous blood kinetics.
Table 5. . Cocaine and benzoylecgonine DBS and venous blood concentrations and SD in simultaneously collected specimens (n = 392 DBS, n = 97 blood) from 13 participants, 30, 60 and 90 min following 25 mg/kg intravenous cocaine administration on three separate days (days 1, 5 and 10).
| Day | Time (min) | Mean (µg/l) | Median (µg/l) | SD (µg/l) | Inter-subject %CV | Median intra-DBS %CV (range) | Minimum (µg/l) | Maximum (µg/l) | Samples (n) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine DBS | 1 | 30 | 81.4 | 62.3 | 50.0 | 61.5 | 20.0 (5.3–40.3) | 19.0 | 232.6 | 47 |
| 60 | 59.7 | 48.5 | 38.5 | 64.5 | 20.7 (7.5–80.4) | 17.4 | 191.6 | 39 | ||
| 90 | 72.1 | 47.9 | 53.8 | 74.6 | 29.7 (6.2–102) | 17.6 | 219.2 | 51 | ||
| 5 | 30 | 77.3 | 65.7 | 46.1 | 59.6 | 16.0 (4.9–54.3) | 37.5 | 231.0 | 42 | |
| 60 | 50.1 | 46.4 | 25.4 | 50.7 | 24.0 (9.2–65.6) | 19.5 | 139.4 | 43 | ||
| 90 | 40.2 | 32.2 | 25.8 | 64.2 | 23.4 (7.2–122) | 4.4 | 113.2 | 42 | ||
| 10 | 30 | 68.2 | 59.4 | 52.7 | 77.2 | 15.4 (6.1–120) | 2.9 | 232.4 | 34 | |
| 60 | 49.0 | 36.6 | 32.1 | 35.7 | 18.1 (7.3–62.3) | 15.1 | 145.3 | 35 | ||
| |
|
90 |
56.7 |
31.7 |
60.1 |
105.9 |
25.2 (12.1–46.2) |
3.8 |
215.7 |
31 |
| Cocaine blood | 1 | 30 | 90.3 | 92.6 | 23.6 | 26.1 | – | 56.0 | 130.0 | 13 |
| 60 | 53.9 | 53.0 | 23.1 | 43.0 | – | 30.2 | 115.3 | 13 | ||
| 90 | 34.2 | 34.4 | 9.6 | 28.0 | – | 18.6 | 49.6 | 12 | ||
| 5 | 30 | 91.6 | 93.2 | 23.6 | 25.8 | – | 57.4 | 121.9 | 10 | |
| 60 | 48.2 | 43.3 | 14.5 | 30.1 | – | 25.3 | 72.8 | 11 | ||
| 90 | 32.2 | 30.8 | 7.2 | 22.5 | – | 18.3 | 42.0 | 11 | ||
| 10 | 30 | 87.6 | 79.8 | 30.7 | 35.1 | – | 52.0 | 137.6 | 9 | |
| 60 | 50.8 | 46.0 | 18.6 | 36.6 | – | 30.0 | 84.9 | 9 | ||
| |
|
90 |
34.5 |
32.8 |
10.8 |
31.3 |
– |
22.5 |
48.1 |
9 |
| BE DBS | 1 | 30 | 83.1 | 81.7 | 27.1 | 32.6 | 7.7 (2.8–26.8) | 40.7 | 159.4 | 48 |
| 60 | 89.4 | 83.4 | 33.0 | 36.9 | 10.0 (4.0–39.0) | 50.1 | 240.4 | 42 | ||
| 90 | 102.5 | 101.5 | 23.8 | 23.3 | 7.2 (1.6–20.5) | 60.7 | 166.4 | 51 | ||
| 5 | 30 | 101.7 | 96.1 | 35.3 | 34.7 | 13.6 (0.1–36.5) | 51.2 | 199.7 | 45 | |
| 60 | 93.8 | 97.5 | 20.1 | 21.4 | 13.6 (5.3–32.9) | 52.1 | 133.2 | 43 | ||
| 90 | 98.7 | 104.5 | 36.3 | 36.8 | 10.8 (2.9–143) | 1.1 | 197.6 | 44 | ||
| 10 | 30 | 88.9 | 92.6 | 45.1 | 50.8 | 7.6 (4.7–119) | 2.9 | 205.5 | 34 | |
| 60 | 103.1 | 101.2 | 28.6 | 27.7 | 13.1 (4.7–35.3) | 51.1 | 175.5 | 35 | ||
| |
|
90 |
114.2 |
111.7 |
48.3 |
42.3 |
11.1 (7.1–27.9) |
17.6 |
224.0 |
31 |
| BE blood | 1 | 30 | 86.6 | 82.3 | 22.6 | 26.1 | – | 57.2 | 132.6 | 13 |
| 60 | 121.7 | 113.4 | 33.2 | 27.3 | – | 70.1 | 186.1 | 13 | ||
| 90 | 133.5 | 127.5 | 30.1 | 22.6 | – | 77.9 | 180.5 | 12 | ||
| 5 | 30 | 96.2 | 100.2 | 16.1 | 16.7 | – | 58.7 | 115.6 | 10 | |
| 60 | 132.9 | 139.7 | 29.7 | 22.4 | – | 76.4 | 178.2 | 11 | ||
| 90 | 136.3 | 141.2 | 32.6 | 23.9 | – | 85.0 | 189.3 | 11 | ||
| 10 | 30 | 94.4 | 94.0 | 23.1 | 24.5 | – | 60.3 | 126.9 | 9 | |
| 60 | 132.8 | 129.9 | 19.8 | 14.9 | – | 92.3 | 166.0 | 9 | ||
| 90 | 141.2 | 142.0 | 24.0 | 17.0 | – | 111.9 | 193.4 | 9 |
This table does not include 27 cocaine and 19 BE DBS that quantified above the ULOQ (>200 µg/l) and 1 cocaine DBS detected below the LOQ.
BE: Benzoylecgonine.
Figure 2. . Median cocaine and benzoylecgonine concentrations in DBS and venous blood from 13 participants 30, 60 and 90 min following 25 mg/kg intravenous cocaine administrations on three separate days (days 1, 5 and 10).
Error bars represent one standard deviation constrained to 0.
BE: Benzoylecgonine.
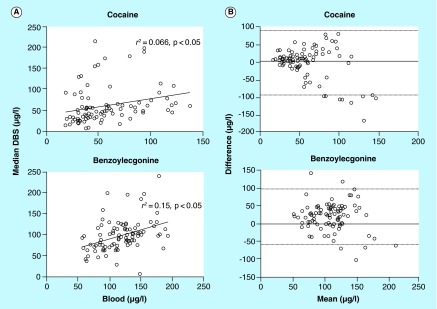
Individual cocaine and BE venous blood concentrations were compared with median DBS concentrations, as up to five DBS were collected at each sampling time, versus a single blood sample. There were 87 and 90 whole blood-DBS pairs for cocaine and BE, respectively, to investigate agreement between the two matrices. Due to the high intra-DBS-variability, DBS median concentrations (for up to five spots) were employed for comparison of DBS and venous blood. Cocaine and BE blood and DBS concentrations significantly moderately correlated (Spearman's r s 0.43 and 0.40, respectively; p < 0.0001). Least-squares regression analyses also demonstrated a significant correlation between blood and DBS concentrations (p < 0.05) with r 2 = 0.066 and 0.15 for cocaine and BE, respectively (Figure 3A); equations of the fitted lines were: y = 0.3882x + 40.04 for cocaine and y = 0.4314x + 47.24. Bland-Altman analysis plots yielded mean differences between blood and DBS cocaine concentrations of -5.10 ± 46.7 and 20.2 ± 39.4 µg/l for BE, with 95% limits of agreement between -96.6–86.4 and -56.9–97.3 µg/l, respectively (Figure 3B).
Figure 3. . Comparison between venous blood and DBS cocaine and benzoylecgonine concentrations in simultaneously collected specimens from 13 participants 30, 60 and 90 min following 25 mg/kg intravenous cocaine administration on three separate days (days 1, 5 and 10).
Only paired venous blood-DBS specimens within the linear range were included in correlations and Bland-Altman analysis plots: cocaine (n = 87) and benzoylecgonine (BE) (n = 90). (A) Correlation of venous blood with median DBS cocaine and BE concentrations. Regression was calculated by least-squares regression analysis. (B) Bland-Altman analysis plots comparing blood and DBS collection methods for cocaine and BE detection. Dotted lines represent the 95% limits of agreement (mean difference ± 2SD).
Discussion
The use of DBS as an alternative matrix in clinical and forensic toxicology is increasing as specimens can be collected easily in a less invasive manner (via finger puncture), with little potential for adulteration, increased stability over other matrices including blood, and have the potential to identify recent drug consumption. Here, we describe cocaine and BE concentrations in DBS and simultaneously collected venous blood specimens following controlled intravenous cocaine administration, allowing evaluation of agreement between capillary DBS and blood concentrations. Previous studies did not investigate capillary DBS (obtained via finger puncture) compared with blood specimens collected simultaneously under controlled cocaine administration.
Fully validated analytical methods for the detection of cocaine, BE and norcocaine in DBS (LC–HRMS) and cocaine and BE in whole blood (2D GC–MS) were developed to determine if capillary DBS correlate well with simultaneously collected blood specimens. A different analytical approach was utilized for the detection of DBS cocaine and metabolites than the technique implemented for venous blood to achieve the required low LOQs. Previous studies investigated cocaine and metabolites in DBS using LC–based methods [10–12,15,17,23,25,28–30], with reported LOQs ranging from 0.25 to 50 µg/l. In the present method, cocaine, BE and norcocaine quantification in DBS was achieved with low LOQs (1 µg/l) determined empirically, with lower analyte concentrations required to meet identification and quantification criteria. One previous study quantified cocaine with a lower LOQ (0.25 µg/l); however, this was determined empirically by calculating five-times LOD [23]. Norcocaine was not previously analyzed in other studies. Sensitive LOQs of 1 µg/l also were achieved for cocaine and BE in blood utilizing a 2D GC–MS method, similarly to previous publications [37–40]. Norcocaine was not quantified in venous blood in the current study as it chromatographed poorly and did not achieve clinically relevant sensitivity.
DBS sample preparation for this method required two steps: first, extraction of analytes from DBS paper (3 mm punched discs) with 1% formic acid in water, followed by SPE. Initially, a simple one-step solvent extraction procedure, as described in the literature [11,41,42], was attempted utilizing different solvents including methanol, methanol + 0.1% formic acid, acetonitrile, acetonitrile + 0.1% formic acid, water + 0.1% formic acid. Significant ion suppression was observed for all investigated solvents; therefore, a SPE procedure was employed to reduce matrix effects. Although we employed this additional step, ion suppression ranged from 2.4 to 43.9% (%CV < 24.2). Matched deuterated IS were included to help compensate for observed DBS matrix effects (IS-corrected matrix effects ranged from -15.1 to 16.9%). Despite low extraction efficiencies (35.2–64.2%) and ion suppression (2.4–43.9%), a sensitive LOQ of 1 µg/l was achieved for all DBS analytes.
All analytes were stable in DBS under all tested conditions: room temperature for 24 h, at 4 and -20°C for 72 h and after three freeze–thaw cycles. These results demonstrated that DBS for measurement of cocaine and metabolites could be collected, shipped and stored at ambient temperature, avoiding expensive refrigeration costs. Cocaine and BE also were stable in venous blood under all tested conditions (<-13.2%), except for cocaine at 4°C for 72 h that demonstrated greater instability (25.0% loss). In authentic specimens, the loss observed in fortified stability samples may be different due to the presence of anticoagulants and preservatives in the gray-top blood collection tubes. It is possible that esterase activity would continue in authentic DBS specimens (which do not contain sodium fluoride), reducing cocaine concentrations; however, when tested, we did not observe a difference between fortified DBS specimens allowed to dry the minimum 3 h (normal procedure) compared with specimens analyzed immediately following fortification with no drying time (n = 5 at each QC concentration). Stability of DBS compared with blood was previously reported for cocaine [12] and other substances, such as phosphatidylethanol [13], zopiclone [16] and benzodiazepines. In this study, blood and DBS were stored at -20°C until analysis to minimize analyte degradation.
One DBS method limitation was the limited amount of blood volume spotted onto cards and the small 3 mm punch from the DBS. The entire DBS eluent was evaporated to dryness to concentrate analytes and improve sensitivity. DBS were not reanalyzed if concentrations were greater than the ULOQ (200 µg/l). It is possible to dilute the extract with mobile phase, so that concentrations initially greater than the ULOQ fall within the linear range if the entire extract is not injected. Cocaine concentrations in 6.9% of DBS samples were greater than the ULOQ, while 4.8% of BE concentrations were greater than the ULOQ (n = 392). High assay sensitivity was needed to quantify analytes in the small sample and obtain low LOQ. Increased sensitivity also was achieved by utilizing the QExactive mass spectrometer that offers high resolution and high mass accuracy, increasing specificity and sensitivity by improving the signal-to-noise ratio.
Another issue that was encountered was increased blood cocaine concentrations when norcocaine was present at high concentrations (500 µg/l); however, no interference was observed at the upper limit of linearity (200 µg/l). Norcocaine concentrations are generally much lower than 200 µg/l in blood, and certainly after 25 mg iv. cocaine. A study examining cocaine and metabolite concentrations from patients admitted to the emergency department reported mean norcocaine concentration after intravenous cocaine administration of 30 ± 100 µg/l (n = 3) [35]; however, dosing information was unknown. Previously, after subcutaneous cocaine administration (121.3–228.2 mg) mean norcocaine concentrations were only 7.5 ± 2.0 µg/l (n = 7) [34].
Previous studies applied analytical DBS methods for the detection of cocaine and metabolites in authentic specimens including newborn screening [10,15,29], cocaine and polydrug users [11,30], DUID cases [25] and postmortem cases [17]; however, this study is the first to investigate cocaine and BE in capillary DBS compared with simultaneously collected blood specimens following controlled cocaine administration. Several studies reported good agreement between blood dispersed onto DBS cards utilizing authentic venous specimens [17,25,30], but these studies were primarily conducted to determine if DBS could be utilized for an alternative sample preparation procedure (reduce sample volume and sample preparation). Thus, capillary DBS obtained via finger puncture were not evaluated against venous blood concentrations. Mercolini et al. detected cocaine, BE and cocaethylene in capillary DBS and corresponding plasma specimens. They found DBS concentrations were in good agreement with plasma concentrations once hematocrit variability was considered [11]. They applied an arbitrary hematocrit correction factor (1.62 for women and 1.92 for men) to plasma concentrations; although results were only documented for one case and one time point involving a cocaine user.
Both cocaine and BE were detected in all DBS and blood specimens collected 30, 60 and 90 min after controlled intravenous cocaine administration. Norcocaine was not detected in DBS and concentrations in venous blood were undetermined as this analyte was not included in the assay. In general, median cocaine and BE DBS concentrations were slightly lower than their respective blood concentrations. This study also found that cocaine DBS concentration ranges were wider than blood concentrations ranging between 2.9–232.6 and 18.3–137.6 µg/l, and BE concentrations between 2.9–240.4 µg/l for DBS and 57.2–193.4 µg/l for blood, respectively. In addition, large intra-DBS- and inter-subject-variability was observed in cocaine and BE DBS concentrations as compared with their corresponding blood concentrations (Table 5). It is unlikely that observed discrepancies between the capillary DBS and venous blood cocaine and BE concentrations are a result of utilizing different analytical platforms, as both methodologies were successfully validated. Extraction efficiencies could contribute to observed differences. Additionally, it is not recommended to add more than 5% organic solvent to blood when preparing DBS, as it could affect the spreading of the spot and subsequent concentrations detected. Although 10% acetonitrile was added in this study during fortification, authentic DBS specimens quantified lower than calibrators, hence it does not appear to be a contributor to the concentration differences observed in this case. Variability in DBS concentrations also could be attributed to several factors, including volume of blood spotted onto the card, the hematocrit of the blood and the homogeneity of the spot [8,43]. Blood hematocrit levels can substantially affect DBS concentrations, as blood with higher hematocrit will contain less water per given volume of blood (or punch diameter), and therefore possibly increase analyte concentration, compared with blood with lower hematocrit [43,44]. Nonhomogeneity of the blood spots also could contribute to the variability of DBS concentrations observed in this study, as several studies reported variation in analyte concentration across the spot, especially around the edges [45,46]. Extracting the entire blood spot and not just a fixed diameter can largely avoid hematocrit bias and nonhomogeneity of blood spots [8,43]; however, often the blood volumes spotted onto the cards in this study were inconsistent.
It is unlikely that hematocrit played a significant role in the observed discrepancy between DBS and venous blood concentrations. Typically, this is only a major issue in cases with strongly deviating hematocrit. Participants in this study were considered to have normal hematocrit values (38.5–50.0% men, 34.9–44.5% women), with hemoglobin levels greater than 12.5 g/dl. Participants had hematocrit values between 38.2 and 46.0% at the time of screening for the study, within 9 weeks of cocaine administration. Additionally, blank blood obtained from the NIH blood bank for calibrators and QC samples had hematocrit values of at least 37.5%, similar to the population studied. To investigate if variable blood spot volumes or sample homogeneity contributed to intra-DBS variability, different volumes (50, 40, 30, 20 and 10 µl) of fortified venous blood were spotted onto DBS cards at low and high QC concentrations (n = 3) and analyzed. In addition, peripheral punches also were obtained from the 50 µl specimens to assess sample homogeneity. No differences were observed between central and peripheral punches suggesting that sample homogeneity was unlikely a contributor to the observed DBS variability; however, small blood spot volumes (10 µl) demonstrated up to -20.9% decrease in concentration compared with 50 µl spots. This confirms our hypothesis that blood spot volumes contribute to the observed DBS variability.
In addition, to investigate if the DBS variability was due to the DBS approach itself, venous blood was fortified (n = 5) at each of three QC concentrations, spotted onto the DBS cards and analyzed according to the previously described procedures. The venous blood also was analyzed. There was a significantly strong correlation between venous DBS (vDBS) and venous blood for cocaine (rs = 0.93, R2 = 0.9953) and BE (rs = 0.89, R2 = 0.9885) concentrations. This suggests that the DBS approach per se was not the primary source of the observed variability; however, authentic vDBS may behave differently than fortified vDBS and should be investigated in future studies.
When DBS were statistically compared with corresponding blood concentrations, Spearman's correlation indicated that the two methods were significantly moderately correlated (r s = 0.43 cocaine, r s = 0.40 BE) to a degree unlikely due to random sampling error; however, linear regression analysis yielded r 2 of only 0.066 and 0.15 for cocaine and BE, indicating that one cannot accurately predict venous blood concentrations from those of DBS. Additionally, Bland-Altman analysis plots were conducted to further determine, if the difference between the two collections methods were clinically acceptable, as Garcia Boy et al. did when evaluating venous DBS and blood for morphine [14]. We showed a mean difference between blood and DBS of -5.1 ± 46.7 µg/l for cocaine, with a 95% CI spanning 183 µg/l in total. Although the mean difference between blood and DBS was close to zero, suggesting the two methods are similar, the wide CI limits clinical usefulness for comparing analyte concentrations from two different collection methods. Additionally, the plot indicated inconsistent comparability between the two methods, in other words, scatter increased as the mean concentrations of blood and median DBS increased. The comparison for BE concentrations showed somewhat greater mean difference than for cocaine (20.2 ± 39.4 µg/l) and a wide 95% CI for BE (154.2 µg/l), especially in comparison to the linear range evaluated (1–200 µg/l). These statistical data suggest poor agreement between blood and DBS cocaine and BE concentrations.
The discrepancy between capillary DBS and venous blood cocaine and BE concentrations is primarily due to concentration differences between venous and capillary blood, based on results of vDBS and venous blood comparisons. Mohammed et al. reported significant differences in paracetamol capillary DBS and venous blood concentrations during absorption following oral administration [47]. The authors also stated that equivalent PK will only result after distribution equilibrium is attained. Drugs that are low in molecular weight, lipophilic and with low protein binding can traverse capillary walls more readily, thus contributing to concentration differences during the absorption phase [48]. It is possible that the observed discrepancies between cocaine and BE concentrations in capillary DBS and venous blood were due to inherent concentration differences between these two matrices, especially since sampling occurred rapidly after cocaine administration. We suggest that for cocaine and BE greater clinical acceptability for the capillary DBS method awaits resolution of inconsistent blood volume spots on the cards and further investigation of differences between capillary and venous blood following controlled drug administration.
Conclusion
Sensitive and specific methods for the detection of cocaine, BE and norcocaine in DBS (LC–HRMS) and cocaine and BE in blood (2D GC–MS) were developed and validated to determine if capillary DBS concentrations correlate well with venous blood concentrations following controlled intravenous cocaine administration. These first data indicate that although cocaine and BE were detected in all DBS after controlled cocaine administration, there was significant variability (intra- and inter-) among DBS concentrations collected at the same time (up to five spots per card). There was not a good agreement in cocaine marker concentrations between DBS and blood collection methods. DBS are an alternative matrix to blood for detecting recent cocaine consumption; however, DBS variability must be addressed and further investigation of differences between capillary and venous blood following controlled drug administration is warranted.
Future perspective
Future research must address whether extracting the entire DBS, as opposed to only a 3 mm disc as in this study, would help eliminate or reduce the variability observed in DBS. If DBS variability can be minimized, this collection method may prove advantageous for clinical and forensic toxicology applications. Sensitive LOQs are required to detect analyte concentrations in DBS, which was accomplished in this study. Another promising approach in utilizing DBS as an alternative matrix to blood is volumetric absorptive microsampling, believed to overcome hematocrit bias and homogeneity issues observed with conventional DBS sampling by sampling a fixed volume of blood [43]. Although cocaine and BE were detected in DBS, the large variability between DBS concentrations and lack of agreement between the two methods warrant further investigation.
Key terms.
Cocaine: Widely abused CNS stimulant found in Erythroxylon coca that blocks the dopamine transporter, preventing presynaptic reuptake of dopamine, norepinephrine and serotonin.
Benzoylecgonine: Inactive major metabolite of cocaine formed by enzymatic or chemical hydrolysis.
LC–HRMS: Offers enhanced selectivity and sometimes sensitivity over conventional LC–MS technology by acquiring ions with high mass accuracy.
2D GC–MS: Technique utilizing two analytical columns coupled to a single quadrupole MS to achieve higher sensitivity than traditional GC–MS by reducing noise and matrix effects.
Executive summary.
Background
Cocaine is a widely abused stimulant that is unstable in whole blood.
DBS were investigated as an alternative matrix to venous blood due to their less invasive, easy collection and increased stability.
Results
For the first time, cocaine and benzoylecgonine (BE) concentrations in DBS (n = 392) and simultaneously collected venous blood specimens (n = 97), following controlled intravenous cocaine administration, were evaluated and compared to determine agreement between capillary DBS and blood. No measurable norcocaine was detected in DBS.
Cocaine and BE were detected in all specimens at each time point; however, DBS concentrations were lower and range wider than their respective venous blood concentrations with large variability (both intra and inter) observed among DBS concentrations.
DBS discrepancies (and highly variable DBS concentrations) could be attributed to blood volumes spotted on the cards, differences in extraction efficiency and/or possible venous-capillary blood differences.
Future research should address DBS variability and possible differences between capillary and venous blood to improve the method's clinical usefulness.
Supplementary Material
Acknowledgements
The authors acknowledge the contributions of the clinical staff of the NIDA-IRP and the Clinical Research Unit, Johns Hopkins Bayview Medical Center, as well as the University of Maryland, Baltimore, a member of the Graduate Partnership Program, NIH.
Footnotes
Financial & competing interests disclosure
This work was supported by the Intramural Research Program (IRP), National Institute on Drug Abuse (NIDA), NIH. JL da Costa received a postdoctoral fellowship from Brazilian National Counsel of Technological and Scientific Development (CNPq) to conduct this research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.United Nations Office on Drugs and Crime (UNODC) World Drug Report 2014. Vienna, Austria: 2014. www.unodc.org/documents/data-and-analysis/WDR2014/World_Drug_Report_2014_web.pdf [Google Scholar]
- 2.U.S. Drug Enforcement Administration (DEA) National Forensic Laboratory Information System: 2013 Annual Report. Springfiled, Virginia: 2014. www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2014StatMethodology.pdf [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Annual Report 2012: The State of the Drugs Problem in Europe. Lisbon, Portugal: 2012. www.emcdda.europa.eu/publications/annual-report/2012 [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, Maryland: 2013. www.samhsa.gov/data/sites/default/files/NSDUHresults2012/NSDUHresults2012.pdf [Google Scholar]
- 5.Jatlow P. Cocaine: analysis, pharmacokinetics, and metabolic disposition. Yale J. Biol. Med. 1988;61:105–113. [PMC free article] [PubMed] [Google Scholar]
- 6.Ropero-Miller JD, Huestis MA, Stout PR. Cocaine analytes in human hair: evaluation of concentration ratios in different cocaine sources, drug-user populations and surface-contaminated specimens. J. Anal. Toxicol. 2012;36(6):390–398. doi: 10.1093/jat/bks050. [DOI] [PubMed] [Google Scholar]
- 7.Skopp G, Klingmann A, Potsch L, Mattern R. In vitro stability of cocaine in whole blood and plasma including ecgonine as a target analyte. Ther. Drug Monit. 2001;23:174–181. doi: 10.1097/00007691-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Sadones N, Capiau S, De Kesel PM, Lambert WE, Stove CP. Spot them in the spot: analysis of abused substances using dried blood spots. Bioanalysis. 2014;6(17):2211–2227. doi: 10.4155/bio.14.156. [DOI] [PubMed] [Google Scholar]; •• Recent review highlighting benefits, associated limitations and challenges, and recent developments and future perspectives of DBS analysis of abused substances.
- 9.Li W, Tse FL. Dried blood spot sampling in combination with LC–MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010;24(1):49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- 10.Henderson LO, Powell MK, Hannon WH, et al. An evaluation of the use of dried blood spots from newborn screening for monitoring the prevalence of cocaine use among childbearing women. Biochem. Mol. Med. 1997;61(2):143–151. doi: 10.1006/bmme.1997.2609. [DOI] [PubMed] [Google Scholar]
- 11.Mercolini L, Mandrioli R, Gerra G, Raggi MA. Analysis of cocaine and two metabolites in dried blood spots by liquid chromatography with fluorescence detection: a novel test for cocaine and alcohol intake. J. Chromatogr. A. 2010;1217(46):7242–7248. doi: 10.1016/j.chroma.2010.09.037. [DOI] [PubMed] [Google Scholar]; • Reports good correlation between cocaine and metabolite concentrations in capillary DBS and plasma specimens.
- 12.Alfazil AA, Anderson RA. Stability of benzodiazepines and cocaine in blood spots stored on filter paper. J. Anal. Toxicol. 2008;32(7):511–515. doi: 10.1093/jat/32.7.511. [DOI] [PubMed] [Google Scholar]; • Stability of cocaine in DBS was investigated compared with corresponding venous blood specimens stored under similar conditions.
- 13.Faller A, Richter B, Kluge M, Koenig P, Seitz HK, Skopp G. Stability of phosphatidylethanol species in spiked and authentic whole blood and matching dried blood spots. Int. J. Legal Med. 2013;127(3):603–610. doi: 10.1007/s00414-012-0799-y. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Boy R, Henseler J, Mattern R, Skopp G. Determination of morphine and 6-acetylmorphine in blood with use of dried blood spots. Ther. Drug Monit. 2008;30(6):733–739. doi: 10.1097/FTD.0b013e31818d9fdb. [DOI] [PubMed] [Google Scholar]
- 15.Henderson LO, Powell MK, Hannon WH, et al. Radioimmunoassay screening of dried blood spot materials for benzoylecgonine. J. Anal. Toxicol. 1993;17(1):42–47. doi: 10.1093/jat/17.1.42. [DOI] [PubMed] [Google Scholar]
- 16.Jantos R, Vermeeren A, Sabljic D, Ramaekers JG, Skopp G. Degradation of zopiclone during storage of spiked and authentic whole blood and matching dried blood spots. Int. J. Legal Med. 2013;127(1):69–76. doi: 10.1007/s00414-012-0696-4. [DOI] [PubMed] [Google Scholar]
- 17.Versace F, Déglon J, Lauer E, Mangin P, Staub C. Automated DBS extraction prior to Hilic/RP LC–MS/MS target screening of drugs. Chromatographia. 2013;76(19–20):1281–1293. [Google Scholar]
- 18.Aburuz S, Millership J, Mcelnay J. Dried blood spot liquid chromatography assay for therapeutic drug monitoring of metformin. J. Chromatogr. B. 2006;832(2):202–207. doi: 10.1016/j.jchromb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 19.Koal T, Burhenne H, Römling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Comm. Mass Spec. 2005;19(21):2995–3001. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm AJ, Den Burger JCG, Chahbouni A, Vos RM, Sinjewel A. Analysis of mycophenolic acid in dried blood spots using reversed phase high performance liquid chromatography. J. Chromatogr. B. 2009;877(30):3916–3919. doi: 10.1016/j.jchromb.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Hinchliffe E, Adaway JE, Keevil BG. Simultaneous measurement of cyclosporin A and tacrolimus from dried blood spots by ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2012;883:102–107. doi: 10.1016/j.jchromb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman JT, Rossi SS, Espina-Quinto R, Letendre S, Capparelli EV. Determination of efavirenz in human dried blood spots by reversed-phase high-performance liquid chromatography with UV detection. Ther. Drug Monit. 2013;35(2):203–208. doi: 10.1097/FTD.0b013e31827fb72b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas A, Geyer H, Schanzer W, et al. Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/orbitrap mass spectrometer. Anal. Bioanal. Chem. 2012;403(5):1279–1289. doi: 10.1007/s00216-011-5655-2. [DOI] [PubMed] [Google Scholar]
- 24.Mercolini L, Mandrioli R, Sorella V, et al. Dried blood spots: liquid chromatography-mass spectrometry analysis of delta(9)-tetrahydrocannabinol and its main metabolites. J. Chromatogr. A. 2013;1271:33–40. doi: 10.1016/j.chroma.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 25.Saussereau E, Lacroix C, Gaulier JM, Goulle JP. On-line liquid chromatography/tandem mass spectrometry simultaneous determination of opiates, cocainics and amphetamines in dried blood spots. J. Chromatogr. B. 2012;885:1–7. doi: 10.1016/j.jchromb.2011.11.035. [DOI] [PubMed] [Google Scholar]; • Reports significant correlations between venous DBS and venous blood concentrations of various drugs of abuse in suspected driving under the influence of drugs (DUID) cases.
- 26.Jantos R, Veldstra JL, Mattern R, Brookhuis KA, Skopp G. Analysis of 3,4-methylenedioxymetamphetamine: whole blood versus dried blood spots. J. Anal. Toxicol. 2011;35(5):269–273. doi: 10.1093/anatox/35.5.269. [DOI] [PubMed] [Google Scholar]
- 27.Ingels AS, Lambert WE, Stove CP. Determination of gamma-hydroxybutyric acid in dried blood spots using a simple GC–MS method with direct “on spot” derivatization. Anal. Bioanal. Chem. 2010;398(5):2173–2182. doi: 10.1007/s00216-010-4183-9. [DOI] [PubMed] [Google Scholar]
- 28.Deglon J, Thomas A, Mangin P, Staub C. Direct analysis of dried blood spots coupled with mass spectrometry: concepts and biomedical applications. Anal. Bioanal. Chem. 2012;402(8):2485–2498. doi: 10.1007/s00216-011-5161-6. [DOI] [PubMed] [Google Scholar]
- 29.Sosnoff CS, Ann Q, Bernert JT, Jr, et al. Analysis of benzoylecgonine in dried blood spots by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J. Anal. Toxicol. 1996;20:179–184. doi: 10.1093/jat/20.3.179. [DOI] [PubMed] [Google Scholar]
- 30.Antelo-Dominguez A, Cocho JA, Tabernero MJ, Bermejo AM, Bermejo-Barrera P, Moreda-Pineiro A. Simultaneous determination of cocaine and opiates in dried blood spots by electrospray ionization tandem mass spectrometry. Talanta. 2013;117:235–241. doi: 10.1016/j.talanta.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J. Chromatogr. A. 2010;1217(9):1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewitte K, Fierens C, Stockl D, Thienpont LM. Application of the Bland-Altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin. Chem. 2002;48(5):799–801. author reply 801– 792. [PubMed] [Google Scholar]
- 33.Scientific Working Group for Forensic Toxicology (SWGTOX) SWGTOX: standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013;37(7):452–474. doi: 10.1093/jat/bkt054. [DOI] [PubMed] [Google Scholar]; • Specific recommendations for method validation in forensic toxicology which were followed for both DBS and venous blood analytical methods developed in this current research.
- 34.Kolbrich E, Barnes A, Gorelick DA, Boyd SJ, Cone EJ, Huestis MA. Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. J. Anal. Toxicol. 2006;30:501–510. doi: 10.1093/jat/30.8.501. [DOI] [PubMed] [Google Scholar]
- 35.Blaho K, Logan B, Winbery S, Park L, Schwilke E. Blood cocaine and metabolite concentrations, clinical findings, and outcome of patients presenting to an ED. Am. J. Emerg. Med. 2000;18(5):593–598. doi: 10.1053/ajem.2000.9282. [DOI] [PubMed] [Google Scholar]
- 36.Jufer RA, Wstadik A, Walsh SL, Levine BS, Cone EJ. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J. Anal. Toxicol. 2000;24:467–477. doi: 10.1093/jat/24.7.467. [DOI] [PubMed] [Google Scholar]
- 37.Pelicao FS, Peres MD, Pissinate JF, De Martinis BS. A one-step extraction procedure for the screening of cocaine, amphetamines and cannabinoids in postmortem blood samples. J. Anal. Toxicol. 2014;38(6):341–348. doi: 10.1093/jat/bku039. [DOI] [PubMed] [Google Scholar]
- 38.Paul BD, Lalani S, Bosy T, Jacobs AJ, Huestis MA. Concentration profiles of cocaine, pyrolytic methyl ecgonidine and thirteen metabolites in human blood and urine: determination by gas chromatography-mass spectrometry. Biomed. Chromatogr. 2005;19(9):677–688. doi: 10.1002/bmc.495. [DOI] [PubMed] [Google Scholar]
- 39.Cardona PS, Chaturvedi AK, Soper JW, Canfield DV. Simultaneous analyses of cocaine, cocaethylene, and their possible metabolic and pyrolytic products. Forensic Sci. Int. 2006;157(1):46–56. doi: 10.1016/j.forsciint.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Gunnar T, Mykkanen S, Ariniemi K, Lillsunde P. Validated semiquantitative/quantitative screening of 51 drugs in whole blood as silylated derivatives by gas chromatography-selected ion monitoring mass spectrometry and gas chromatography electron capture detection. J. Chromatogr. B. 2004;806(2):205–219. doi: 10.1016/j.jchromb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Mommers J, Mengerink Y, Ritzen E, Weusten J, Van Der Heijden J, Van Der Wal S. Quantitative analysis of morphine in dried blood spots by using morphine-d3 pre-impregnated dried blood spot cards. Anal. Chim. Acta. 2013;774:26–32. doi: 10.1016/j.aca.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Jimmerson LC, Zheng JH, Bushman LR, Macbrayne CE, Anderson PL, Kiser JJ. Development and validation of a dried blood spot assay for the quantification of ribavirin using liquid chromatography coupled to mass spectrometry. J. Chromatogr. B. 2014;944:18–24. doi: 10.1016/j.jchromb.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denniff P, Spooner N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal. Chem. 2014;86(16):8489–8495. doi: 10.1021/ac5022562. [DOI] [PubMed] [Google Scholar]; • Highlights advantages of volumetric absorptive microsampling as a promising approach in utilizing DBS as an alternative matrix to whole blood in comparison to DBS cards.
- 44.De Kesel PM, Sadones N, Capiau S, Lambert WE, Stove CP. Hemato-critical issues in quantitative analysis of dried blood spots: challenges and solutions. Bioanalysis. 2013;5(16):2023–2041. doi: 10.4155/bio.13.156. [DOI] [PubMed] [Google Scholar]
- 45.Cobb Z, De Vries R, Spooner N, et al. In-depth study of homogeneity in DBS using two different techniques: results from the EBF DBS-microsampling consortium. Bioanalysis. 2013;5(17):2161–2169. doi: 10.4155/bio.13.171. [DOI] [PubMed] [Google Scholar]
- 46.O'mara M, Hudson-Curtis B, Olson K, Yueh Y, Dunn J, Spooner N. The effect of hematocrit and punch location on assay bias during quantitative bioanalysis of dried blood spot samples. Bioanalysis. 2011;3(20):2335–2347. doi: 10.4155/bio.11.220. [DOI] [PubMed] [Google Scholar]; • Evaluates the impact of sample hematocrit and punch location on assay bias in DBS sampling.
- 47.Mohammed BS, Cameron GA, Cameron L, Hawksworth GH, Helms PJ, Mclay JS. Can finger-prick sampling replace venous sampling to determine the pharmacokinetic profile of oral paracetamol? Br. J. Clin. Pharmacol. 2010;70(1):52–56. doi: 10.1111/j.1365-2125.2010.03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports significant paracetamol concentration differences between capillary DBS and venous blood during absorption following oral administration.
- 48.Upton RN, Mather LE, Runciman WB, Nancarrow C, Carapetis RJ. The use of mass balance principles to describe regional drug distribution and elimination. J. Pharmacokinet. Biopharm. 1988;16(1):13–29. doi: 10.1007/BF01061860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.