Abstract
A 48-year-old female was admitted to our hospital presenting with a chief complaint of progressive swelling because of diabetic nephrotic syndrome. Dapagliflozin seemed to play a role in accelerating the patient’s urinary sodium excretion as well as reducing gross fluid retention despite the fact that her nephrotic condition was resistant to furosemide. Our experience emphasizes a potential novel approach to overcoming loop diuretic resistance using this agent among some subsets of type 2 diabetic subjects complicated with severe volume accumulation. We believe that combination treatment consisting of dapagliflozin and furosemide may produce diuretic synergy via sequential nephron blockade. The accumulation of more experience with additional cases similar to ours requires continuous and careful attention.
Keywords: nephrotic syndrome, SGLT-2, dapagliflozin, diabetes, furosemide
Introduction
Dapagliflozin, a sodium–glucose cotransporter 2 (SGLT-2) inhibitor, which recently received marketing approval as a novel therapeutic option for type 2 diabetes, promotes glycosuria via an osmotic diuretic effect.1 In this report, we describe our serendipitous experience with a case of type 2 diabetes accompanied by nephrotic syndrome in which dapagliflozin seemed to play a role in controlling diuretic-resistant fluid retention.
Case Report
A 48-year-old female was admitted to our hospital presenting with a complaint of progressive swelling of her legs. She had gained ~20 kg in the past four months. At 35 years of age, she was found to have type 2 diabetes with a hemoglobin A1c (HbA1c) level of 9.4%, for which she had received sporadic medical care. Two months before admission, when she was found to have hypertension and hypercholesterolemia as well as uncontrolled diabetes with a serum HbA1c level of 7.1%, treatment with furosemide at a dose of 60 mg/day combined with alogliptin at a dose of 25 mg/day, irbesartan at a dose of 100 mg/day, amlodipine at a dose of 10 mg/day, and rosuvastatin at a dose of 2.5 mg/day was started; however, her generalized edema persisted and subsequently worsened. Therefore, she was referred and admitted for a further workup. She neither smoked nor drank alcohol and denied using any drugs.
A physical examination completed on admission revealed that the patient’s face was swollen, with significant edema noted in the upper and lower extremities. Her blood pressure (BP) was 153/87 mmHg, her pulse was 90 beats/minute, and her temperature was 36.0°C. Although the oxygen saturation was 98% while she breathed ambient air, the presence of bilateral pleural effusion and ascites was confirmed on chest X-ray and/or computed tomography scans. No findings suggestive of heart failure were observed on echocardiography. A laboratory evaluation revealed the following results: Hb, 9.5 g/dL; platelet count, 33.6 × 104/μL; total protein, 5.5 g/dL; serum albumin, 2.0 g/dL; blood urea nitrogen, 14.5 mg/dL; creatinine (Cr), 1.01 mg/dL; sodium, 141 mmol/L; potassium, 4.0 mmol/L; chloride, 105.1 mmol/L; aspartate aminotransferase, 20 U/L; alanine aminotransferase, 9 U/L; fasting plasma glucose, 180 mg/dL; HbA1c, 6.6%; and C-reactive protein, 0.3 mg/dL. The patient’s urine contained 7.0 g of protein in a 24-hour specimen, and the sediment contained five to nine red blood cells per high-power field. The Cr clearance was 29.0 mL/minute. An ophthalmologic analysis revealed severe nonproliferative diabetic retinopathy.
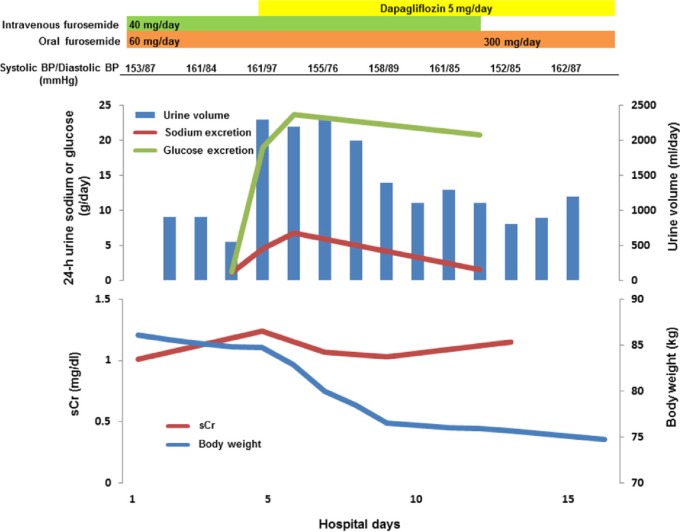
Based on the clinical picture and laboratory findings, the patient was thus diagnosed as having nephrotic syndrome due to diabetic nephropathy. Despite treatment with an increased dose of furosemide, given both orally and intravenously, her body weight and daily urine volume remained almost constant, keeping her grossly edematous. She then received treatment with oral dapagliflozin at a dose of 5 mg/day on hospital day 5, which resulted in a remarkable increase in her urine volume as well as the amount of urinary excreted sodium and glucose, with the gradual disappearance of the generalized edema, despite the almost constant levels of her systolic and diastolic BP during the observation period (Fig. 1). Finally, her body weight settled at around 65 kg under the treatment with sodium and fluid restriction and the same dose of dapagliflozin combined with oral furosemide (220 mg/day). At three months of follow-up, she is currently doing well with an HbA1c level of 6.1% despite protracted nephrotic-range proteinuria at approximately 5 g/day.
Figure 1.
Clinical course. On hospital day 12, intravenous furosemide was terminated, while oral furosemide was continued with an increased dose of 300 mg/day, and the patient was discharged on hospital day 16. Note that the levels of BP and serum Cr (sCr) were almost constant during the observation period despite prominent elevation of the daily urine output after the commencement of oral dapagliflozin.
Discussion
Nephrologists occasionally face difficulties in controlling fluid retention despite the availability of various types of diuretic agents.2,3 The pathogenic processes responsible for diuretic resistance are multifactorial, and the albumin present in the luminal content as a result of the disease process is capable of binding the diuretic drug and thereby eliciting resistance in subjects with nephrotic syndrome.4 Consequently, one may argue that some of the clinical manifestations and therapeutic conundrums observed in our patient are too common to be described in the literature; however, the clinical significance of the current report should be evaluated carefully in terms of assessing the therapeutic potential of dapagliflozin for the management of refractory edema in the cases of diabetic nephrotic syndrome.
Comprehensive insight into the role of SGLT-2, which is located mainly in the brush border membrane of the early proximal tubule,5 in glucose handling within the kidney has led to the development of selective orally available sodium–glucose transport inhibitors as a means of regulating the serum glucose level.1,6 Recent clinical trials of these agents have demonstrated favorable safety profiles, whereas neither major hypoglycemic events nor adverse changes in the renal function were reported.7,8 On the other hand, there are several observations suggesting that the promising benefits of these drugs may extend beyond glycemic control. The blockade of SGLT-2 has been shown to result in glycosuria-mediated calorie wasting, leading to weight loss.7 Such treatment may also lead to reduced sodium reabsorption in the proximal tubule, thereby accelerating sodium excretion.9 Although too few studies have reported the cases of urinary sodium excretion to allow for a substantial analysis,10–12 the association between the use of SGLT-2 inhibitors and the significant decreases in BP demonstrated in several recent studies should encourage researchers to pursue further investigations regarding the diuretic-like antihypertensive actions of these medications, which should be linked to natriuresis as well as glucose-mediated osmotic diuresis.1,9,13 At present, we have no idea why we failed to confirm such an effect in the present patient; however, it may be reasonable to consider that her volume overload status might have masked the diuresis-dependent antihypertensive properties.14 Otherwise, what subtypes of diabetic subjects are vulnerable to these kinds of agents also needs to be assessed in more detail. A case of volume depletion and prerenal azotemia that required rehydration and the withdrawal of angiotensin-converting inhibitor and diuretic treatment was recently reported in a phase II trial of dapagliflozin.15
Not surprisingly, dapagliflozin was effective and well tolerated as an adequate therapeutic option for modulating the present patient’s level of glycemic control. Our experience with this agent in the current patient rather emphasizes a potential approach for overcoming loop diuretic resistance among some subsets of type 2 diabetic subjects complicated with severe volume accumulation. Indeed, dapagliflozin seemed to play a role in accelerating urinary sodium excretion as well as reducing the considerable fluid retention in the current patient despite the fact that her nephrotic condition was resistant to furosemide. The fact that the natriuretic effect of dapagliflozin did not persist may be ascribed to the establishment of a new steady state, in which the patient’s depleted volume status and increased activity of the renin angiotensin system stimulated renal sodium uptake through alternate pathways and compensated for the natriuretic effect of the agent.6,16 Finally, we believe that combination treatment consisting of dapagliflozin and furosemide may have produced diuretic synergy via the sequential blockade of solute reabsorption at the proximal tubules and at the thick ascending limb of the loop of Henle in our patient. The lack of longitudinal data regarding the gross daily urine output, as well as the amount of urinary excreted sodium after the commencement of oral dapagliflozin treatment in the previous studies, precludes us from evaluating the validity of our findings.10–12 Nevertheless, List et al, demonstrated that the administration of dapagliflozin in treatment-naive patients with type 2 diabetes resulted in an increased urinary output of between 107 mL/day and 470 mL/day, equating to ~0.3–1.5 additional voids per day.1,17 This highlights the need for further investigation regarding the impact of the diuretic properties of this agent on the overall management of diabetic patients.9 Obviously, the accumulation of more experience with additional cases similar to ours requires continuous and careful attention, and such a strategy would aid in the establishment of a novel approach to treating fluid retention as well as investigating the therapeutic impact of SGLT-2 inhibitors in diabetic patients with nephrotic syndrome.
Footnotes
ACADEMIC EDITOR: Anuj Chauhan, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 358 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Drafted the manuscript: TI and TA. Made contributions to the acquisition of the clinical data: CI and TM. Provided a detailed review of the contents and structure of the manuscript, resulting in significant changes to the original document: DN. All authors have read and approved the final manuscript.
REFERENCES
- 1.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onoyama K, Kumagai H, Fujishima M. Hemodynamic and volume changes by ultrafiltration in refractory edema of diabetic nephrotic syndrome with severe renal insufficiency. Clin Nephrol. 1987;27(1):21–25. [PubMed] [Google Scholar]
- 3.Davenport A. Ultrafiltration in diuretic-resistant volume overload in nephrotic syndrome and patients with ascites due to chronic liver disease. Cardiology. 2001;96(3–4):190–195. doi: 10.1159/000047403. [DOI] [PubMed] [Google Scholar]
- 4.Brater DC. Diuretic resistance: mechanisms and therapeutic strategies. Cardiology. 1994;84(suppl 2):57–67. doi: 10.1159/000176458. [DOI] [PubMed] [Google Scholar]
- 5.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12(2):78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012;44(4):375–393. doi: 10.3109/07853890.2011.560181. [DOI] [PubMed] [Google Scholar]
- 8.Shah NK, Deeb WE, Choksi R, Epstein BJ. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2012;32(1):80–94. doi: 10.1002/PHAR.1010. [DOI] [PubMed] [Google Scholar]
- 9.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85(5):513–519. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 11.Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14(6):539–545. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 12.Devineni D, Vaccaro N, Polidori D, Rusch S, Wajs E. Effects of hydrochlorothiazide on the pharmacokinetics, pharmacodynamics, and tolerability of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Clin Ther. 2014;36(5):698–710. doi: 10.1016/j.clinthera.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262–275. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Meltzer JI, Keim HJ, Laragh JH, Sealey JE, Jan KM, Chien S. Nephrotic syndrome: vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Ann Intern Med. 1979;91(5):688–696. doi: 10.7326/0003-4819-91-5-688. [DOI] [PubMed] [Google Scholar]
- 15.Wilding JP, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32(9):1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas MC, Jandeleit-Dahm K, Bonnet F. Beyond glycosuria: exploring the intrarenal effects of SGLT-2 inhibition in diabetes. Diabetes Metab. 2014;40(6 suppl 1):S17–S22. doi: 10.1016/S1262-3636(14)72691-6. [DOI] [PubMed] [Google Scholar]
- 17.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011;79(suppl 120):S20–S27. doi: 10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]