Abstract
Background
Neurodegeneration is characterized by progressive neurological deficits due to selective neuronal loss in the nervous system. Huntington’s disease (HD) is an incurable neurodegenerative disorder. Neurodegeneration in HD patients shows aging-dependent pattern. Our previous study has suggested that a herbal formula B401 may have neuroprotective effects in the brains of R6/2 mice.
Objective
To clarify possible mechanisms for neurodegeneration, which improves the understanding the aging process. This study focuses on clarifying neurodegenerative mechanisms and searching potential therapeutic targets in HD patients.
Methods
The oxidative stress and apoptosis were compared in the brain tissue between R6/2 HD mice with and without oral B401 treatment. Expressions of proteins for oxidative stress and apoptosis in the brain tissue of R6/2 HD mice were examined by using immunostaining and Western blotting techniques.
Results
R6/2 HD mice with oral B401 treatment significantly reduced reactive oxygen species levels in the blood, but markedly increased expressions of superoxide dismutase 2 in the brain tissue. Furthermore, R6/2 HD mice with oral B401 treatment significantly increased expressions of B-cell lymphoma 2 (Bcl-2), but significantly reduced expressions of Bcl-2-associated X protein (Bax), calpain, and caspase-3 in the brain tissue.
Conclusion
Our findings provide evidence that the herbal formula B401 can remedy for aging-dependent neurodegeneration of R6/2 mice via suppressing oxidative stress and apoptosis in the brain. We suggest that the herbal formula B401 can be developed as a potential health supplement for ameliorating aging-dependent neurodegeneration.
Keywords: neuroprotection, reactive oxygen species, superoxide dismutase 2, B-cell lymphoma 2, Bcl-2-associated X protein, calpain, caspase-3, transgenic mouse model, Huntington’s disease
Introduction
Herbal treatment is an alternative form of healing/therapy in curing aging-dependent diseases in both human beings and animals. Because neurodegenerative disease onset is usually in the later years of their lifetime, prevalence is increasing with the aged population. To clarify possible mechanisms for neurodegeneration, which improves the understanding of the aging process. As of today, many natural compounds discovered within and extracted from plants have provided numerous clinically useful drugs for treating aging-dependent neurodegenerative diseases.1 Huntington’s disease (HD) is the most common genetic cause of chorea.2 A mutation in the Huntingtin gene causes the aggregation of the mutant protein huntingtin, which in turn produces cytotoxicity in the brain.3 Huntingtin aggregations are observed in the pathogenetic process in basal ganglia neurons of HD patients.4,5 Oxidative stress and mitochondrial dysfunction have been suggested to be contributing factors in the processes of huntingtin aggregation leading to neuronal death.6,7 Thus, clearance of huntingtin-containing aggregates represents a powerful therapeutic approach in the treatment of HD.8
From our previous study, the herbal formula B401 may provide new insights or new leads into advancing HD therapy, because regular oral B401 treatment has been shown to effectively reduce huntingtin aggregation in the brain of R6/2 HD mice.9 The herbal formula B401 is a Taiwan-US patent formula and consists of six herbal ingredients. Pharmacological studies have shown that regular oral B401 treatment could significantly improve life expectancy, motor ability, and maintain body weight retention in R6/2 mice. Furthermore, oral B401 treatment could increase expressions of brain-derived neurotrophic factor in the brain tissue of these mice.9 The neuroprotective effects of the herbal formula B401 for R6/2 mice are obvious, but further molecular mechanisms are still unclear.
Many common pathological mechanisms such as oxidative stress and apoptosis have been suggested to be involved in this neurodegenerative disease.10,11 Thus, we further study whether oral B401 treatment could ameliorate the neurodegenerative symptoms associated with HD via suppressing oxidative stress and apoptosis. In this study, expressions of proteins for oxidative stress (reactive oxygen species, ROS; superoxide dismutase 2, SOD2) and apoptosis B-cell lymphoma 2, Bcl-2; Bcl-2-associated X protein, Bax; calpain; caspase-3 in the brain tissue of R6/2 HD mice were examined and compared by using immunostaining and Western blotting techniques. Results from our study have proven our hypothesis and serve as an example in elucidating the great potential botanical extracts have a potential health supplement for ameliorating aging-dependent neurodegeneration such as HD.
Materials and methods
Chromatographic fingerprint analysis of the herbal formula B401
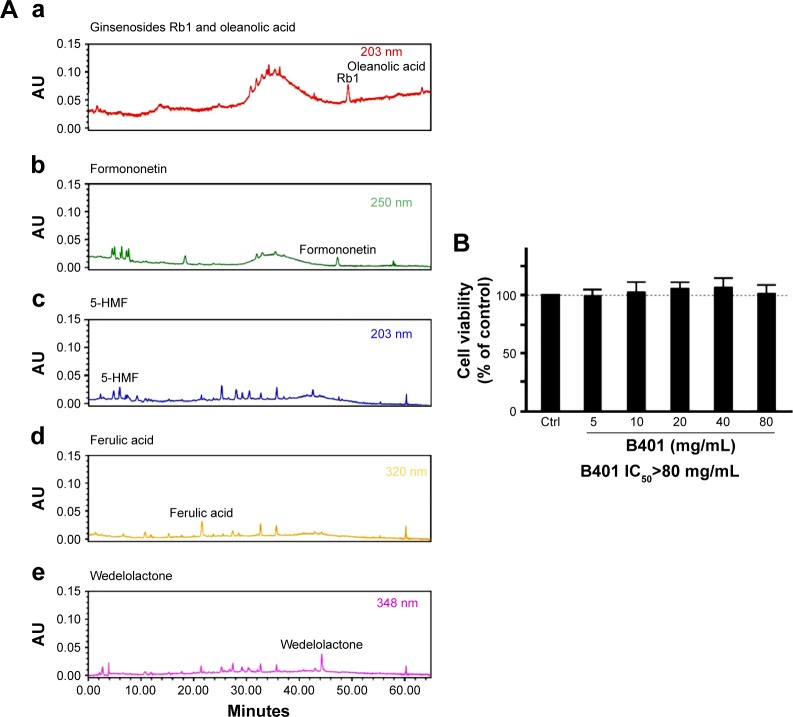
The herbal formula B401 (US patent, No 7838048B2, November 23, 2010) was supplied by Sun-Ten Pharmaceutical Company (New Taipei City, Taiwan). B401 contains six herbal ingredients, including extracts from Panax ginseng, Astragalus membranaceus, Angelica sinensis, Rehmannia glutinosa, Ligustri fructus, and Eclipta prostrata in specific ratios, and has been developed as a health supplement. High-performance liquid chromatography (HPLC) charts with different wavelengths in Figure 1Ai–Av show the six ingredients of B401. The charts include ginsenosides Rb1 (molecular formula: C54H92O23; molecular weight: 1,109.3 g/mol; extracts from P. ginseng), oleanolic acid (molecular formula: C30H48O3; molecular weight: 456.7 g/mol; extracts from L. fructus), formononetin (molecular formula: C16H12O4; molecular weight: 268.3 g/mol; extracts from A. membranaceus), 5-hydroxymethylfurfural (molecular formula: C6H6O3; molecular weight: 126.1 g/mol; extracts from R. glutinosa), ferulic acid (molecular formula: C10H10O4; molecular weight: 194.2 g/mol; extracts from L. fructus), and wedelolactone (molecular formula: C16H10O7; molecular weight: 314.3 g/mol; extracts from E. prostrata). HPLC-grade acetonitrile was purchased from Burdick & Jackson (Gyeonggi-do, Korea), along with methanol from Avantor (Center Valley, PA, USA). Water was purified by a Milli-Q water purification system (EMD Millipore, Billerica, MA, USA). All chemicals used were of analytical grade and solubilized in distilled H2O/MeOH. The half maximal inhibitory concentration (IC50) of the herbal formula B401 was evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.
Figure 1.
Chromatographic fingerprint analysis and cell viability assay for the herbal formula B401.
Notes: HPLC fingerprint of the herbal formula B401. Characteristic peaks of B401, ie, (Aa) ginsenosides Rb1 (from Panax ginseng) and oleanolic acid (from Ligustri fructus), (Ab) formononetin (from Astragalus membranaceus), (Ac) 5-HMF (from Rehmannia glutinosa), (Ad) ferulic acid (from L. fructus), (Ae) wedelolactone (from Eclipta prostrata), were identified and marked at the corresponding peaks in the fingerprint. (B) Cell viabilities of RA-induced SH-SY5Y cells in the absence (Ctrl) or presence of the B401 at indicated doses. The herbal formula B401 has no cytotoxicity in SH-SY5Y cells under treatment at a dose of less than 80 mg/mL.
Abbreviations: AU, arbitrary unit; IC50, half maximal inhibitory concentration; 5-HMF, 5-hydroxymethylfurfural; RA, retinoic acid; Ctrl, control group.
MTT assay for the herbal formula B401
Human neuroblastoma SH-SY5Y cells were plated in 96-well culture plates at a density of 3.0×104 cells/well for complete attachment at 37°C with 5% CO2 for 24 hours. The cells were then treated with the herbal formula B401 at doses of 5, 10, 20, 40, and 80 mg/mL for 24 hours to determine the IC50 of cytotoxicity. The culture medium was then removed, followed by incubation with 100 mL of MTT solution (0.5 mg/mL) at 37°C for 3 hours. Then 100 µL of 10% sodium dodecyl sulfate (SDS)–0.01 N HCl solution was added into each well and incubated at 37°C overnight to dissolve the formazan. The absorbency was measured at 570 nm with an ELISA reader (uQuant, bio-tek Inc., Winooski, VT, USA), and the results were expressed as the relative cell viability of treated cells against those of the controls. From Figure 1B, we found that the herbal formula B401 has no cytotoxicity in SH-SY5Y cells under treatment at a dose of less than 80 mg/mL.
R6/2 transgenic mouse model
In this study, male R6/2 mice (B6CBA-Tg(HDexon1)62Gpb/1J; HD exon 1 of the HTT gene with an expanded CAG repeat) with HD and their wild-type (WT) littermate controls were used to compare and elucidate the neuroprotective effect of B401. R6/2 mice and their WT were bred within an animal facility of National Taiwan Normal University. Breeder pairs of R6/2 mice in the experiment were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Genotyping of the R6/2 mice was conducted by polymerase chain reaction at 4 weeks of age. The neurological phenotype including choreiform-like or unintentional movements, tremor, and epilepsy progressed with age in R6/2 mice.12 We observed that some of the phenotypes in R6/2 mice were similar to the quality of HD patients, to include movement disorders, uncommon vocalization, and muscle atrophy. All mice were housed and maintained on a 12:12 hours light–dark cycle with water and food ad libitum. We chose 8-week-old R6/2 mice to be given the oral treatment of either B401 extract (30 mg/mL, the pH value was closed to 7.0) or their vehicle (dimethyl sulfoxide) in their drinking water twice daily for 2 consecutive weeks. All doses were adjusted according to individual weight and water consumption, which averaged 30 mL/day. The dosage of B401 extract given was much lower than the dosage of IC50 for mice. We compared motor performance, brain immunohistochemistry (IHC) staining, and Western blotting analysis in 10-week-old R6/2 mice and their WT. In this study, all animal experiments were conducted according to the international guidelines for care and use of laboratory animals and have been approved by the Committee on Animal Research of National Taiwan Normal University. These experiments were implemented under the guidelines of the Committee (protocol number: NTNU/Animal Use/No 13017/November 26, 2013).
Lifespan, body weight, and motor analysis in R6/2 mice
Life span and body weight of R6/2 mice and their WT were monitored daily. All the mice in the experiment were checked daily so as to determine their life span. We judged the mice as being euthanized by their lack of movement even after prodding, lying on their side, and lack of righting reflex. For comparing motor ability and coordination in R6/2 mice and their WT, a footprint test was performed. To obtain footprints, the hind- and forefeet of the mice were coated with purple and orange nontoxic paints, respectively. The animals were then allowed to walk along a 50 cm long, 10 cm wide runway with 10 cm high walls into an enclosed box. All mice had three training runs and were then given one run per week. To collect the footprints, a fresh sheet of white paper was placed on the floor of the runway for each run. The footprint patterns were analyzed for four step parameters. Stride length was measured as the average distance of forward movement between each stride.
ROS analysis of R6/2 mice
For determining blood levels of ROS in R6/2 mice, lucigenin-and luminol-amplified chemiluminescence (CL) methods were used to measure O2•− and H2O2 activity. As described previously,13 the lucigenin-enhanced CL method provides a reliable assay for ROS. A heparinized 0.2 mL sample of whole blood was taken from the left femoral artery of each mouse. ROS blood levels in R6/2 mice were measured by a CL analyzer (CLA-ID3 chemiluminescence analyzer; Tohoku Electronic Industrial Co., Ltd., Sendai, Japan) after administration of 1.0 mL of 0.1 mM lucigenin in phosphate-buffered saline (pH 7.4) into the tested samples. The assay was duplicated for each sample, and total CL counts in 600 seconds were calculated by integrating the area under the curve.
Brain histological and immuno histochemistry stains of R6/2 mice
For brain histological and immunohistochemistry (IHC) stains, anesthetized R6/2 mice and their WT were first brain perfused with phosphate-buffered saline containing 4% formaldehyde. Then brain tissue was removed and fixed with 4% formaldehyde (EM grade). Brain specimens were embedded in paraffin and cut into tissue sections of 5 µm thick. These brain tissue sections were then mounted on slides for histological and IHC analysis. General brain morphology was assessed using hematoxylin and eosin staining with a kit-based approach (Sigma-Aldrich Corporation, St Louis, MO, USA). By using the heat-induced epitope retrieval method, brain tissue sections were separately stained at room temperature for 1 hour with antibodies of mutant huntingtin (mHtt) (Abcam Inc., Cambridge, MA, USA), SOD2 (Cell Signaling Technology Inc., Danvers, MA, USA), B-cell lymphoma 2 (Bcl-2) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), Bcl-2 associated X protein (Bax) (Thermo Fisher Scientific Inc., Waltham, MA, USA), calpain (Cell Signaling Technology Inc.), and caspase 3 (Cell Signaling Technology Inc.). As immunostaining controls for each antibody, serial 5 µm cross sections were treated with the unanimous staining protocol. Immunostaining detection was executed by incubation with biotinylated secondary antibodies (Novolink™ polymer detection system l) at room temperature for 30 minutes, then by incubation with avidin–biotin–HRP (horseradish peroxidase) complex (Novolink™ polymer detection system l, Leica Biosystems Newcastle Ltd, Newcastle, United Kingdom) for 30 additional minutes. Immunostaining visualization was performed with DAB Chromogen kit (Novolink™ polymer detection system l) and counterstained with hematoxylin (Novolink™ polymer detection system l) following the supplier’s protocol.
Brain Western blotting of R6/2 mice
In preparing for the brain Western blotting analysis, the removed brain tissue was homogenized in a buffer solution containing 0.05 M tris (hydroxymethyl) aminomethane (Tris, pH 8.0, Bionovas Pharmaceuticals Inc., Washington DC, USA), 0.15 M sodium chloride (Bionovas Pharmaceuticals Inc.), 0.02 M ethylenediaminetetraacetic acid (Bionovas Pharmaceuticals Inc.), 1% deoxycholic acid (Bionovas Pharmaceuticals Inc.), 1% nonidet P40 (Bionovas Pharmaceuticals Inc.), 0.1% SDS (Bionovas Pharmaceuticals Inc.), 1% protease inhibitor cocktail for full range (Bionovas Pharmaceuticals Inc.), 1% serine/threonine phosphatase inhibitor cocktail (Bionovas Pharmaceuticals Inc.), and 1% tyrosine phosphatase inhibitor cocktail (Bionovas Pharmaceuticals Inc.). The homogenized buffer solution was placed on ice for 1 hour and then centrifuged at 4°C for 13,000 rpm for another 20 minutes, causing the supernatant solution to separate. The separated solution was quantified using BCA Protein Assay Kit (Thermo Fisher Scientific Inc.). Thirty micrograms of the total protein was denatured at 95°C for 5 minutes with 5× sample dye, that included 0.25 M tris (hydroxymethyl) aminomethane hydrochloride, pH 6.8 (Bionovas Pharmaceuticals Inc.), 10% SDS (Bionovas Pharmaceuticals Inc.), 0.5% bromophenol blue (Bionovas Pharmaceuticals Inc.), 50% glycerol (Bionovas Pharmaceuticals Inc.), and 5% β-mercaptoethanol (Bionovas Pharmaceuticals Inc.). The electrophoresis was done with a 12.5% discontinuous SDS-polyacrylamide gel. The proteins were then electroblotted onto a 0.2 µm polyvinylidene difluoride (GE Healthcare Life Sciences, Barrington, IL, USA) membrane for 120 minutes at 100 V. The membranes were reacted with a blocking buffer (5% skim milk in Tris-buffered saline with 0.1% Tween 20 buffer) for 1 hour at the ambient temperature, and then they were blocked. The brain protein was subjected to SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Antibodies of β-actin (Thermo Fisher Scientific Inc.), mHtt (Abcam Inc.), SOD2 (Cell Signaling Technology Inc.), Bcl-2 (Santa Cruz Biotechnology Inc.), Bax (Thermo Fisher Scientific Inc.), calpain (Thermo Fisher Scientific Inc.), and caspase 3 (Thermo Fisher Scientific Inc.) were used to identify expression levels of these proteins in the brain tissue by means of an HRP-linked secondary antibody. In addition, Western blotting detection reagents (GE Healthcare Life Sciences) were utilized to make immunoreactive bands perceivable. An ImageQuant LAS-4000 biomolecular imager (GE Healthcare Life Sciences) was used to detect the CL. ImageJ software (version 1.48t, Wayne Rasband, Washington, DC, USA) was used to perform densitometric assessments of the bands.
Statistical analysis
The data of cell viability assay were obtained from at least six independent experiments, each done in triplicate, whereas the data from Western blotting analysis were obtained from at least six independent experiments. The values of all data were expressed as the mean ± standard error of the mean (SEM). Differences between groups were evaluated by one- or two-way analysis of variance followed by Student–Newman–Keuls multiple comparisons posttest. The P-values of at least less than 0.05 were considered significant.
Results
Effects of oral B401 treatment on life, body weight, and motor ability of R6/2 mice
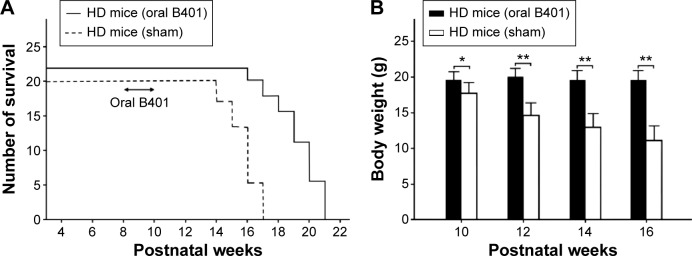
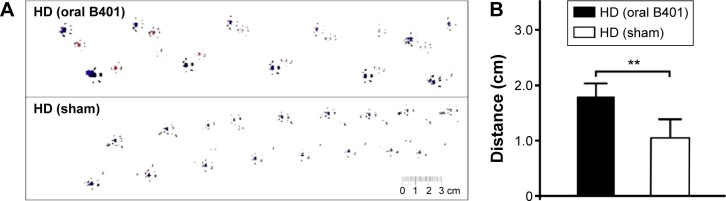
A total of 42 male R6/2 mice (22 R6/2 mice received oral B401 treatment and 20 R6/2 mice received sham treatment) were observed in this study. We compared the life span and body weight of R6/2 mice with and without oral B401 treatment. Our results had shown that oral B401 treatment lengthened life span and assisted in maintaining the body weight of R6/2 mice (Figure 2). The survival duration of the R6/2 mice given the oral B401 treatment was greater than the mice having received the sham treatment (Figure 2A). The R6/2 mice given the oral B401 treatment all died having reached 21 weeks of age (Figure 2A, solid line), whereas R6/2 mice given the sham treatment all died at 17 weeks of age (Figure 2A, dotted line). We compared the body weight of R6/2 mice given both the oral B401 and sham treatments. The body weights of R6/2 mice given the oral B401 treatment were significantly higher than those of R6/2 mice given the sham treatment from 10 weeks of age and thereafter (Figure 2B, P<0.01–0.05). We compared stride lengths of R6/2 mice with and without oral B401 treatment by using gait analysis (Figure 3A). Stride lengths of paw placement records of 10-week-old R6/2 mice given the oral B401 treatment were significantly longer than those of mice given the sham treatment (Figure 3B, P<0.01). Hindlimb gait abnormalities were apparent from 8 weeks and were followed at later ages by dragging of the hind legs. At 10 weeks of age, R6/2 mice given the oral B401 treatment placed their hind paws in almost the same spots as those occupied by the preceding forepaws during walking, whereas the steps of R6/2 mice given the sham treatment were irregular (Figure 3B).
Figure 2.
Oral B401 treatment prolongs life and maintains body weight of R6/2 (HD) mice.
Notes: (A) Survival duration of R6/2 mice given the oral B401 treatment was longer than those given the sham treatment. (B) Averaged body weight of R6/2 mice given the oral B401 treatment was significantly higher when compared with those given the sham treatment from 10 weeks of age and thereafter. The number of R6/2 mice under oral B401 and sham treatments were 22 and 20, respectively. Values are mean ± SEM (*P<0.05, **P<0.01, one-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: HD, Huntington’s disease; ANOVA, analysis of variance; SEM, standard error of the mean.
Figure 3.
Oral B401 treatment enhances motor ability of R6/2 (HD) mice.
Notes: (A) Gait analysis of 10-week-old R6/2 mice given the oral B401 and sham treatments. (B) Averaged stride lengths of paw placement records of the 10-week-old R6/2 mice given the oral B401 treatment were significantly better than those given the sham treatment. The number of R6/2 mice under oral B401 and sham treatments were eight for each group. Values are mean ± SEM (**P<0.01, one-way ANOVA followed by a Student–Newman–Keuls multiple comparisons posttest).
Abbreviations: HD, Huntington’s disease; ANOVA, analysis of variance; SEM, standard error of the mean.
Effects of oral B401 treatment on brain huntingtin expressions of R6/2 mice
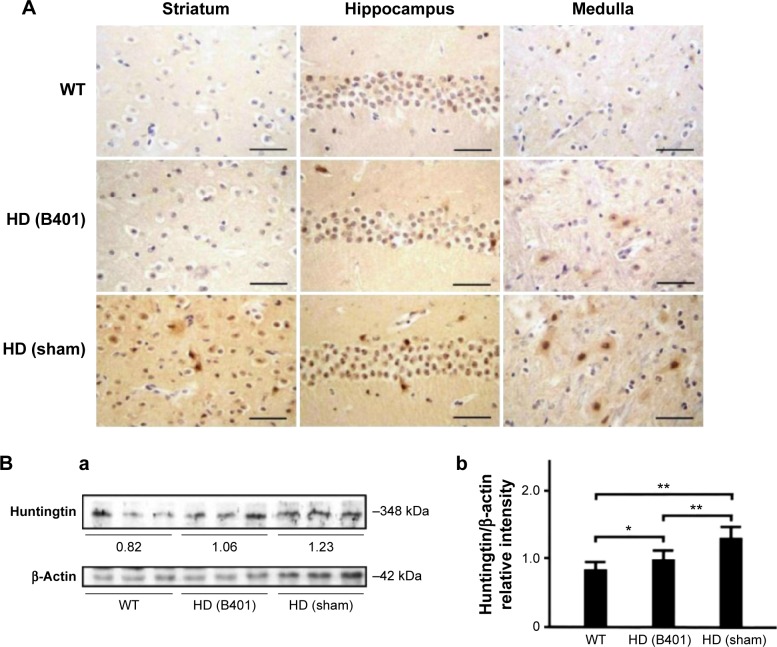
We compared aggregated mHTT in the brains of 10-week-old R6/2 mice under the oral B401 treatment, sham treatment, and their WT by IHC stain and Western blotting analysis in Figure 4. The deposition of mHTT is a neuropathological hallmark of HD.14 As observed from IHC staining of the brain, expressions of mHtt aggregation were obvious in the striatum, hippocampus, and medulla of R6/2 mice given the sham treatment, whereas they were not obvious in the striatum and hippocampus of R6/2 mice given the oral B401 treatment, and their WT (Figure 4A). As analyzed from Western blotting analysis of the brain, quantified brain huntingtin levels in 10-week-old R6/2 mice given the sham treatment were significantly higher than those in 10-week-old R6/2 mice given the oral B401 treatment and their WT (Figure 4Bi and Bii, P<0.01). Huntingtin levels of 10-week-old R6/2 mice given the oral B401 treatment were significantly lower than those of their WT (Figure 4Bi and Bii, P<0.05).
Figure 4.
Huntingtin aggregations in the brain tissue of R6/2 (HD) mice were reduced under oral B401 treatment.
Notes: (A) IHC staining shows that the expressions of huntingtin in the striatum, hippocampus, and medulla of the 10-week-old R6/2 mice given the oral B401 treatment were obviously weaker than those given the sham treatment but were obviously more intense than their WT. Scale bars: 30 µm. Western blotting analysis shows the following: (Ba) expression levels of huntingtin in whole brain tissue of the 10-week-old R6/2 mice given both the oral B401 and sham treatments, and their WT and (Bb) a significant increase in quantified brain huntingtin levels in the 10-week-old R6/2 mice in comparison with their WT, and a significant reduction under oral B401 treatment. The number of R6/2 mice under oral B401 and sham treatments and their WT was six for each group. Values are mean ± SEM (*P<0.05, **P<0.01, two-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: HD, Huntington’s disease; IHC, immunohistochemistry; WT, wild-type littermate; ANOVA, analysis of variance; SEM, standard error of the mean.
Effects of oral B401 treatment on oxidative stress in the brain of R6/2 mice
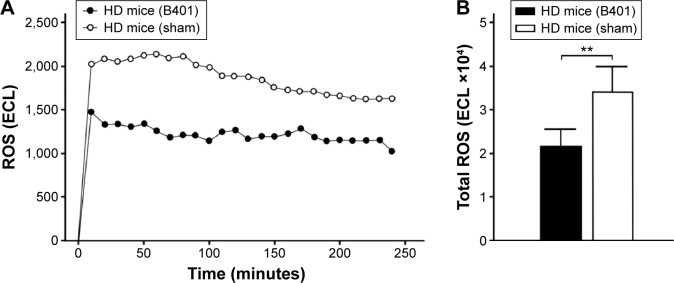
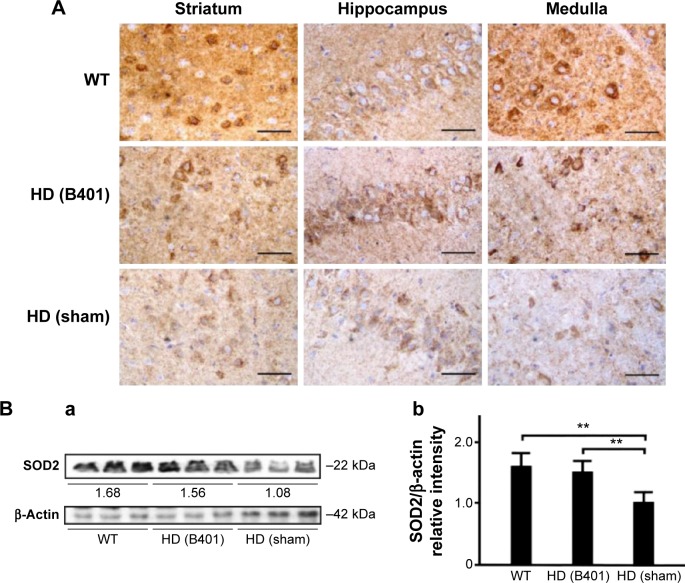
In comparing the effect of B401 treatment on oxidative stress, we examined the blood levels of ROS in 10-week-old R6/2 mice given the oral B401 and sham treatments by using a CLA-ID3 chemiluminescence analyzer. We observed that blood levels of ROS in the 10-week-old R6/2 mice given the sham treatment greatly increased beyond those of R6/2 mice given the oral B401 treatment (Figure 5A). Furthermore, total counts of blood ROS in the 10-week-old R6/2 mice given the sham treatment significantly increased beyond those of the R6/2 mice given the oral B401 treatment (Figure 5B, P<0.01). In this study, we also compared SOD2 in the brains of the 10-week-old R6/2 mice given the oral B401 and sham treatments, and their WT by IHC staining and Western blotting analysis in Figure 6. SOD2 is an important antioxidant enzyme for oxidative stress.15 As observed from IHC staining of the brain, expressions of SOD2 were not obvious in the striatum, hippocampus, and medulla of R6/2 mice given the sham treatment, but were obvious in the stratum, hippocampus, and medulla of R6/2 mice given the oral B401 treatment, and their WT (Figure 6A). As analyzed from Western blotting analysis of the brain, quantified SOD2 levels in the 10-week-old R6/2 mice given the sham treatment were significantly reduced when compared with those of the 10-week-old R6/2 mice given the oral B401 treatment, and their WT (Figure 6Bi and Bii, P<0.01). SOD2 levels of the 10-week-old R6/2 mice given the oral B401 treatment were not significant in comparison with those of their WT (Figure 6Bi and Bii, P>0.05).
Figure 5.
ROS levels in the blood of the R6/2 (HD) mice were reduced under oral B401 treatment.
Notes: (A) Chemiluminescence analysis shows that the expressions of ROS in the blood of the 10-week-old R6/2 mice given the oral B401 treatment were obviously weaker than those given the sham treatment but were obviously more intense than their WT. (B) The quantified expression blood levels of ROS in the 10-week-old R6/2 mice given the oral B401 treatment were significantly lower than those mice given the sham treatment. The number of R6/2 mice under oral B401 and sham treatments was six for each group. Values are mean ± SEM (**P<0.01, one-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: ECL, electro-chemiluminescence; HD, Huntington’s disease; ROS, reactive oxygen species; WT, wild-type littermate; ANOVA, analysis of variance; SEM, standard error of the mean.
Figure 6.
Expressions of anti-oxidative stress-related SOD2 in the brain tissue of the R6/2 (HD) mice were enhanced under oral B401 treatment.
Notes: (A) IHC staining shows that the expressions of SOD2 in the striatum, hippocampus, and medulla of the 10-week-old R6/2 mice given the oral B401 treatment were obviously more intense than those given the sham treatment but were obviously weaker than their WT. Scale bars: 30 µm. Western blotting analysis shows the following: (Ba) expression levels of SOD2 in whole brain tissue of the 10-week-old R6/2 mice given both oral B401 and sham treatments, and their WT and (Bb) quantified brain SOD2 levels of the 10-week-old R6/2 mice were significantly lower than their WT and significantly higher under oral B401 treatment. The number of R6/2 mice under oral B401 and sham treatments and their WT was six for each group. Values are mean ± SEM (**P<0.01, two-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: HD, Huntington’s disease; IHC, immunohistochemistry; SOD2, superoxide dismutase 2; WT, wild-type littermate; ANOVA, analysis of variance; SEM, standard error of the mean.
Effects of oral B401 treatment on apoptosis in the brain of R6/2 mice
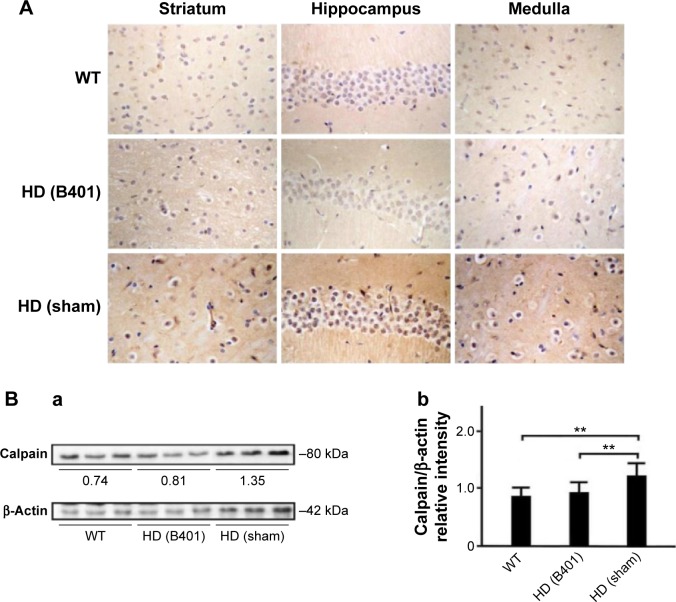
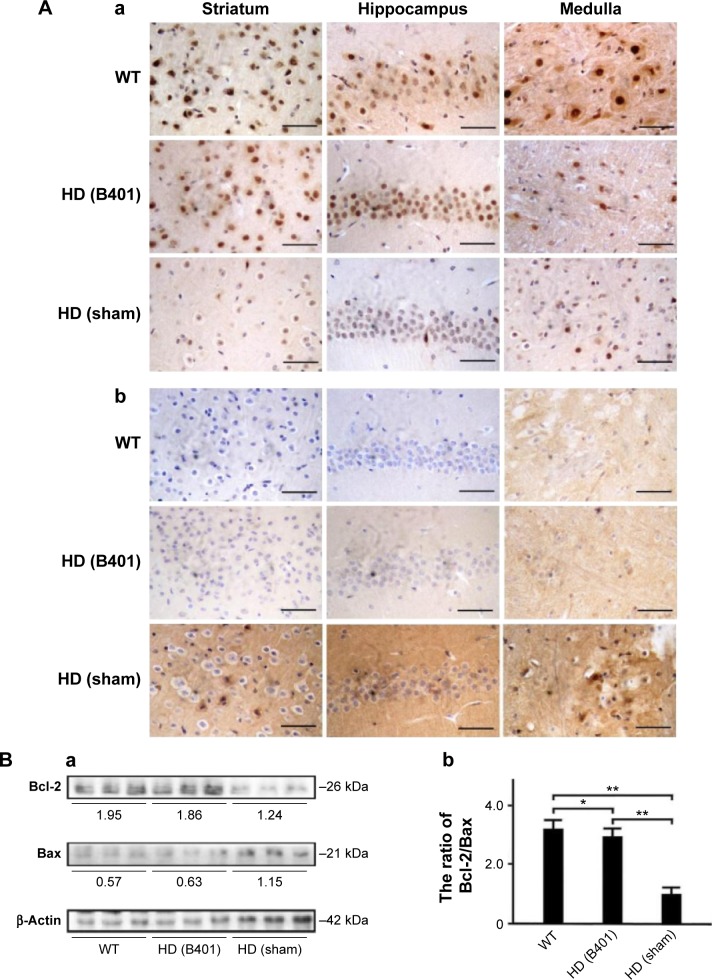
Apoptosis is believed to be involved in the pathogenesis and progression of HD.16 We examined whether the Chinese herbal formula B401 alleviates apoptosis in the brain of R6/2 mice. As observed from IHC staining of the brain, expressions of endoplasmic reticulum (ER) stress-related calpain were obvious in the stratum, hippocampus, and medulla of R6/2 mice given the sham treatment, but were not obvious in those of R6/2 mice given the oral B401 treatment, and their WT (Figure 7A). Furthermore, expressions of anti-apoptosis-related Bcl-2 were not obvious in the stratum, hippocampus, and medulla of R6/2 mice given the sham treatment, but were obvious in those of R6/2 mice given the oral B401 treatment, and their WT (Figure 8Ai and Aii). On the contrary, expressions of mitochondrial dysfunction-related Bax and caspases-3 were obvious in the stratum, hippocampus, and medulla of R6/2 mice given the sham treatment, but were not obvious in those of R6/2 mice given the oral B401 treatment, and their WT (Figures 8Ai, Aii, and 9A). As analyzed from Western blotting analysis of the brain, the quantified ratio of Bcl-2/Bax of the 10-week-old R6/2 mice given the sham treatment was significantly reduced when compared with those of the 10-week-old R6/2 mice given the oral B401 treatment, and their WT (Figure 8Bi and Bii, P<0.01). Quantified brain calpain and caspases-3 levels in the 10-week-old R6/2 mice given the sham treatment significantly increased beyond those of the 10-week-old R6/2 mice given the oral B401 treatment, and their WT (Figures 7Bi, Bii, and 9Bi, Bii, P<0.01). Calpain and caspases-3 levels in the 10-week-old R6/2 mice given the oral B401 treatment were not significant when compared with those of their WT (Figures 7Bi, Bii, and 9Bi, Bii, P>0.05).
Figure 7.
Expressions of ER-stress-related calpain in the brain tissue of the R6/2 (HD) mice were reduced under oral B401 treatment.
Notes: (A) IHC staining shows that the expressions of calpain in the striatum, hippocampus, and medulla of the 10-week-old R6/2 mice given the oral B401 treatment were obviously weaker than those given the sham treatment but had shown no difference in their WT. Scale bars: 30 µm. Western blotting analysis shows the following: (Ba) expression levels of calpain in whole brain tissue of the 10-week-old R6/2 mice given both oral B401 and sham treatments, and their WT and (Bb) quantified brain calpain levels of the 10-week-old R6/2 mice were significantly higher than their WT and significantly lower under oral B401 treatment. The number of R6/2 mice under oral B401 and sham treatments, and their WT was six for each group. Values are mean ± SEM (**P<0.01, two-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: ER, endoplasmic reticulum; HD, Huntington’s disease; IHC, immunohistochemistry; WT, wild-type littermate; ANOVA, analysis of variance; SEM, standard error of the mean.
Figure 8.
The ratio of anti-apoptosis-related Bcl-2/Bax in the brain tissue of the R6/2 (HD) mice was increased under oral B401 treatment.
Notes: IHC staining shows that (Aa) the expressions of Bcl-2 were in the striatum, hippocampus, and medulla of the 10-week-old R6/2 mice given the oral B401 treatment were obviously greater than those given the sham treatment, but (Ab) the expressions of Bax were obviously weaker than those given the sham treatment. Western blotting analysis shows the following: (Ba) expression levels of Bcl-2 and Bax in whole brain tissue of the 10-week-old R6/2 mice given both oral B401 and sham treatments, and their WT and (Bb) the quantified brain ratio of Bcl-2/Bax in the 10-week-old R6/2 mice was significantly lower than their WT and significantly higher under oral B401 treatment. The number of R6/2 mice under oral B401 and sham treatments and their WT was six for each group. Values are mean ± SEM (*P<0.05, **P<0.01, two-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; HD, Huntington’s disease; IHC, immunohistochemistry; WT, wild-type littermate; ANOVA, analysis of variance; SEM, standard error of the mean.
Figure 9.
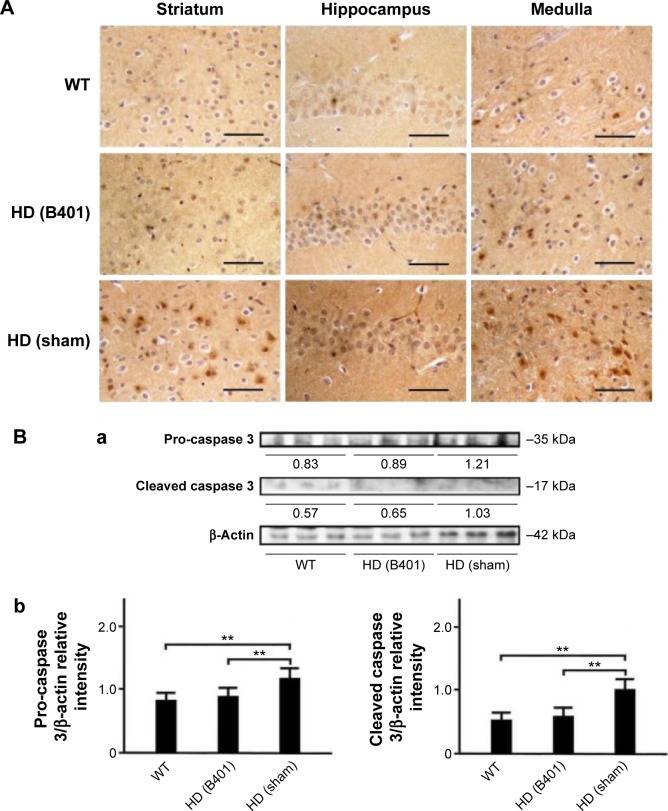
Expressions of apoptosis-related caspase 3 in the brain tissue of R6/2 (HD) mice were reduced under oral B401 treatment.
Notes: (A) IHC staining shows that the expressions of caspase 3 in the striatum, hippocampus, and medulla of 10-week-old R6/2 mice with oral B401 treatment were obviously weaker than those given the sham treatment but had shown no difference in their WT. Scale bars: 30 µm. Western blotting analysis shows the following: (Ba) expression levels of caspase 3 in whole brain tissue of the 10-week-old R6/2 mice given both oral B401 and sham treatments, and their WT and (Bb) quantified brain pro-caspase 3 (35 kDa) and cleaved caspase 3 (17 kDa) levels of the 10-week-old R6/2 mice were significantly higher than their WT and significantly lower under oral B401 treatment. The number of R6/2 mice under oral B401 and sham treatments and their WT was six for each group. Values are mean ± SEM (**P<0.01, two-way ANOVA followed by a Student–Newman–Keuls multiple comparison posttest).
Abbreviations: HD, Huntington’s disease; IHC, immunohistochemistry; WT, wild-type littermate; ANOVA, analysis of variance; SEM, standard error of the mean.
Discussion
In this study, we investigated the neuroprotective capabilities of the herbal formula B401 in the brain of R6/2 mice. R6/2 mice possess several advantages, such as a well-described neuropathology and availability for direct evaluation of mHtt protein in their brain tissue. We found that life span, body weight, and motor ability of the R6/2 mice were reduced when compared with their WT, whereas chronic oral B401 treatment obviously enhanced their life span, body weight, and motor ability (Figures 2 and 3). Neuroprotection of the herbal formula B401 in the brain of R6/2 mice may occur via inhibition of oxidative stress and apoptosis (Figures 5–9). Our study can provide a possible alternative therapy for neurodegeneration in HD patients.
Here we found that regular B401 treatment produced significant improvements in survival rates and motor activity in the R6/2 mice with HD. The herbal formula B401 mainly contains ginsenosides Rb1 extracted from P. ginseng, oleanolic acid extracted from L. fructus, formononetin extracted from A. membranaceus, 5-hydroxymethylfurfural extracted from R. glutinosa, ferulic acid extracted from L. fructus, and wedelolactone extracted from E. prostrata. It has been known that all of these compounds have antioxidative properties. For example, ginsenoside Rb1 has been widely used as a traditional herbal medicine, as it has been shown to be a powerful antioxidant and inhibitor of apoptosis.17 Formononetin has been reported to exhibit neuroprotective effects in cellular excitotoxicity.18 These studies reveal the multi-protective mechanisms of Chinese herbal formulas in treating the neurodegeneration associated with HD.
The neuropathology of HD involves neuronal loss, which occurs most markedly in the striatum and deep layers of the cerebral cortex.19 R6/2 mice have been widely used to study neurodegenerative pathology of HD, because those mice are correlated with mHtt protein across brain regions. Our results indicate a ubiquitous abundance of mHtt protein across various brain regions, including the striatum, hip-pocampus, and medulla. Oral B401 treatment may effectively reduce mHtt aggregation in the striatum and hippocampus of R6/2 mice (Figure 4). The importance of ROS has received increased attention within the last decade, because these molecules are exacerbating factors in neural damage and aging processes.20 Previous findings suggest the role of oxidative damage in the HD pathogenesis.10 It has been demonstrated that the expression of mHtt in neuronal cells may cause increased ROS, which contributes to neuronal death.21 Evidence from our study shows that oral B401 treatment may effectively reduce blood ROS and enhance brain SOD2 expressions (Figures 5 and 6). Our data provide further evidence that oxidative damage may contribute to HD pathogenesis in R6/2 mice. We raise the possibility that the antioxidative activity of the herbal formula B401 may be a useful therapeutic agent in slowing the progression of neurodegeneration in HD.
Along with oxidative stress, apoptosis is believed to be intimately involved in the pathogenesis and progression of HD.11 We found that oral B401 treatment may also effectively enhance the Bcl-2/Bax ratio within the brain and reduce brain calpain and caspase-3 expressions (Figures 7–9). Calpain is a key marker of ER-stress-related apoptosis.22 Here we found that expressions of calpain in the brain tissue of the R6/2 mice were greater in comparison with their WT but were reduced under oral B401 treatment (Figure 7). Mitochondria play a key role in apoptotic signal transduction.23 Bcl-2 and Bax are members of the Bcl-2 family, and both regulate apoptosis by controlling mitochondrial integrity. Bcl-2 acts to inhibit apoptosis, whereas Bax counteracts this effect. The Bcl-2/Bax ratio indicated the tendency of apoptosis. Our data reported that the Bcl-2/Bax ratio in the brain tissue of R6/2 HD mice was significantly lower in comparison with that of their WT, but was significantly higher under oral B401 treatment (Figure 8). Moreover, enhanced calpain expressions and a reduced Bcl-2/Bax ratio in brain tissue may activate the downstream effector caspases, such as caspase-3, which eventually leads to apoptosis. Here we observed that expressions of caspase-3 in the R6/2 mice were significantly higher than those of their WT but were significantly lower under oral B401 treatment (Figure 9). Based on the earlier discussion, we suggested that both mitochondrial dysfunction and ER-stress-related apoptosis can be involved in neuropathogenesis of R6/2 mice, whereas apoptosis damage in the brain tissue of R6/2 mice can be ameliorated under oral B401 treatment.
Conclusion
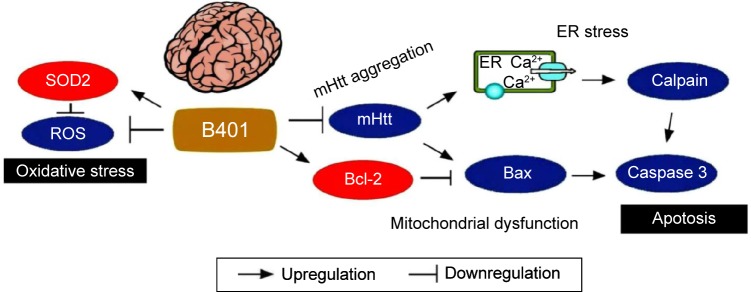
As summarized in Figure 10, our studies demonstrated that oral B401 treatment may improve neuroprotection in R6/2 mice via enhancing anti-oxidative (marked by SOD2) and anti-apoptosis activity (marked by the Bcl-2/Bax ratio), while suppressing mHtt aggregation, ROS production, ER-stress-related apoptosis (marked by calpain), and mitochondrial dysfunction-related apoptosis (marked by caspase 3) in the brain. We suggest that the herbal formula B401 can be developed as a potential health supplement for ameliorating aging-dependent neurodegeneration.
Figure 10.
The schematic diagram illustrates the possible neuroprotective pathways in the brain under oral B401 treatment. Oral B401 treatment may improve neuroprotection in R6/2 mice via enhancing anti-oxidative stress (marked by SOD2) and anti-apoptosis (marked by Bcl-2), while suppressing mutant huntingtin aggregation, ROS production, ER stress-related apoptosis (marked by calpain), and mitochondrial dysfunction-related apoptosis (marked by Bax and caspase 3) in the brain.
Abbreviations: Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; ER, endoplasmic reticulum; mHtt, mutant huntingtin; ROS, reactive oxygen species; SOD2, superoxide dismutase 2.
Acknowledgments
We thank Professor Chiung-Mei Chen (Department of Neurology, College of Medicine, Chang-Gung University, Taoyuan, Taiwan) for providing the R6/2 HD mice, and Sun-Ten Pharmaceutical Company for the technical assistance. This research was supported by the Industry-University Cooperative Grant of Brion Research Institute of Taiwan, Top University Project of National Taiwan Normal University (NTNU), and the Transnational Research Centers Grant (103T3040B04) from NTNU. The funding agency had no role in the study design, data collection and analysis, and the decision to publish the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6(12):81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Huntington’s Disease Collaboration Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 3.Bano D, Zanetti F, Mende Y, et al. Neurodegenerative processes in Huntington’s disease. Cell Death Dis. 2011;2:e228. doi: 10.1038/cddis.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies SW, Turmaine M, Cozens BA, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90(3):537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 5.Schilling G, Becher MW, Sharp AH, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8(3):397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- 6.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38(3):357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins BG, Koroshetz WJ, Beal MF, et al. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology. 1993;43(12):2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Sanjuan I, Bates GP. The importance of integrating basic and clinical research toward the development of new therapies for Huntington disease. J Clin Invest. 2011;121(2):476–483. doi: 10.1172/JCI45364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SE, Lin CL, Hsu CH, et al. Treatment with a herbal formula B401 enhances neuroprotection and angiogenesis in the R6/2 mouse model of Huntington’s disease. Drug Des Dev Ther. 2015;9:887–900. doi: 10.2147/DDDT.S78015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beal MF. Mitochondria, free radicals and neurodegeneration. Curr Opin Neurobiol. 1996;6(5):661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 11.Portera-Cailliau C, Hedreen JC, Price DL, et al. Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J Neurosci. 1995;5(Pt 2):3775–3787. doi: 10.1523/JNEUROSCI.15-05-03775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangiarini L, Sathasivam K, Seller M, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87(3):493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 13.Chien CT, Fan SC, Lin SC, et al. Glucagon-like peptide-1 receptor agonist activation ameliorates venous thrombosis-induced arteriovenous fistula failure in chronic kidney disease. Thromb Haemost. 2014;112(5):1051–1064. doi: 10.1160/TH14-03-0258. [DOI] [PubMed] [Google Scholar]
- 14.Gutekunst CA, Li SH, Yi H, et al. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J Neurosci. 1999;19(7):2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Hickey MA, Chesselet MF. Apoptosis in Huntington’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):255–265. doi: 10.1016/S0278-5846(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen XC, Zhu YG, Zhu LA, et al. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473(1):1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Li S, Yang H, et al. A novel liquid chromatography/tandem mass spectrometry based depletion method for measuring red blood cell partitioning of pharmaceutical compounds in drug discovery. Rapid Commun Mass Spectrom. 2005;19(2):250–254. doi: 10.1002/rcm.1777. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante RJ, Gutekunst CA, Persichetti F, et al. Heterogeneous topographic and cellular distribution of huntingtin expression in the normal human neostriatum. J Neurosci. 1997;17(9):3052–3063. doi: 10.1523/JNEUROSCI.17-09-03052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 21.Wyttenbach A, Sauvageot O, Carmichael J, et al. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11(9):1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 22.Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16(6):653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatton WG, Olanow CW. Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta. 1999;1410(2):195–213. doi: 10.1016/s0005-2728(98)00167-4. [DOI] [PubMed] [Google Scholar]