Abstract
Secondary hyperalgesia is believed to be a key feature of “central sensitization” and is characterized by enhanced pain to mechanical nociceptive stimuli. The aim of the present study was to characterize, using EEG, the effects of pinprick stimulation intensity on the magnitude of pinprick-elicited brain potentials [event-related potentials (ERPs)] before and after secondary hyperalgesia induced by intradermal capsaicin in humans. Pinprick-elicited ERPs and pinprick-evoked pain ratings were recorded in 19 healthy volunteers, with mechanical pinprick stimuli of varying intensities (0.25-mm probe applied with a force extending between 16 and 512 mN). The recordings were performed before (T0) and 30 min after (T1) intradermal capsaicin injection. The contralateral noninjected arm served as control. ERPs elicited by stimulation of untreated skin were characterized by 1) an early-latency negative-positive complex peaking between 120 and 250 ms after stimulus onset (N120-P240) and maximal at the vertex and 2) a long-lasting positive wave peaking 400–600 ms after stimulus onset and maximal more posterior (P500), which was correlated to perceived pinprick pain. After capsaicin injection, pinprick stimuli were perceived as more intense in the area of secondary hyperalgesia and this effect was stronger for lower compared with higher stimulus intensities. In addition, there was an enhancement of the P500 elicited by stimuli of intermediate intensity, which was significant for 64 mN. The other components of the ERPs were unaffected by capsaicin. Our results suggest that the increase in P500 magnitude after capsaicin is mediated by facilitated mechanical nociceptive pathways.
Keywords: capsaicin, secondary hyperalgesia, central sensitization, pinprick, evoked potentials
cutaneous tissue injury is often associated with the development of increased pain sensitivity in the area of actual tissue injury (referred to as primary hyperalgesia) and in the surrounding uninjured skin (referred to as secondary hyperalgesia).
A hallmark of secondary hyperalgesia is enhanced pain to mechanical nociceptive stimuli (e.g., pinprick stimuli; Ali et al. 1996; Magerl et al. 1998; Raja et al. 1984). It can be induced experimentally by activating nociceptors in a sustained and intense fashion, for example, with intradermal injection or topical application of capsaicin, a substance that selectively activates primary nociceptive afferents via the TRPV1 receptor (LaMotte et al. 1991; Magerl et al. 1998, 2001; Ziegler et al. 1999). Some studies suggest that secondary pinprick hyperalgesia is primarily mediated by capsaicin-insensitive Aδ fibers, which include high-threshold mechanoreceptors (HTM) and type I A-fiber mechano-heat nociceptors (AMH-I) (Magerl et al. 2001; Ziegler et al. 1999), and results from “central sensitization” (Baumann et al. 1991; LaMotte et al. 1991; Latremoliere and Woolf 2009; Simone et al. 1991), which is defined by the International Association for the Study of Pain (IASP) as “increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input” (Loeser and Treede 2008).
Experimentally induced secondary pinprick hyperalgesia seems very similar to the observed hyperalgesia in patients with pain following lesions of the peripheral and central nervous systems (Jensen and Finnerup 2014). Until now the measurement of pinprick hyperalgesia has relied predominantly on the subjective report of pain intensity. It would thus be of great value to have a noninvasive clinical and research laboratory tool that is able to reliably assess sensitization of mechanical nociceptive pathways. One of these tools could be the recording of pinprick event-related brain potentials (ERPs).
Recently, Iannetti et al. (2013) recorded for the first time ERPs elicited by mechanical pinprick stimulation before and after intradermal injection of capsaicin in the area of secondary hyperalgesia in healthy volunteers. The mechanical pinprick stimulation was performed by quickly applying and removing a sharp-tipped probe (0.25-mm diameter). The probe could slide freely inside a tube handheld by the experimenter. A calibrated weight on top of the load was used such that the normal force was 128 mN when the needle was maintained against the skin. The stimulus elicited a biphasic ERP waveform (N120 and P240 waves) with latencies compatible with the conduction of myelinated Aβ- or Aδ-fiber afferents. After capsaicin injection, they observed an enhancement of both the pain ratings and the magnitude of the pinprick-evoked N120 wave.
However, only one force was used in this seminal study, leaving unexplored the possibility that pinprick-evoked potentials are modulated differently depending on the applied force, both in untreated skin and in the area of secondary hyperalgesia. Therefore, the aim of the present study was to characterize the ERPs elicited by different intensities of pinprick stimulation before and after inducing secondary hyperalgesia. Different weights were used to deliver a range of stimulation intensities extending between 16 and 512 mN. Secondary hyperalgesia was induced by intradermal injection of capsaicin.
METHODS
Participants
Nineteen healthy volunteers took part in the experiment (9 men and 10 women; aged 19–29 yr, mean ± SD age: 23.6 ± 2.3 yr; 18 right handed, 1 left handed). Approval for the experiment was obtained from the local Ethical Committee. All participants signed an informed consent form.
Experimental Design
Participants were comfortably seated in a reclining chair with their arms resting on armrests. Two concentric circles were drawn on the left and right ventral forearms, with diameters of 1.5 cm and 2.5 cm, respectively.
Intradermal Injection of Capsaicin
A 10 mM solution of capsaicin (40 μg capsaicin dissolved in 12.5 μl 16% Tween 80 in normal saline; Magerl et al. 2001) was injected in the dermis at the center of the circles drawn on the ventral forearm of the nondominant arm (Fig. 1). Immediately after the injection, participants were asked to rate the magnitude of the capsaicin-induced pain every 10 s for the first minute and then every 30 s until 15 min after injection, using a numeric rating scale (NRS) ranging from 0 (no pain) to 100 (most intense pain imaginable). A placebo saline injection was not included in this study, as the aim was not to ascertain whether capsaicin induces hyperalgesia but rather to examine the relationship between this well-known phenomenon and pinprick ERPs.
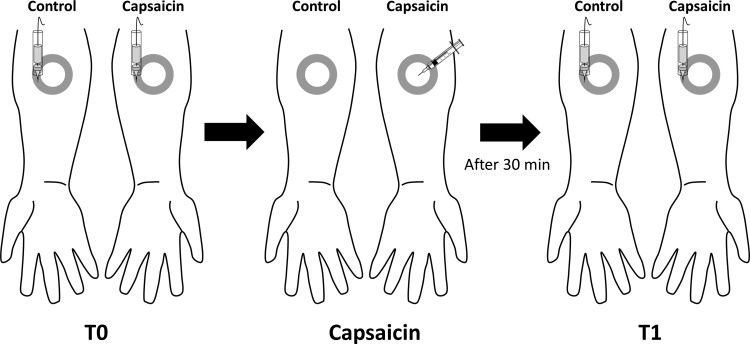
Fig. 1.
Experimental setup. The effect of capsaicin on the responses elicited by 6 different intensities of mechanical pinprick stimulation (16, 32, 64, 128, 256, and 512 mN) was assessed at 2 different time points: before (T0) and 30 min after (T1) intradermal capsaicin injection. The pinprick stimuli were delivered within a 1-cm-wide area defined by 2 circles drawn on the forearm (gray circle), i.e., at a distance ranging between 1.5 and 2.5 cm from the site of capsaicin injection on both the capsaicin-treated arm and the contralateral arm, which served as control.
Pinprick Stimulation
Pinprick-elicited ERPs were recorded before (T0) and 30 min after (T1) capsaicin injection, with six different intensities of mechanical pinprick stimulation: 16, 32, 64, 128, 256, and 512 mN. The pinprick stimuli were delivered to the capsaicin-treated and control arms within the 1-cm-wide area defined by the two circles drawn on the forearms at a distance ranging between 1.5 and 2.5 cm from the site of capsaicin injection on the treated arm (Fig. 1).
For each intensity of pinprick stimulation and for each arm, a total of 20 stimuli were administered in separate blocks. Stimulation of the control and capsaicin-treated arms was performed in an alternating fashion (block-by-block design), but the capsaicin-treated arm was always stimulated first. The order of the different stimulation intensities was balanced across participants. The stimuli were delivered with a random interstimulus interval ranging from 7 to 10 s. To avoid sensitization of the stimulated skin, the target of the pinprick stimulus was displaced by the experimenter after each stimulus.
The pinprick stimuli were generated by a set of six probes consisting of a cylindrical stainless steel 0.25-mm-diameter flat tip (uniform tip geometry) mounted on a plastic rod (MRC Systems, Heidelberg, Germany). The plastic rod moved freely inside a polished handheld stainless steel tube. Each pinprick stimulus was delivered by applying the needle in a vertical direction onto the skin and moving the tube downward and upward with a total duration of ∼1 s. The normal force applied by the needle against the skin (16, 32, 64, 128, 256, and 512 mN) was adjusted by placing inside the tube and on top of the plastic rod one of a set of six calibrated cylindrical weights.
Instead of applying the pinprick stimulus as fast as possible as in the study of Iannetti et al. (2013), the pinprick stimulus was applied and removed slowly and maintained against the skin for at least 1 s. The reason for doing this is that the actual force that is applied onto the skin is dependent on the velocity at which the stimulus is delivered. To be sure that the actual delivered force is the same as the planned force, the pinprick stimulus should be applied onto the skin in a slow fashion (www.mrc-systems.de/englisch/products/pinprick.html).
Intensity of Perception
The effect of capsaicin on the intensity of perception elicited by pinprick stimulation was assessed by asking participants to rate the intensity of “pinprick pain” on a NRS ranging from 0 (no pain) to 100 (most intense pain imaginable). Participants were instructed to distinguish the perception of pain (i.e., sharp or slightly pricking or burning sensation) from touch or pressure by rating the stimulus > 0 (Rolke et al. 2006). Subjects were free to use integers as well as fractions. Ratings of the intensity of perception were obtained 3 s after each individual pinprick stimulus.
Electroencephalography
The electroencephalogram (EEG) was recorded with five Ag-AgCl electrodes (Easycap; Brain Products) mounted in an elastic electrode cap and located at Fz, Cz, Pz, T3, and T4 according to the International 10-10 system. Linked earlobes (A1A2) served as reference. Eyeblinks were recorded with a pair of surface electrodes placed at the upper and lower sides of the right eye. Impedance was kept <5 kΩ for all leads. The signals were amplified and digitized at a 200-Hz sampling rate with a BrainAmp system (Brain Products). During the recording, participants were instructed to keep their gaze fixed on a reference point at a distance of ∼1 m at eye level and to sit as still as possible without making any movements.
The experiment was carried out with a modified set of pinprick probes (MRC Systems). The stimulators were equipped with an electrically conductive connection from the needle tips via the internal sliding body to an external cable. As soon as the probe came in contact with the skin, the electrical circuit detected the change in circuit impedance and generated a trigger signal.
Data Analysis
Intensity of perception.
Intensity ratings of capsaicin- and pinprick-evoked pain were transformed into decadic logarithmic values to obtain a secondary normal distribution. A small constant of 0.1 was added to pain ratings to avoid a loss of zero values (Magerl et al. 1998).
To characterize the effect of capsaicin on the intensity of the percept elicited by pinprick stimulation, a general linear model (GLM) repeated-measures ANOVA analysis (Statistica 4.5) was performed on log-transformed pain ratings with three within-subject factors: time (2 levels: T0, T1), treatment (2 levels: control arm, capsaicin arm), and stimulation intensity (6 levels: 16, 32, 64, 128, 256, 512 mN). The level of significance was set at P < 0.05 (2 sided). Post hoc comparisons were performed with paired-sample t-tests. P values were corrected for the number of tests with the Scheffé test.
To illustrate parts of the outcomes of the statistical procedure pain ratings were normalized 1) to baseline by building the difference between log-transformed pain ratings to pinprick stimuli (averaged across stimulus intensities) before and after capsaicin injection and 2) to unconditioned control site by building the difference of baseline-normalized ratings between test and contralateral control sites (separately for each stimulus intensity). Building the difference of log-transformed data is equivalent to building a ratio of the nontransformed pain ratings.
Pinprick-elicited ERPs.
The signals were analyzed offline with Brain Vision Analyzer v. 1.05 (Brain Products). After DC correction and filtering with a 0.5- to 30-Hz zero-phase Butterworth band-pass filter and a 50-Hz notch filter, the continuous EEG recordings were segmented into epochs extending from −500 to +1,500 ms relative to stimulus onset. Epochs containing ocular artifacts (i.e., eyeblinks) were corrected with the Gratton-Coles method. The method uses a linear regression to estimate the relationship between EOG and EEG recordings (Gratton et al. 1983). After baseline correction (reference interval: −500 to 0 ms), segments with amplitude values exceeding ±100 μV were rejected, as these were likely to be contaminated by artifacts. Average pinprick-evoked ERPs were computed for each participant, time point (T0 and T1), stimulation site (capsaicin and control), and intensity (16, 32, 64, 128, 256, 512 mN). In one subject, the EEG signal of one condition (the 128 mN stimulus intensity on the control arm at T0) contained too many artifacts. As a consequence, after artifact rejection no trials remained for calculating a subject-specific ERP for that condition. Because it involved only one condition, we decided to keep this subject in the analyses for the remaining conditions.
Statistical analysis of the obtained average waveforms was performed with MATLAB 2012b. Because we had no a priori assumption regarding the ERPs that would be elicited by the different intensities of stimulation and the effect of capsaicin on these ERPs, we used a nonparametric cluster-based permutation approach for statistical testing (Maris and Oostenveld 2007), also commonly used in neuroimaging (Bullmore et al. 1999; Nichols and Holmes 2002). The technique assumes that true neural activity will tend to generate signal changes over contiguous time points (Groppe et al. 2011). First, the ERP waveforms of the different conditions were compared by means of a point-by-point paired-sample t-test or F-statistic. Then, clusters of contiguous time points above the critical t- or F-value for a parametric two-sided test were identified, and an estimate of the magnitude of each cluster was obtained by computing the sum of the t-values or F-values constituting each cluster (cluster-level statistic). Random permutation testing (2,000 times) of the subject-specific ERP waveforms of the different conditions (performed independently for every subject) was then used to obtain a reference distribution of maximum cluster magnitude. Finally, the proportion of random partitions that resulted in a larger cluster-level statistic than the observed one (i.e., P value) was calculated. Clusters in the observed data were regarded as significant if they had a magnitude exceeding the threshold of the 95th percentile of the permutation distribution when the F-statistic was used and the 97.5th and 2.5th percentiles when the t-statistic was used (corresponding to a 2-sided test).
EFFECT OF STIMULATION INTENSITY.
For each subject, the baseline recordings (T0) were used to compute separate ERP waveforms for each stimulation intensity, averaged across stimulation sides (control and capsaicin-treated arms). The effect of stimulation intensity on the ERP waveforms was assessed with a point-by-point F-test with six levels (16, 32, 64, 128, 256, 512 mN).
EFFECT OF CAPSAICIN SENSITIZATION.
For each subject, separate ERP waveforms were computed for each session (before vs. after capsaicin treatment; T0 vs. T1) and arm (control vs. capsaicin-treated arm), averaged across the different stimulation intensities. Subsequently, difference waveforms were computed, assessing the change in ERP waveform after vs. before treatment at the control arm (control armT1 − control armT0) and at the capsaicin-treated arm (capsaicin armT1 − capsaicin armT0). The main effect of treatment on the ERP waveforms was then assessed with a point-by-point t-test for dependent samples applied on the difference waveforms (control vs. capsaicin arm).
Finally, to test whether capsaicin sensitization exerted a differential effect on the responses elicited by different intensities of stimulation, the same analysis was performed using the ERP waveforms obtained for each stimulation intensity.
RESULTS
Capsaicin-Induced Pain
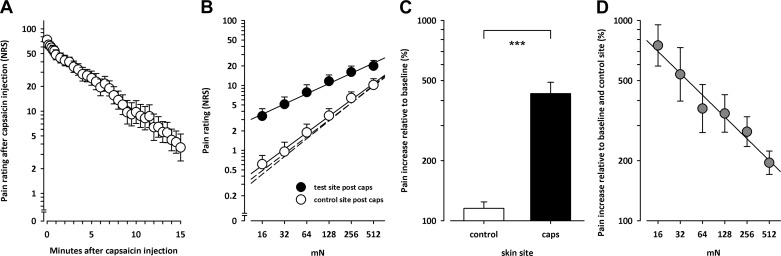
Capsaicin injection caused a mean peak pain sensation of 73.7/100 (log 1.8672 ± 0.0218, mean ± SE; Fig. 2A). Immediately after the injection, the pain sensation decayed log-linearly to 3.6/100 NRS (log 0.5589 ± 0.1638) at the end of the 15-min observation period.
Fig. 2.
A: time course of pain sensation evoked by intradermal capsaicin injection. Capsaicin-evoked pain decayed log-linearly to 3.6/100 after the 15-min observation period. NRS, numeric rating scale. B: stimulus-response (S/R) functions to pinprick elicited pain after capsaicin injection. The S/R function for punctate probes did not differ between test sites and was approximately linear in log-log coordinates in untreated skin (short dashed lines; symbols are omitted for clarity). After capsaicin injection there was a parallel upward shift of this function adjacent to the capsaicin injection site (secondary hyperalgesia; filled circles). There was no hyperalgesia adjacent to the unstimulated contralateral control site (open circles). Each circle represents average pain ratings across 19 subjects on a 100-point numerical rating scale. C: pain to pinpricks was increased by 331% adjacent to the capsaicin injection site (filled bars), whereas pain at the contralateral control site was largely unchanged [+15%, nonsignificant (ns); open bars]. Pain ratings were normalized to individual baseline. D: the relative pain increase after capsaicin injection depends on stimulus strength of the punctate probes (P < 0.001). Pain ratings were normalized to individual baseline as well as to individual control site. ***Significant pain increase after capsaicin injection (post hoc Scheffé). Mean ± SE values across 19 subjects.
Pinprick Pain Perception Before and After Capsaicin Sensitization
Pain evoked by the pinprick stimuli increased exponentially with stimulus intensities in untreated and hyperalgesic skin [ANOVA intensity, F(5,90) = 159.7, P < 0.001], resulting in a linear stimulus-response (S/R) function in double-logarithmic space (Fig. 2B).
At baseline, i.e., before capsaicin injection, the S/R function to pinprick stimuli did not differ between test sites [not significant (ns); dashed lines in Fig. 2B]. After capsaicin injection there was an upward shift of the S/R function to pinprick stimuli by 331% (log 0.6342 ± 0.0565; P < 0.001) in the area adjacent to the capsaicin injection site, suggesting the induction of secondary hyperalgesia [time × treatment interaction: F(1,18) = 84.7, P < 0.001; Fig. 2B], whereas pain ratings at the contralateral control site were largely unaffected by capsaicin injection (+15%, log 0.0614 ± 0.0328, ns; Fig. 2C).
The degree of hyperalgesia after capsaicin injection depended on the intensity of the pinprick stimulus [time × treatment × intensity interaction: F(5,90) = 5.75, P < 0.001; Fig. 2D]. Whereas the increase after capsaicin injection at 16 mN was +651% (log 0.8755 ± 0.1032; P < 0.001) the pain enhancement decreased log-linearly to +95% (log 0.2897 ± 0.0583) at 512 mN (ns, post hoc Scheffé; Fig. 2D).
Pinprick ERPs Elicited from Untreated Skin Before Capsaicin Sensitization
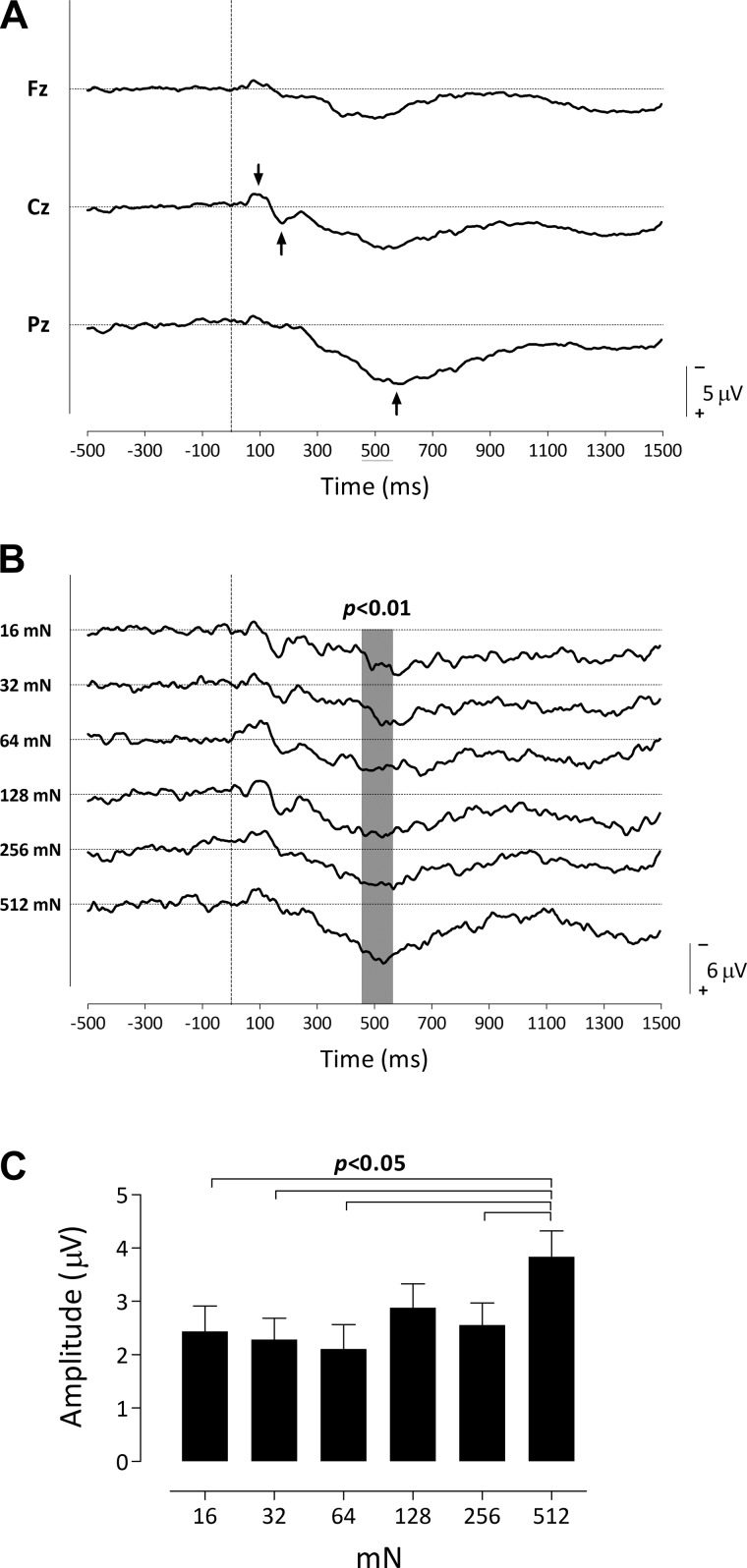
Figure 3A shows the group-level average pinprick-evoked waveforms at baseline (T0) merged for both arms and all intensities, at frontal (Fz), central (Cz), and posterior (Pz) electrodes. In these waveforms one can visually identify 1) an early-latency negative-positive complex (N120 and P240 waves) that is maximal at Cz and 2) a long-lasting positive wave peaking ∼400–600 ms after stimulus onset and maximal at Pz (P500). The cluster-based permutation testing revealed a significant difference in ERP activity across intensity at Cz between 455 and 570 ms (P < 0.01; Fig. 3B). Figure 3B shows the group-level average pinprick-evoked waveforms for each intensity (16, 32, 64, 128, 256, 512 mN) at Cz. Figure 3C shows the mean (and SE) change in amplitude relative to baseline of the signals obtained within the time window 455 to 570 ms. Post hoc testing revealed that only the P500 elicited by the 512-mN pinprick stimulus was significantly larger than the P500 elicited by the 16-, 32-, 64-, and 256-mN stimuli (P < 0.05; paired t-tests, uncorrected). Figure 4, however, suggests that the relationship between pinprick pain and the P500 at baseline can be approximated by a linear regression.
Fig. 3.
A: pinprick evoked event-related potentials (ERPs) at the frontal (Fz), central (Cz), and posterior (Pz) electrodes (n = 19). The 2 arrows at the Cz waveform indicate the early-latency N-P complex that is maximal at this electrode, whereas the arrow at the Pz waveform indicates the large positive wave maximal at Pz. B: pinprick ERPs elicited by different intensities of stimulation observed at Cz (n = 18). The waveforms show the group-level average ERP waveforms (averaged across the 2 arms) of the signals measured before capsaicin treatment from Cz vs. A1A2, after pinprick stimulation with 16, 32, 64, 128, 256, and 512 mN normal force. Gray shading indicates the significant differences across stimulation intensity. C: group-level mean (and SE) of the average ERP amplitude within the cluster identified in B, as a function of stimulation intensity.
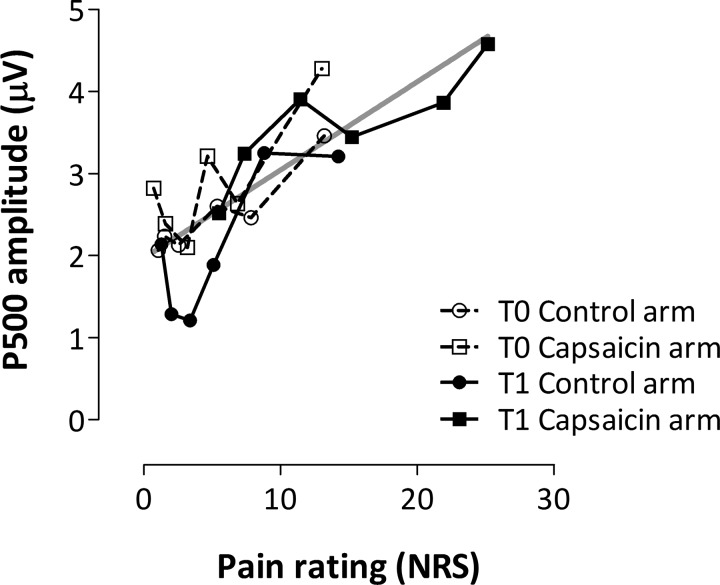
Fig. 4.
Relationship between pain ratings and P500 magnitude. Group-level average (untransformed) pain ratings and P500 magnitude (mean value of the cluster shown in Fig. 3B) for each pinprick intensity (16–512 mN represented as symbols in successive order) within each condition (n = 18). Gray line shows a linear regression calculated across all 4 conditions (r2 = 0.67, P < 0.0001).
Effect of Capsaicin Sensitization on Pinprick-Elicited ERPs
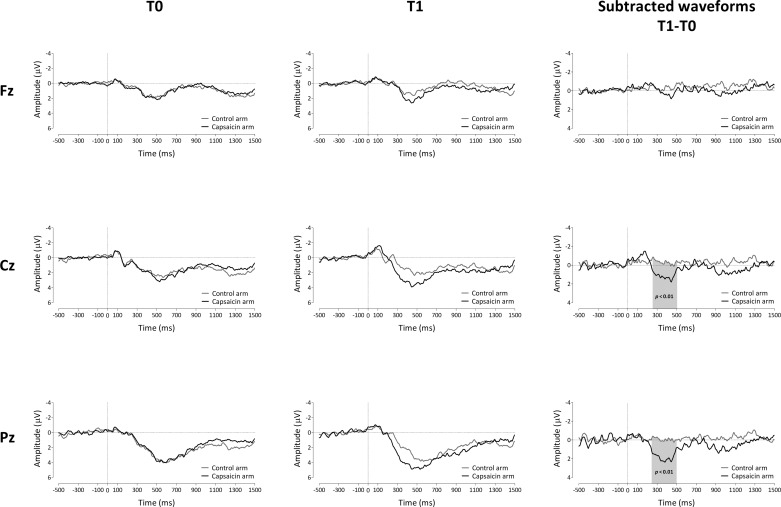
Figure 5 shows the group-level average ERP waveforms (averaged across stimulation intensities) obtained at the control and capsaicin-treated arms, before (T0) and after (T1) treatment, for the three midline electrodes Fz, Cz, and Pz. The cluster-based permutation test revealed a significant difference between the control and capsaicin-treated arms after capsaicin treatment between 255 and 505 ms relative to stimulus onset at Cz (P < 0.01) and between 245 and 500 ms at Pz (P < 0.01; Fig. 5). This increase may be reflected in a shift of the relationship between P500 and pinprick pain (Fig. 4) along the approximated regression line.
Fig. 5.
Effect of capsaicin on the ERPs elicited by pinprick stimulation applied in the area of secondary hyperalgesia (n = 19). Left and center: group-level average ERP waveforms of the signals measured from Fz, Cz, and Pz vs. A1A2, before capsaicin injection (T0; left) and 30 min after capsaicin treatment (T1; center) for both the control arm and the capsaicin-treated arm (black) and averaged across pinprick intensities. Right: group-level average subtracted (post − pre) waveforms for the 2 arms (capsaicin treated and control). Gray shading indicates the time window for which the 2 arms significantly differ after capsaicin treatment.
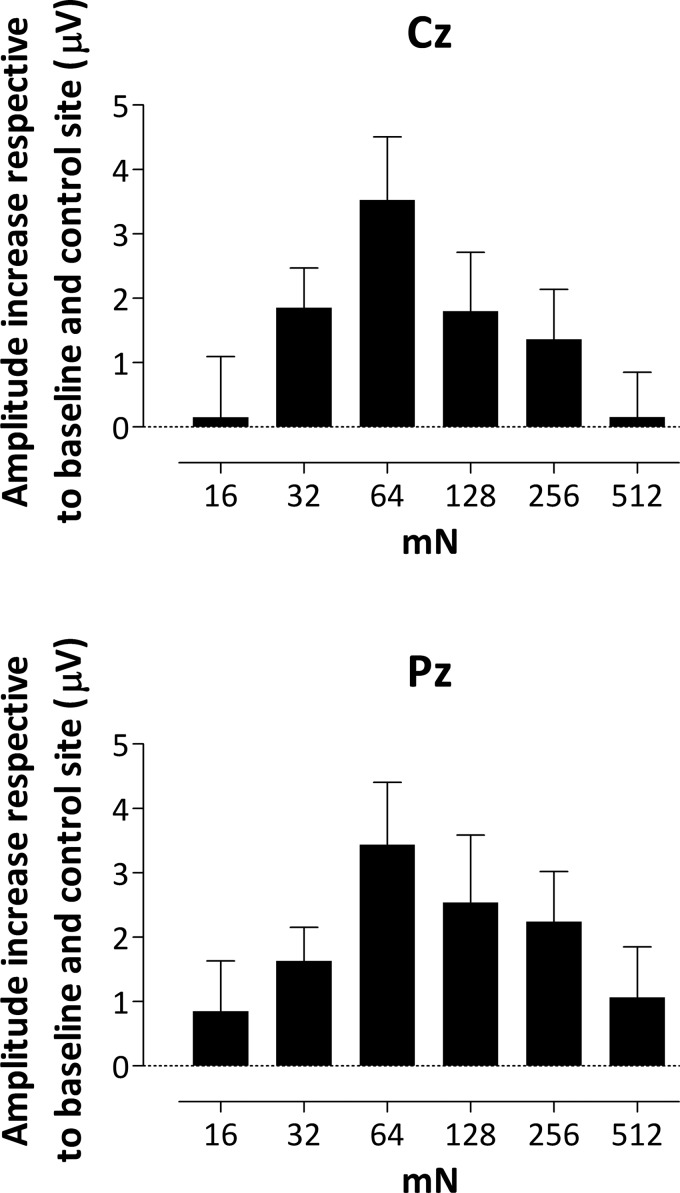
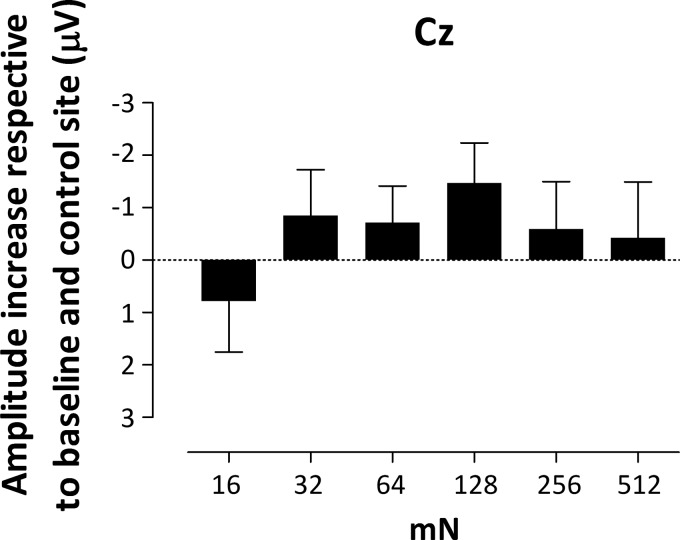
Figure 6 shows the mean (and SE) amplitude increase respective to baseline and control site within the cluster identified at Cz and Pz electrodes for each intensity.
Fig. 6.
Group-level mean (and SE) ERP activity representing the difference between the 2 arms (subtracted from baseline) of the mean activity calculated within the significant time window (as shown in Fig. 5) identified at Cz and Pz for each intensity (n = 18 for 128 mN). Note that the increase in amplitude observed after capsaicin treatment is maximal at intermediate intensities.
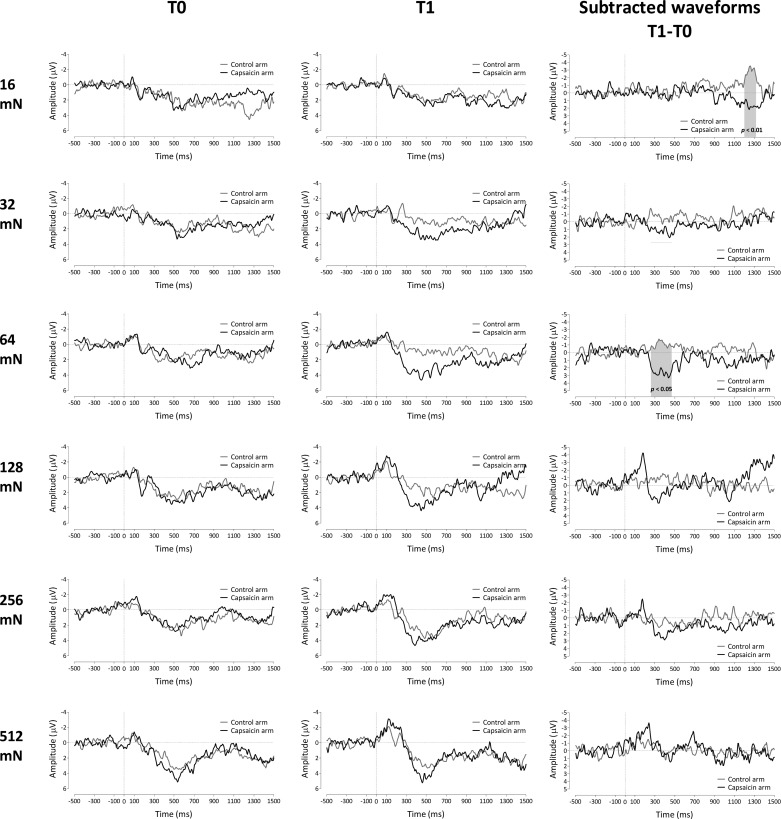
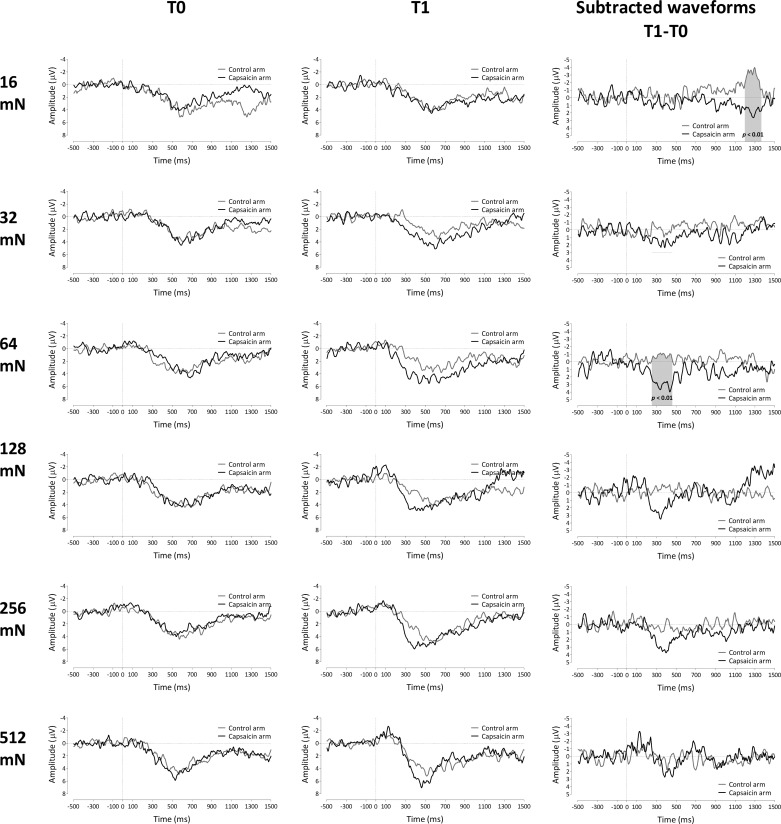
The results of the permutation tests performed on the difference waveforms (T1 − T0) of both arms for each stimulus intensity are shown in Fig. 7 (for electrode Cz) and Fig. 8 (for electrode Pz). For the lowest stimulation intensity (16 mN), the permutation test revealed a significant difference between control and capsaicin-treated arms between 1,200 and 1,325 ms (electrode Cz) and between 1,195 and 1,370 ms (electrode Pz) relative to stimulus onset (P < 0.01). For the intermediate stimulation intensity of 64 mN, the permutation test revealed a significant difference between 260 and 475 ms at electrode Cz (P < 0.05) and 250 and 465 ms at electrode Pz (P < 0.01). There were no statistically significant differences between capsaicin-treated and control arms for the 32-, 128-, 256-, and 512-mN pinprick intensities.
Fig. 7.
Left and center: group-level average ERP waveforms of the signals measured from Cz vs. A1A2, before capsaicin injection (T0; left) and 30 min after capsaicin treatment (T1; center) for both the control arm and the capsaicin-treated arm for each pinprick intensity. Right: group-level average subtracted (T1 − T0) waveforms for the 2 arms (capsaicin treated and control) and each pinprick intensity. Gray shading indicates the time window for which the 2 arms significantly differ after capsaicin treatment (n = 18 for 128 mN).
Fig. 8.
Left and center: group-level average ERP waveforms of the signals measured from Pz vs. A1A2, before capsaicin injection (T0; left) and 30 min after capsaicin treatment (T1; center) for both the control arm and the capsaicin-treated arm for each pinprick intensity. Right: group-level average subtracted (T1 − T0) waveforms for the 2 arms (capsaicin treated and control) and each pinprick intensity. Gray shading indicates the time window for which the 2 arms significantly differ after capsaicin treatment (n = 18 for 128 mN).
Post Hoc Analysis
In contrast to Iannetti et al. (2013), we did not observe a significant increase of the early-latency N120 wave after capsaicin. This could have been the consequence of the cluster-based statistical test used. For the cluster-based test statistic we calculated the maximum cluster level statistic (Maris and Oostenveld 2007). The reason for using the maximum cluster level statistic is that it has been shown that this test statistic is the most sensitive statistic in detecting statistically significant differences between conditions compared with other non-cluster-based test statistics (see Supplementary Material in Maris and Oostenveld 2007).
Moreover, the maximum cluster level statistic controls the family-wise error rate (FWER; i.e., type I error) for all clusters (from largest to smallest); however, it does so at the expense of a reduced sensitivity for smaller clusters (reduced compared with a statistical test that is specific for the second, third, etc. largest cluster-level statistic; Maris and Oostenveld 2007). Therefore, it is possible that the sensitivity of the test to detect the cluster covering the latency window of the N120 peak was reduced because of the larger cluster between 250 and 500 ms.
To test whether in fact there was a significant increase of the N120 peak after capsaicin, we calculated baseline to peak values of the N120 amplitude for both arms at all intensities at Cz and performed a GLM repeated-measures ANOVA analysis (SPSS 18; SPSS, Chicago, IL) using three within-subject factors: time (2 levels: T0, T1), treatment (2 levels: control arm, capsaicin arm), and stimulation intensity (6 levels: 16, 32, 64, 128, 256, 512 mN). The assumption of sphericity was tested with Mauchly's test of sphericity. In those cases where the data violated the assumption of sphericity, F-values were corrected with the Greenhouse-Geisser procedure. The level of significance was set at P < 0.05 (2-sided). The ANOVA revealed no significant interactions. Figure 9 shows the group-level mean (and SE) N120 amplitude increase respective to baseline and control site after capsaicin injection for all intensities.
Fig. 9.
Group-level mean (and SE) N120 amplitude increases after capsaicin injection relative to baseline and control site for all intensities (n = 18).
DISCUSSION
The aim of the present study was to characterize pinprick ERPs elicited with a range of stimulation intensities (16–512 mN) before and after intradermal injection of capsaicin. Before capsaicin sensitization, pinprick stimulation elicited an early-latency N120-P240 complex that was maximal at the scalp vertex (electrode Cz) and similar to the ERP components reported by Iannetti et al. (2013). In addition to this early-latency component, pinprick stimulation also elicited a long-lasting positive deflection peaking between 450 and 600 ms (P500), which amplitude was maximal more posterior than the early-latency response and whose magnitude was significantly dependent on stimulation intensity.
Intradermal capsaicin injection enhanced the intensity of pinprick pain and exerted a significant effect on the pinprick ERP waveforms. Most interestingly, the effect of capsaicin sensitization on the pinprick ERP waveforms was dependent on the intensity of pinprick stimulation. Pinprick ERPs elicited by stimulating the area of secondary hyperalgesia were enhanced (between 250 and 500 ms) compared with baseline and control site. This enhancement was most pronounced at intermediate intensities and only significant for 64 mN.
Primary Afferents Mediating Pinprick-Elicited Pain and ERPs in Untreated Skin
The present data on pinprick-evoked pain are consistent with previously reported data in animals and humans. In primates, it has been shown that high-intensity mechanical punctate stimuli are capable of activating both A- and C-fiber mechanosensitive nociceptors (Slugg et al. 2000, 2004; see also Garell et al. 1996 for similar findings in cat). Both types of nociceptors exhibited stronger discharge rates when stronger forces were applied to the skin (Slugg et al. 2000). However, the stimulus-response function of A fibers was steeper than that of C fibers (Slugg et al. 2000).
Using a 0.4-mm-diameter cylindrical probe, Slugg et al. (2004) also showed that C-fiber nociceptors responded more vigorously than A-fiber nociceptors to a 20-mN stimulus, whereas A-fiber nociceptors responded more vigorously than C-fiber nociceptors to a 100-mN stimulus. This suggests that C-fiber afferents could respond more strongly relative to A fibers to pinprick stimuli of low intensity whereas A-fiber afferents could respond more strongly relative to C-fiber afferents to high-intensity pinprick stimuli.
In human volunteers, both Ziegler et al. (1999) and Magerl et al. (2001) showed a monotonic increase in pain perception to pinprick stimuli of increasing force delivered with a 0.25-mm-diameter cylindrical probe. The same authors also demonstrated that after the conduction of myelinated afferents was blocked via prolonged pressure to the superficial branch of the radial nerve pain elicited by pinprick stimuli was substantially reduced (75%). The remaining C fiber-mediated pain still showed a relationship with stimulus intensity, but this relationship was reduced compared with when the conduction of A fibers was intact (Ziegler et al. 1999). Moreover, Magerl et al. (2001) showed that pretreatment of the skin with capsaicin in order to induce a denervation of capsaicin-sensitive epidermal free nerve endings leads to a significant but small (32%) reduction of pinprick pain within the capsaicin-treated skin, suggesting that the pain elicited by pinprick stimuli is primarily mediated by capsaicin-insensitive afferents. Taken together, these human studies indicate that pinprick pain is primarily mediated by capsaicin-insensitive mechanosensitive Aδ-fiber afferents such as AMH-I and HTM.
The pinprick ERPs in the present study are characterized by an early-latency N120 and P240 wave and a long-lasting positive wave peaking ∼400–600 ms after stimulus onset (P500). The early-latency biphasic waveform (N120-P240) is similar to that described by Iannetti et al. (2013). Its latency (N120: 111 ± 8 ms; P240: 245 ± 17 ms) is compatible with the conduction velocity of myelinated fibers, i.e., tactile Aβ fibers and/or fast-conducting nociceptive Aδ fibers, in particular, AMH-I (25.4 ± 15.7 m/s, extending between 8.2 and 70.0 m/s) (Treede et al. 1998; but see also Djouhri and Lawson 2004).
In contrast to perception, there was no clear parametric positive relationship between the magnitude of the early-latency N120-P240 or P500 and the intensity of pinprick stimulation at baseline. However, visual inspection of the waveforms in Fig. 3B seems to suggest that the P240 component of the early-latency complex decreases when the intensity of the pinprick stimulus increases. In contrast, the P500 was significantly increased at the highest pinprick intensity compared with lower intensities (Fig. 3C) and, importantly, its magnitude was related to the intensity of perception (Fig. 4). This relationship may become more apparent when more elaborate analysis techniques are used, such as time-frequency analyses or dipole source analyses, but because of the small number of stimulus repetitions and electrodes these are not appropriate for the present data set. The fact that, at the highest intensity of stimulation (512 mN), the P240 is almost absent but the P500 is maximal might suggest that the two brain responses are mediated by different afferents. A possible explanation could be that the N120-P240 reflects activity mediated by nonnociceptive Aβ fibers and that the P240 is masked by an overlapping Aδ-mediated negative wave (van den Broeke and Mouraux 2014) that would become more prominent at higher intensities of stimulation.
Interestingly, the signal deflection observed around 1,200-1,300 ms when the untreated skin was stimulated with the 16-mN intensity may reflect brain activity related to the activation of unmyelinated mechanosensitive C fibers, as its latency is compatible with the conduction velocity of these fibers.
Primary Afferents Mediating Pinprick Hyperalgesia and Increased Pinprick-Elicited ERPs After Capsaicin Injection
In human volunteers, it has been shown that secondary hyperalgesia is induced by activation of capsaicin-sensitive C fibers, whereas the increase in pain to pinprick stimuli is mainly mediated by capsaicin-insensitive Aδ fibers (Magerl et al. 2001; Ziegler et al. 1999).
Pinprick stimulation applied in the area of secondary hyperalgesia only increased the magnitude of the P500. The amplitude increase was present at midline electrodes Cz and Pz, suggesting that it could be related to the P2 wave of vertex potentials (van den Broeke and Mouraux 2014) and/or a later P3 component (Legrain et al. 2002; Michie et al. 1987; Siedenberg and Treede 1996; Yamaguchi and Knight 1991).
Notably, this increase was nonlinearly related (inverted U-shape) with pinprick intensity and was only significant for the 64-mN stimulus. Contrasting with this relationship, the increase in pinprick pain was present at all intensities and maximal at the lowest intensities of stimulation. This dissociation suggests that the increase in P500 magnitude reflects only a fraction of the cortical activity generated by the pinprick stimulus. At baseline, at the lower intensities the activation of the mechanosensitive afferents responsible for the P500 wave is not sufficient to elicit a reliable ERP P500; however, the signal-to-noise ratio improves after capsaicin injection when the Aδ-fiber pathway is facilitated, with exception of the 16-mN stimulus. Conversely, the lack of increase at higher intensities might be due to a ceiling effect.
In addition to the P500 increase, there was a significant effect of capsaicin on the ERP waveforms elicited by pinprick stimulation with the 16-mN intensity (Fig. 7 and Fig. 8). The latency of this effect, peaking around 1,200-1,300 ms, suggests that capsaicin injection exerted a significant effect on the responsiveness of mechanosensitive C-fiber afferents, possibly leading to a disappearance of the C fiber-related brain response, as this response was observed only at the control site and at baseline.
Recently, Iannetti et al. (2013) also recorded pinprick ERPs in the area of secondary hyperalgesia induced by intradermal capsaicin injection. They observed an enhancement of pinprick pain as well as an increase of the N120 wave magnitude after capsaicin injection, which was attributed to an amplification of the responses to type I AMH mechano-nociceptive input. However, the enhanced N120 wave could also have been related, at least in part, to an amplification of the responses triggered by nonnociceptive low-threshold mechanoreceptors as has been shown for nonnociceptive vibrotactile stimulation selectively activating Aβ fibers (N120: 129 ± 12 ms; P240: 246 ± 39 ms; van den Broeke and Mouraux 2014) in the area of secondary hyperalgesia after high-frequency electrical stimulation (van den Broeke and Mouraux 2014).
The absence of a similar increase in magnitude of the early-latency N120 wave in the present study as well as the presence of an additional wave (P500) could be explained by differences in the velocity and/or duration of the applied pinprick stimulus. Indeed, in the study of Iannetti et al. (2013) the pinprick stimulus was applied and removed as fast as possible, whereas in the present study the pinprick stimulus was applied more slowly and, most importantly, was maintained against the skin for ∼1 s. The longer duration of the pinprick stimulus in the present study may thus have resulted in a more robust recruitment of mechanosensitive nociceptors. In contrast, very fast application of the pinprick stimuli may have resulted in a predominant activation of nonnociceptive Aβ-fiber afferents.
Conclusions
The present study shows for the first time that pinprick stimulation elicits, besides an early-latency N120-P240 wave, a long-lasting positive wave that peaks ∼400–600 ms after stimulus onset (P500). After capsaicin injection, the magnitude of the P500—but not the earlier N120-P240—was significantly increased when elicited in the area of secondary hyperalgesia, suggesting that the P500 is mediated by facilitated mechanical nociceptive pathways and potentially useful for evaluating the presence of hyperalgesia.
GRANTS
E. van den Broeke is supported by the Belgian National Foundation for Scientific Research (FNRS). A. Mouraux is supported by an ERC “Starting Grant” (PROBING PAIN 336130). T. Klein and R.-D. Treede were supported by Deutsche Forschungsgemeinschaft (DFG; Tr236/19-1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.N.v.D.B and A.M. analyzed data; E.N.v.D.B, A.M., and T.K. interpreted results of experiments; E.N.v.D.B, A.M., and R.-D.T. prepared figures; E.N.v.D.B, A.M., and T.K. drafted manuscript; E.N.v.D.B, A.M., A.H.G., D.B.P., R.-D.T., and T.K. edited and revised manuscript; E.N.v.D.B, A.M., A.H.G., D.B.P., R.-D.T., and T.K. approved final version of manuscript; A.H.G., D.B.P., R.-D.T., and T.K. conception and design of research; A.H.G. and D.B.P. performed experiments.
REFERENCES
- Ali Z, Meyer RA, Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following capsaicin injection in hairy skin. Pain 68: 401–411, 1996. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 66: 212–227, 1991. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer M. Global, voxel and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42, 1999. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev 46: 131–145, 2004. [DOI] [PubMed] [Google Scholar]
- Garell PC, McGillis SL, Greenspan JD. Mechanical response properties of nociceptors innervating feline hairy skin. J Neurophysiol 75: 1177–1189, 1996. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55: 468–484, 1983. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields. I. A critical tutorial review. Psychophysiology 48: 1711–1725, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Baumgärtner U, Tracey I, Treede RD, Magerl W. Pinprick-evoked brain potentials: a novel tool to assess central sensitization of nociceptive pathways in humans. J Neurophysiol 110: 1107–1116, 2013. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 13: 924–935, 2014. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66: 190–211, 1991. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10: 895–926, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain 99: 21–39, 2002. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain 137: 473–477, 2008. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 124: 1754–1764, 2001. [DOI] [PubMed] [Google Scholar]
- Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain 74: 257–268, 1998. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. [DOI] [PubMed] [Google Scholar]
- Michie PT, Bearpark HM, Crawford JM, Glue LC. The effects of spatial selective attention on the somatosensory event-related potential. Psychophysiology 24: 449–463, 1987. [DOI] [PubMed] [Google Scholar]
- Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain 107: 1179–1188, 1984. [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 123: 231–243, 2006. [DOI] [PubMed] [Google Scholar]
- Siedenberg R, Treede RD. Laser evoked potentials: exogenous and endogenous components. Electroencephalogr Clin Neurophysiol 100: 240–249, 1996. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses to spinothalamic tract neurons. J Neurophysiol 66: 228–246, 1991. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Campbell JN, Meyer RA. The population response of A- and C-fiber nociceptors in monkey encodes high-intensity mechanical stimuli. J Neurosci 24: 4649–4656, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli. J Neurophysiol 83: 2179–2191, 2000. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol 80: 1082–1093, 1998. [DOI] [PubMed] [Google Scholar]
- van den Broeke EN, Mouraux A. High-frequency electrical stimulation of the human skin induces heterotopical mechanical hyperalgesia, heat hyperalgesia, and enhanced responses to nonnociceptive vibrotactile input. J Neurophysiol 111: 1564–1573, 2014. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroencephalogr Clin Neurophysiol 78: 50–55, 1991. [DOI] [PubMed] [Google Scholar]
- Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 122: 2245–2257, 1999. [DOI] [PubMed] [Google Scholar]