Abstract
Background
Statins are at the forefront of strategies to manage hypercholesterolemia. However 10% to 15% of patients are intolerant to any statin drugs, even at low daily doses and almost one-third of statin users discontinue therapy within 1 year. Some nutraceuticals are prescribed as lipid-lowering substances, but doubts remain about their efficacy and tolerability.
Objectives
We aimed to investigate the efficacy and the safety of a nutraceutical combination consisting mainly of 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, and 10 mg policosanols (MBP-NC) in patients with low-moderate risk hypercholesterolemia.
Methods
In this single centre, randomized, double-blind, placebo-controlled study 60 consecutive outpatients (29 men and 31 women; age range = 18–60 years), with newly diagnosed primary hypercholesterolemia not previously treated, after a run-in period of 3 weeks on a stable hypolipidic diet, were randomized to receive a pill of MBP-NC (n = 30) or placebo (n = 30) once a day after dinner, in addition to the hypolipidic diet. The efficacy and the tolerability of the proposed nutraceutical treatment were fully assessed after 4, 12, and 24 weeks of treatment.
Results
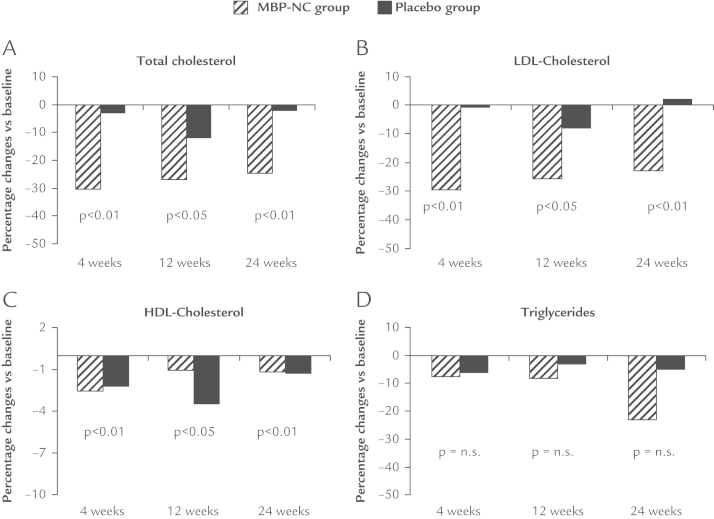
In the MBP-NC group both total cholesterol and LDL-C already showed a significant reduction at Week 4 (–30.3% ± 33.9% and –29.4% ± 35.3%, respectively) that remained substantially unchanged at Week 12 (–26.7% ± 33.1% and –25.6% ± 31.5%, respectively) and at Week 24 (–24.6% ± 32.1% and –23.7% ± 32.6%, respectively). The between-groups differences were significant at all time points for both total cholesterol and LDL-C. There were no significant changes in HDL-C, fasting glucose, and triglyceride serum levels in either group. MBP-NC was also safe and well tolerated.
Conclusions
In patients with low- to moderate-risk hypercholesterolemia a nutraceutical combination in association with a hypolipidic diet significantly reduced total cholesterol and LDL-C levels and may favor the reaching the recommended cholesterol targets. ClinicalTrials.gov identifier: NCT02078167.
Key words: berberine, hypercholesterolemia, monacolin, policosanols, red yeast rice, tolerability
Introduction
Many epidemiologic studies have shown that serum cholesterol levels are strongly related to cardiovascular risk.[1], [2] Consequently lowering cholesterol levels is a fundamental prognostic goal in the primary and secondary prevention of cardiovascular events. The 3-hydroxy-3-methylglutaryl-coenzime A reductase inhibitors, commonly known as statin drugs, are the drugs of first choice to lower serum cholesterol levels, especially in patients at high or very-high risk of cardiovascular diseases, in view of their established efficacy in reducing cardiovascular mortality and morbidity in both primary and secondary prevention.[3], [4] Statin drugs are generally well tolerated and in controlled trials the adverse events were similar in patients treated with statins and those treated with placebo.4 However, in clinical practice, 10% to 15% of patients are found to be intolerant to any statin drugs, even at low daily doses and almost one-third of statin users discontinue therapy within 1 year.5 Furthermore some other patients, especially in primary prevention, refuse statin drugs because of the fear of possible side effects. Some nutraceutical products may represent an alternative treatment to be considered for the above-mentioned cases, above all in patients with marginally high hypercholesterolemia.[4], [6] Because the use of the full dose of nutraceuticals entail some tolerability concerns, a combination of nutraceuticals with different but synergic mechanisms of action at lower and safer dosages could be preferable. In particular, in recent years there has been growing interest in a nutraceutical combination containing monacolin (the biologically active component of red yeast rice), berberine, and policosanols (MBP-NC). The cholesterol-lowering effect of MBP-NC consumed in conjunction with a standard Mediterranean healthy diet has been observed in a large Italian study carried out by general practitioners7 in patients intolerant to >1 statin,8 in patients with metabolic syndrome or who are overweight,[9], [10] and in elderly patients with hypercholesterolemia.11 Moreover, the MBP-NC mixture has been reported to have some direct protective vascular effects, similar to pharmacologic lipid-lowering agents, such as improvement in endothelial dysfunction12 and improvement in aortic stiffness.13 Another recent study reported that a 2-month treatment with MBP-NC improved insulin sensitivity in patients with metabolic syndrome.14
Hitherto the cholesterol-lowering effect of MBP-NC has not been evaluated in long-term double-blind, placebo-controlled studies. The aim of our single-center, randomized, double-blind, placebo-controlled study was to evaluate the efficacy and the safety of a 24-week treatment with a MBP-NC mixture in patients with low-moderate risk hypercholesterolemia.
Materials and Methods
Population
A cohort of 66 consecutive outpatients with newly diagnosed primary hypercholesterolemia not previously treated who applied to the lipid clinic of the Department of Internal Medicine at the University of Siena, Italy, were considered for enrolment in our study. The inclusion criteria were age between 18 and 60 years, body mass index between 18.5 and 29.9, serum LDL-C >150 mg/dL (3.88 mmol/L), and an estimated 10-year cardiovascular risk <20% according to Framingham risk scoring. The exclusion criteria were history of cardiovascular disease or coronary risk equivalents; secondary hyperlipidemia caused by diabetes mellitus, renal, liver, or thyroid diseases; alcohol consumption >40 g/d; estimated 10-year cardiovascular risk >20% according to Framingham risk scoring; and muscular diseases or abnormally elevated creatine phosphokinase (CPK) levels or drug treatment with antiplatelet, anti-inflammatory, or hypolipidemic agents, or hormone replacement therapy either ongoing or any time during the previous 2 months. Instead, the patients stable while taking antihypertensive treatment for at least 3 months were included. All patients were instructed to maintain their habitual physical activity during the study period.
At the screening visit, all patients were instructed to follow a hypolipidic diet (ie, low-cholesterol/low-saturated fat diet approximately consisting of 55% carbohydrates, 20% proteins, and 25% lipids) during a run-in period of 3 weeks, after which all patients who met the inclusion criteria (29 men and 31 women), were randomized to receive a pill of MBP-NC (n=30) or placebo (n=30) once a day after dinner, in addition to the hypolipidic diet. The placebo pills, identical in taste and appearance to the MBP-NC pills, consisted of inactive compound. Randomization and blinding were provided by Rottapharm Madaus SpA (Monza, Italy). The composition of the patented proprietary combination of nutraceuticals investigated was 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, 10 mg policosanol, 0.2 mg folic acid, 2 mg coenzyme Q10, and 0.5 mg asthaxantin (Armolipid Plus, Rottapharm Madaus SpA).
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, as revised in 2000 and 2008, and the study protocol was approved by the Ethics Committee of the University Hospital of Siena. Written informed consent was obtained from each patient.
Clinical and anthropometric evaluation
All patients underwent physical examination at baseline and after 4, 12, and 24 weeks of treatment. All the determinations were made at the lipid clinic at 9:00 AM, after an overnight fast of 12 hours. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index was calculated as weight in kilograms divided by height meters2. Brachial blood pressure was measured by a physician with a mercury sphygmomanometer after patient had been seated for at least 10 minutes and the average of 3 measurements was considered for the analysis. Waist circumference was also measured at each visit midway between the lowest rib and the iliac crest using an anthropometric tape.
In all patients, body composition (fat mass percentage, fat-free mass, and fat-free mass to fat mass ratio) was assessed by anthropometry and bioelectrical impedance analysis using a single-frequency 50 kHz bioelectrical impedance analyzer (BIA 101 RJL, Akern Bioresearch, Florence, Italy). All bioelectrical impedance analysis measurements were carried out by the same operator according to the standard tetrapolar technique, with patients in a supine position for at least 20 minutes. The electrodes were placed on the dorsal surface of the right foot and ankle and the right wrist and hand.
Biochemical measurements
In all patients fasting venous blood samples were drawn at baseline and after 4, 12, and 24 weeks to assess serum levels of total cholesterol, triglycerides (TG), HDL-C, and LDL-C. All lipid parameters were measured using a colorimetric method (Autoanalyzer, Menarini, Florence, Italy). In our institution the intra- and interassay coefficients of variation were, respectively, 1.8% and 3.8% for total cholesterol, 2.0% and 3.0% for HDL-C, 1.7% and 2.9% for TG, and 1.5% and 2.3% for LDL-C assessment. To monitor the safety of the MBP-NC in all patients at the same time points serum levels of glucose, uric acid, creatine phospho kinase (CPKw), gamma-glutamiltranferase, and transaminases were also assessed. Tolerability was monitored by recording symptoms. Medication compliance was assessed by counting the number of pills returned at the clinic visits.
Statistical analysis
All values were expressed as mean (SD). Clinical data and initial values of the variables measured in the study groups were compared using Student t test for unpaired data. The Kolmogorov-Smirnov test was used to verify the normality of the distribution of the outcome variables. For all parameters the absolute changes over time for each patient were expressed as a percentage of the baseline values. Paired t test and Wilcoxon matched-pairs signed-rank test were used, where appropriate, to compare the changes with baseline values. Two-way ANOVA for repeated measures was used to compare the response of the study variables to the 2 different treatments. All tests were 2-sided, and P < 0.05 was considered statistically significant. All tests were performed using the SPSS statistical package for Windows version 16.0 (IBM-SPSS Inc, Armonk, New York).
Results
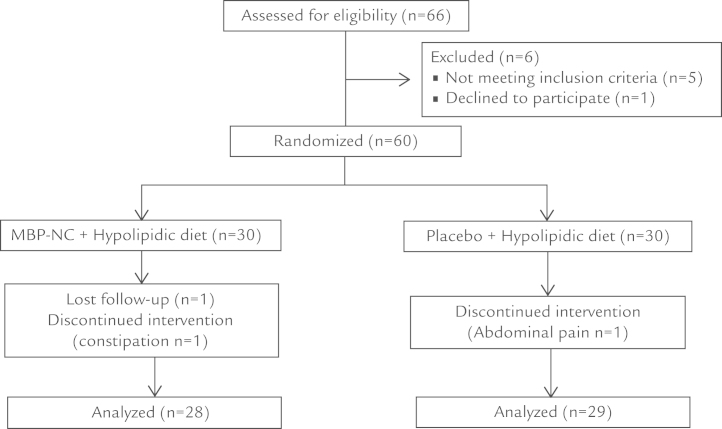
The demographic and clinical characteristics of the 60 patients with hypercholesterolemia who were stable on the hypolipidic diet, 30 assigned to MBP-NC and 30 to placebo, are shown in Table I. There were no significant differences between the 2 groups in baseline characteristics. Fifty-seven patients (28 in the MBP-NC group and 29 in the placebo group) completed the 24-week study period. One patient in the MBP-NC group withdrew from the study for problems unrelated to the study drugs, whereas 2 patients (1 in the MBP-NC group and 1 in the placebo group) withdrew for not serious adverse events (Figure 1). All outcome variables were distributed normally and there was no need for log transformation. The percentage changes of lipid parameters with respect to baseline in the 2 study groups are shown in Figure 1. In the MBP-NC group both total cholesterol and LDL-C levels already showed a significant reduction at Week 4 (–30.3% ± 33.9% and –29.4% ± 35.3%, respectively), which remained substantially unchanged at Week 12 (–26.7% ± 33.1% and –25.6% ± 31.5%, respectively) and at Week 24 (–24.6% ± 32.1% and –23.7% ± 32.6%, respectively), whereas there were no significant changes in the placebo arm. The between-groups differences were significant at all time points for both total cholesterol and LDL-C (Figure 2). There were no significant changes in HDL-C and triglyceride serum levels in either group, although at the end of the study period triglyceride levels were more reduced in patients treated with MBP-NC than in those treated with placebo (–22.9% ± 62.3% and –4.1% ± 35.3%, respectively) (Figure 1). A 24-week treatment with either MBP-NC plus diet or placebo plus diet showed similar effect on body weight (–2.8 kg and –3.5 kg, respectively) and on waist circumference (–2.2 cm and –2.6 cm, respectively) (Table II). No significant differences were observed between groups at all time points for anthropometric or body composition parameters and for systolic and diastolic blood pressure (Table II). MBP-NC treatment showed a tendency to reduce serum levels of fasting glucose and of uric acid but without reaching statistical significance.
Table I.
Baseline characteristics of 60 patients with hypercholesterolemia randomized to receive either a nutraceutical combination consisting mainly of 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, and 10 mg policosanols (MBP-NC) or placebo.*
| Characteristic | MBP-NC | Placebo | P value |
|---|---|---|---|
| Men | 15 | 14 | --- |
| Women | 15 | 16 | --- |
| Age, y | 46.4 (9.7) | 46.4 (10.1) | NS |
| Weight, kg | 72.7 (12.7) | 71.1 (12.9) | NS |
| Body mass index | 26.9 (4.9) | 26.4 (4.1) | NS |
| Waist circumference, cm | 89.9 (10.9) | 88.7 (10.9) | NS |
| Glucose, mg/dL | 92.5 (8.8) | 94.4 (10.0) | NS |
| Total cholesterol, mg/dL | 238.4 (26.9) | 248.1 (32.4) | NS |
| HDL-C, mg/dL | 53.1 (13.2) | 55.7 (14.5) | NS |
| LDL-C, mg/dL | 162.0 (22.5) | 165.8 (29.0) | NS |
| Triglycerides, mg/dL | 132.1 (55.2) | 119.0 (50.4) | NS |
| Uric acid, mg/dL | 4.8 (1.5) | 4.1 (1.6) | NS |
NS = not significant.
Values are expressed as mean (SD), except sex, which is expressed as n.
Figure 1.
Flow diagram of the study population. MBP-NC = nutraceutical combination consisting mainly of 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, and 10 mg policosanols.
Figure 2.
Percentage changes from baseline in (A) total cholesterol, (B) LDL-C, (C) HDL-C, and (D) triglycerides in patients with hypercholesterolemia treated for 24 weeks with a nutraceutical combination consisting mainly of 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, and 10 mg policosanols (n =30) or placebo (n=30).
Table II.
Anthropometric, clinical, and biochemical parameters of 60 patients with hypercholesterolemia randomized to receive either a nutraceutical combination consisting mainly of 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, and 10 mg policosanols (MBP-NC) or placebo.*
| Parameter | MBP-NC |
Placebo |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 12 weeks | 24 weeks | Baseline | 4 weeks | 12 weeks | 24 weeks | |
| Weight, kg | 72.7 (12.7) | 71.8 (12.5) | 70.5 (12.6) | 69.9 (13.0) | 71.0 (12.9) | 69.8 (12.9) | 69.6 (13.0) | 67.5 (12.6) |
| Waist circumference, cm | 89.9 (10.9) | 89.1 (11.0) | 87.5 (10.0) | 87.1 (9.1) | 88.6 (10.9) | 87.0 (9.8) | 87.8 (10.4) | 86.0 (9.3) |
| Lean mass/fat mass | 2.7 (1.3) | 2.9 (1.3) | 3.1 (1.5) | 3.1 (1.4) | 2.9 (1.5) | 2.9 (1.3) | 3.0 (1.2) | 3.0 (1.4) |
| Fat percentage | 27.6 (7.9) | 26.7 (7.9) | 26.6 (8.7) | 27.2 (9.2) | 27.0 (7.3) | 26.9 (6.3) | 26.2 (6.1) | 26.9 (6.5) |
| Glucose, mg/dlL | 92.5 (8.8) | 90.4 (9.7) | 92.1 (10.1) | 90.7 (12.9) | 92.4 (10.0) | 94.6 (9.0) | 92.5 (10.0) | 97.4 (12.8) |
| SBP, mm Hg | 120.1 (11.1) | 117.1 (12.5) | 115.4 (11.5) | 117.1 (13.0) | 119.1 (19.7) | 121.2 (13.4) | 117.5 (16.9) | 119.0 (18.4) |
| DBP, mm Hg | 77.2 (7.0) | 74.8 (8.8) | 77.3 (9.3) | 76.2 (6.8) | 75.2 (10.0) | 78.2 (7.7) | 76.5 (8.6) | 75.3 (10.3) |
| Uric acid, mg/dL | 4.8 (1.5) | 4.4 (1.3) | 4.4 (1.3) | 4.1 (1.3) | 4.1 (1.2) | 4.1 (1.5) | 3.9 (1.2) | 3.7 (1.4) |
| CPK, mU/mL | 111.3 (38.0) | 117.8 (72.3) | 125.2 (70.5) | 140.4 (83.0) | 107.6 (41.8) | 118.7 (67.1) | 110.3 (44.4) | 96.8 (39.6) |
| ALT, IU/L | 20.5 (5.3) | 21.2 (5.4) | 21.0 (6.0) | 19.9 (4.1) | 20.3 (6.0) | 20.5 (5.1) | 20.0 (6.1) | 18.8 (4.7) |
| AST, IU/L | 23.0 (10.8) | 23.6 (11.2) | 23.9 (13.0) | 19.9 (8.8) | 20.6 (12.2) | 21.2 (11.2) | 21.3 (12.1) | 21.2 (10.4) |
| ɣGT, IU/L | 24.0 (13.7) | 27.8 (18.7) | 30.3 (21.5) | 27.5 (15.4) | 21.1 (15.4) | 21.5 (16.3) | 21.7 (15.6) | 24.1 (22.6) |
ALT = alanine aminotrasferase; AST = aspartate aminotrasferase; CPK = creatine phosphate kinase; DBP = diastolic blood pressure; ɣGT = ɣ-glutamyltranspeptidase; SBP = systolic blood pressure.
Values are expressed as mean (SD).
The mean values of hepatic parameters were unchanged at all time points compared with baseline in both groups, and none of the patients showed values higher than double the upper limit of normal (ULN) range. Instead, CPK serum levels showed a slight increase in MBP-NC group without reaching statistical significance (Table II). Four patients (3 in the MBP-NC group and 1 in the placebo group) presented an increase in CPK levels higher than 2 times the ULN during the study period, but at the end of the study only a patient of the MBP-NC group presented CPK levels higher than 2 times the ULN; however, none of these patients complained of myalgia. Nine patients (15%) reported symptoms. Symptom intensity was mild for all patients except for a patient in the MBP-NC group who complained of moderate constipation a Week 4 and was withdrawn from the study at Week 12; a patient in the placebo group withdrew for abdominal problems (Table III).
Table III.
Number of patients with symptoms or events judged as probably, possibly, or certainly related to the treatment with either a nutraceutical combination consisting mainly of 200 mg red yeast rice extract (equivalent to 3 mg monacolins), 500 mg berberine, and 10 mg policosanols (MBP-NC) or placebo.*
| Symptom/event | MBP-NC group (n = 30) | Placebo group (n = 30) |
|---|---|---|
| Constipation | 2 | 1 |
| Muscle pain | 0 | 1 |
| Dizziness | 1 | 0 |
| Dyspepsia | 1 | 0 |
| Meteorism/bloating | 1 | 1 |
| Diarrhea | 0 | 1 |
Symptoms intensity for all patients of both groups was mild except for 1 patient in the MBP-NC group who presented moderate constipation.
Discussion
This is 1 of the first randomized, placebo-controlled, double-blind studies of adequate length (24 weeks) to evaluate the safety and efficacy of treatment with a low-dose combination of red yeast rice, berberine, and policosanols in patients with mild-to-moderate hypercholesterolemia. Our study confirms that a combined therapy of low-dosage nutraceuticals with different mechanisms of action may contribute to reducing cholesterol levels more than the different components of the MBP-NC used individually. In a meta-analysis of 93 trials red yeast rice preparations showed short-term beneficial effects on LDL-C levels.15 Moreover, in a large Chinese population with previous myocardial infarction, long-term treatment with an extract of red yeast rice significantly reduced not only LDL-C by about 20% but also the risk of future major coronary events.16 The positive effect of red yeast rice on cholesterol levels is attributed to an inhibition of 3-hydroxy-3-methylglutaryl-coenzime A reductase due to its monacolin-K content, which is chemically identical to lovastatin.17 Moreover, a recent study18 carried out in patients intolerant to statin drugs reported that red yeast rice preparations induced a dose-dependent decrease of LDL-C levels without serious adverse effects. However in all these studies red yeast rice preparations were used in daily doses, ranging from 1.2 to 4.8 g—much higher than that present in the nutraceutical pills administered in our study—accounting for some reports of myopathy in patients treated with high doses of red yeast rice. The cholesterol-lowering efficacy of policosanols is still controversial. Indeed, some Cuban studies reported that policosanols were effective at producing significant reductions in cholesterol levels and also some antiatherogenic effects. However, more recent randomized controlled trials in white patients with hyperlipidemia did not find any lipid-lowering effect of policosanols.19 Berberine, a natural plant extract from Berberis aristata bark, has been reported as able to reduce serum cholesterol levels by increasing LDL-C receptors on the surface of liver cells and in inhibiting triglycerides biosynthesis.[10], [20], [21] Moreover, a recent study has reported that in Asian patients with type 2 diabetes mellitus, berberine ameliorated insulin sensitivity and improved the management of diabetes.22 However the high dosages employed (1.5 g/d) were often associated with gastrointestinal disorders.22 Therefore, berberine and red yeast rice may be expected to have a synergistic effect on inhibition of hepatic lipids synthesis. The results of our study, in terms of total cholesterol and LDL-C reduction, are consistent with other randomized, placebo-controlled studies in which MBP-NC was administered for 4,23 6,12 8,13 or 5611 weeks.
In agreement with some studies,[11], [13], [14], [24] but in disagreement with others[7], [10] we failed to find any significant effect of MBP-NC on both TG and HDL-C levels. This finding might be due to the relatively low dosages of berberine and red yeast rice; moreover, the normal baseline levels of TG and HDL-C might have contributed to further lessen the effect of MBP-NC treatment on these lipid variables. In disagreement with other studies,[10], [14] our population receiving MBP-NC did not show any significant changes in glucose serum levels. This finding might be due to the relatively low dosages of berberine and to the fact that in the present study patients with obesity or prediabetes were excluded. The aforementioned findings have stimulated an interest in defining the clinical conditions in which nutraceuticals could be recommended. According to the more recent guidelines of the European Society of Cardiology, the best indication may be represented by patients with hypercholesterolemia and low-to-moderate cardiovascular risk.4 In these patients the taking nutraceuticals in combination with a hypolipidic diet may facilitate reaching target cholesterol levels. Another indication for MBP-NC might be for those patients who would perhaps need treatment with statins but were previously found to be intolerant to statins or refused treatment with these drugs. In fact, recently Pisciotta et al24 reported that in patients with primary hypercholesterolemia 6-month treatment with MBP-NC was more effective than 10 mg/d ezetimibe in lowering LDL-C and was well tolerated.
A key point for treatment with nutraceuticals is their safety and tolerability. In particular, European Society of Cardiology guidelines have stated that long-term data regarding tolerability of red yeast rice are scarce.4 Our study has confirmed the good tolerability of MBP-NC; in fact, the patients treated with MBP-NC presented a very low incidence of adverse reactions that were mild, except 1 patient who was withdrawn from the study because of moderate constipation.
Our study has some limitations: first, the relatively low sample size; second, a 24-week study period, although it was longer with respect to the majority of previous studies, might be inadequate to evaluate the long-term efficacy of MBP-NC. Finally, we tested the combination mixture effects but not the effects of the single components.
Conclusions
In patients with low-to-moderate-risk hypercholesterolemia the MBP-NC in association with a hypolipidic diet reduces total cholesterol and LDL-C and may favor reaching the recommended cholesterol targets. Further studies are warranted to investigate whether long-term treatment with this kind of nutraceutical combination may prevent cardiovascular complications.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgment
This study was supported by Rottapharm SpA (Monza, Italy), which supplied the drug and placebo.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration. Lewington S., Whitlock G., Clarke R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C., Blackwell L., Emberson J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Catapano A.L., Reiner Z., De Backer G., ESC Committee for Practice Guidelines 2008-2010 and 2010-2012 Committees ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217(Suppl 1):S1–S44. doi: 10.1016/j.atherosclerosis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Jackevicius C.A., Mamdani M., Tu J.V. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 6.Nijjar P.S., Burke F.M., Bloesh A., Rader D.J. Role of dietary supplements in lowering low-density lipoprotein cholesterol: a review. J Clin Lipidol. 2010;4:248–258. doi: 10.1016/j.jacl.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Trimarco B., Benvenuti C., Rozza F. Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med J Nutrition Metab. 2011;4:133–139. doi: 10.1007/s12349-010-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicero A.F.G., Derosa G., Bove M. Long-term effectiveness and safety of a natraceutical based approach to reduce cholesterolemia in statin intolerant subjects with and without metabolic syndrome. Curr Topics Nutrac Res. 2009;7:121–126. [Google Scholar]
- 9.Izzo R., de Simone G., Giudice R. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J Hypertens. 2010;28:1482–1487. doi: 10.1097/HJH.0b013e3283395208. [DOI] [PubMed] [Google Scholar]
- 10.Cicero A.F.G., De Sando V., Benedetto D. Long-term efficacy and tolerability of a multicomponent lipid-lowering nutraceutical in overweight and normoweight patients. Nutrafood. 2012;11:55–61. [Google Scholar]
- 11.Marazzi G., Cacciotti L., Pelliccia F. Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv Ther. 2011;28:1105–1113. doi: 10.1007/s12325-011-0082-5. [DOI] [PubMed] [Google Scholar]
- 12.Affuso F., Ruvolo A., Micillo F. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20:656–661. doi: 10.1016/j.numecd.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Pirro M., Lupattelli G., Del Giorno R. Nutraceutical combination (red yeast rice, berberine and policosanols) improves aortic stiffness in low-moderate risk hypercholesterolemic patients. PharmaNutrition. 2013;1:73–77. [Google Scholar]
- 14.Affuso F., Mercurio V., Ruvolo A. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J Cardiol. 2012;4:77–83. doi: 10.4330/wjc.v4.i3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Zhang J., Shi Y. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin Med. 2006;23:1–4. doi: 10.1186/1749-8546-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Z., Kou W., Du B., Chinese Coronary Secondary Prevention Study Group Li S. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol. 2008;101:1689–1693. doi: 10.1016/j.amjcard.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 17.Heber D., Yip I., Ashley J.M. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69:231–236. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- 18.Halbert S.C., French B., Gordon R.Y. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198–204. doi: 10.1016/j.amjcard.2009.08.672. [DOI] [PubMed] [Google Scholar]
- 19.Berthold H.K., Unverdorben S., Degenhardt R. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial. JAMA. 2006;295:2262–2269. doi: 10.1001/jama.295.19.2262. [DOI] [PubMed] [Google Scholar]
- 20.Cicero A.F.G., Ertek S. Metabolic and cardiovascular effects of berberine: from preclinical evidences to clinical trial results. Clin Lipidol. 2009;4:553–563. [Google Scholar]
- 21.Kong W., Wei J., Abidi P. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Li X., Zou D. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 23.Cicero A.F., Rovati L.C., Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung. 2007;57:26–30. doi: 10.1055/s-0031-1296582. [DOI] [PubMed] [Google Scholar]
- 24.Pisciotta L., Bellocchio A., Bertolini S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 2012;22(11):123. doi: 10.1186/1476-511X-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]