Abstract
Background
Mechanisms underlying the development of phantom limb pain and neuropathic pain after limb amputation and spinal cord injury, respectively, are poorly understood. The goal of this systematic review was to assess the robustness of evidence in support of “maladaptive plasticity” emerging from applications of advanced functional magnetic resonance imaging (MRI).
Methods
Using MeSH heading search terms in PubMed and SCOPUS, a systematic review was performed querying published manuscripts.
Results
From 146 candidate publications, 10 were identified as meeting the inclusion criteria. Results from fMRI investigations provided some level of support for maladaptive cortical plasticity, including longitudinal studies that demonstrated a change in functional organization related to decreases in pain. However, a number of studies have reported no relationship between reorganization, pain and deafferentation, and emerging evidence has also suggested the opposite — that is, chronic pain is associated with preserved cortical function.
Conclusion
Based solely on advanced functional neuroimaging results, there is only limited evidence for a relationship between chronic pain intensity and reorganization after deafferentation. The review demonstrates the need for additional neuroimaging studies to clarify the relationship between chronic pain and reorganization.
Keywords: Spinal cord injury, Amputation, Neuropathic pain, Phantom-limb pain, Functional imaging, Cortical reorganization
Highlights
-
•
There is evidence of a relationship between brain reorganization, deafferentation, and chronic pain.
-
•
Emerging evidence suggests that reorganization in the CNS could be an adaptive process, preventing the emergence of pain.
-
•
Future studies adopting standardized protocols are needed to clarify the role of chronic pain and plasticity in the brain.
1. Introduction
From seminal studies involving individuals with limb amputations (Sica et al., 1984, Yang et al., 1994) and spinal cord injuries (SCI) (Ding et al., 2005, Green et al., 1999, Jain et al., 1997, Jain et al., 2000), neurophysiological evidence supporting sensory and motor plasticity in the adult central nervous system (CNS) began to emerge more than two decades ago. While initially demonstrating a unique potential for change in the CNS, efforts quickly turned to understanding the effects of central plasticity on functional outcomes. In terms of detrimental outcomes, Flor and colleagues were among the first to show that cortical reorganization was associated with phantom limb pain — pioneering the maladaptive plasticity model using magnetoencephalography (MEG) (Flor et al., 1995). The original operational definition of maladaptive plasticity is that afferentated brain areas enlarge or shift activation into somatotopically organized de-afferented brain areas — the extent of the shift positively associated with pain intensity. Based in large part on this knowledge, rehabilitation practices to relieve chronic pain have been developed to target maladaptive cortical organization (Diers et al., 2010, Flor, 2003, Foell et al., 2014, MacIver et al., 2008).
The past twenty years has also seen considerable advances in the field of neuroimaging, including quantifiable functional magnetic resonance imaging (fMRI). Based on a different measure of brain activity, fMRI provides a unique opportunity to re-address the relationship between cortical reorganization after deafferentation and chronic pain originally demonstrated by MEG. The objective of this review was to systematically examine studies that have addressed the relationship between reorganization in the brain after deafferentation and chronic pain. The specific aim was to determine how fMRI studies have supported the original operational definition of maladaptive plasticity and to what extent the definition has been altered. To address this aim, our systematic review focused on findings from fMRI studies involving individuals with phantom limb pain due to amputation or neuropathic pain related to spinal cord injury (SCI).
2. Material and methods
2.1. Search methods for identification of studies
PubMed and SCOPUS were searched using the time range from their individual inception dates 1977 and 1960, respectively, to the 30th of June 2015. The PubMed search was conducted using the methodological subjects heading (MeSH) keywords ‘spinal cord injury’ along with ‘neuropathic pain’ and ‘magnetic resonance imaging’ for SCI related pain, as well as ‘amputation’ along with ‘phantom limb pain’ and ‘magnetic resonance imaging’ for amputation-associated pain. Similarly, SCOPUS search included the same combination of keywords used for PubMed and also different combinations of keywords (e.g., phantom limb pain and magnetic resonance imaging). To identify additional studies that may have been overlooked, bibliographies of identified studies were hand searched.
2.2. Selection of studies
One author (JLK) carried out an initial screening of retrieved articles and applied inclusion criteria. Subsequently, a second reviewer (CRJ) independently reviewed all the studies in order to assure that the publications met all inclusion criteria. All disagreements were discussed and resolved at a consensus meeting with a third reviewer (AC).
2.3. Inclusion and exclusion criteria
All original English language studies using quantifiable functional imaging techniques to investigate neuropathic pain and phantom limb pain following spinal cord injury or amputation, respectively, were included. Included fMRI studies must have performed a statistical analysis specifically focusing on pain and cortical activation. Preclinical studies in species other than humans (e.g., rodents, and monkeys) were excluded. Also excluded were pediatric studies, case studies, and review articles.
2.4. Outcomes
The specific outcomes extracted from each study included: 1) subject characteristics (i.e., age and sex, and time since deafferentation), 2) pain rating (converted to 0–10 if necessary), 3) number of subjects with amputations or SCI with and without pain, 4) number of healthy subjects, 5) imaging parameters (i.e., echo and repetition time), 6) regions of interest examined, 7) statistical approach (i.e., correction for multiple comparisons), and 8) type of pain assessment. We also considered the type of task performed while in the scanner, as well as methods used to analyze differences in patterns of BOLD activation.
2.5. Levels of evidence
Studies were divided into two levels of evidence. 1st level evidence comprised all studies that included a healthy control group, as well as patients (i.e., SCI and amputation) with and without neuropathic or phantom limb pain. Additionally, 1st level evidence was required to explicitly qualify examining the correlation between pain intensity and a measure of cortical reorganization. 2nd level evidence included studies that did not incorporate a healthy control condition and/or individuals that were ‘pain-free’, and thus less well suited to address the concept of maladaptive plasticity. 2nd level evidence studies examined the correlation between pain intensity and reorganization, or group level comparisons without considering the correlation between pain intensity and cortical reorganization. For each publication, the direction of the relationship between pain and reorganization was made based on the task performed (fMRI only), and the area of the brain examined. Evidence used to support a relationship between cortical activity and pain was based on available results and discussion in the original manuscript.
2.6. Quality assessment rating
Based on 10 criteria relevant to the objectives of the review (adapted from Campbell (Campbell et al., 2011)), the outcomes extracted from each study were considered in a descriptive analysis. CRJ and JLK independently performed the quality assessment. Disagreements of ratings were discussed and final scores for each publication were determined.
3. Results
3.1. Included/excluded studies
As illustrated in Fig. 1, 146 candidate publications (54 for SCI, 92 for amputation) were first identified, of which 10 were suitable for review (Dettmers et al., 2001, Diers et al., 2010, Foell et al., 2014, Gustin et al., 2010, Lotze et al., 2001, MacIver et al., 2008, Makin et al., 2013b, Makin et al., 2015b, Philip and Frey, 2014, Wrigley et al., 2009). The majority of excluded studies (n = 137, 51 SCI, 86 amputation) did not meet one of the other inclusion criteria including species investigated, imaging method applied (e.g., MEG, PET, MR spectroscopy, and voxel-based morphometry (VBM)), language, and study design (i.e., case reports and reviews).
Fig. 1.
Diagram of the review procedure.
3.2. Study details and characteristics
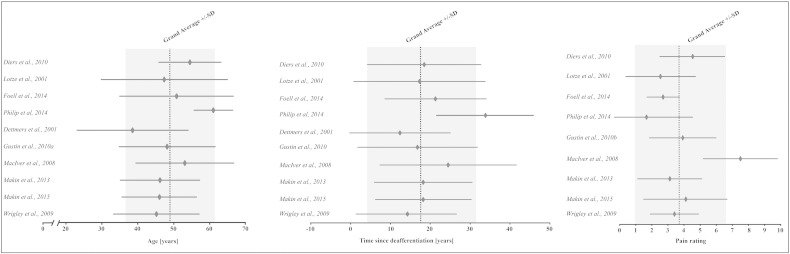
Pain assessment and imaging acquisition parameters for each study are shown in Table 1. The results from the quality assessment are shown in Table 2. Fig. 2 illustrates mean (± standard deviation) age, time since deafferentation, and pain rating summarized for each study.
Table 1.
Characteristics of included studies.
| Study (year) | Study population | Type of pain (n =) | Imaging | Echo time (TE)/Repetition time (TR) | Regions of interest | Statistical correction | Pain assessment |
|---|---|---|---|---|---|---|---|
| Dettmers et al. (2001) | 16 upper arm amputees (14 traumatic, 2 malignant tumors), 6 healthy controls | Phantom-limb pain (8) | 1.5 T, SPM96 | TE: 50 ms TR: 100 ms |
NA | Uncorrected, p < 0.001 | Not reported |
| Diers et al. (2010) | 14 unilateral upper limb amputees (13 traumatic, 1 vascular disease); 9 healthy controls | Phantom-limb pain (7) | 1.5 T, SPM2 | TE: 60 ms TR: 3300 ms |
S1, S2, M1, SMA | Correction for multiple comparisons using FDR | German version of West Haven-Yale Multi-dimensional pain inventory |
| Foell et al. (2014) | 13 unilateral upper limb amputees (10 traumatic, 3 osteosarcoma) | Phantom-limb pain (11) | 3 T, SPM8 | TE: 45 ms, TR: 3300 ms |
S1 and M1 (hand area) |
Correction for multiple comparisons using FWE | (1) German version of West Haven-Yale Multi-dimensional pain inventory (2) a phantom-and-stump interview that included the pain experience scale consisting of 24 pain adjectives from the McGill pain questionnaire (3) Visual analog scale with the endpoints ‘no pain’ and ‘unbearable pain’ |
| Lotze et al. (2001) | 14 unilateral upper limb amputees (11 traumatic, 2 tumors, 1 sepsis); 7 age-matched healthy controls | Phantom-limb pain (7) | 1.5 T, SPM96 | TE: 59 ms, TR: NA |
M1, S1, posterior parietal and dorsolateral prefrontal cortex, basal ganglia, thalamus, cerebellum | Correction for multiple comparisons, a combined test of the peak intensity and the spatial extension of the cluster (Poline et al., 1997) | Multidimensional Phantom Limb Pain Inventory Scale (range 1–6) |
| MacIver et al. (2008) | 13 unilateral upper limb amputees (12 traumatic, 1 bone cancer); 6 age- and sex-matched healthy controls | Phantom-limb pain (13) | 3 T, FEAT 3.3. | TE: 50 ms, TR: 3000 ms |
S1 and M1 (hand and lip areas) |
Correction for multiple comparisons (p < 0.05, cluster-level corrected) | Phantom limb pain questionnaire (Kooijman et al., 2000), numeric rating scale (0 = no pain to 10 = worst pain imaginable) |
| Makin et al., 2013a, Makin et al., 2013b | 29 unilateral upper-limb amputees (18 traumatic, 11 congenital unilateral upper-limb deficit); 22 healthy controls | Phantom-limb pain (17) | 3 T, FSL 5.1 | TE: 30 ms, TR: 2000 ms |
S1 and M1 (hand area) |
Correction for multiple comparisons using FWE | Rating of frequencies of phantom pain & non-painful phantom sensations, as experienced within the last year, and intensity of worst pain experienced during the last week (or in a typical week involving phantom/stump sensations). ‘Pain magnitude’ was calculated by dividing pain intensity (0: ‘no pain’ 10: ‘worst pain imaginable’) by frequency (1 ‘all the time’, 2 ‘daily’, 3 ‘weekly’, 4 ‘several times per month’ and 5 — ‘once or less per month’). |
| Makin et al., 2015a, Makin et al., 2015b | 17 individuals with unilateral upper limb amputees (18 traumatic), 21 age- and handedness-matched | Phantom-limb pain (17) | 3 T, FSL 5.1 | TE: 30 ms, TR: 2040 ms |
S1 and M1 (hand area) |
Correction for multiple comparisons | Intensity and frequencies of phantom/stump pain and non-painful phantom sensations were rated using a 0–10 scale: (i) intensity of worst pain/most vivid sensation experienced during the last week (or in a typical week involving such sensations); (ii) intensity of phantom pain on average over the last week (or in a typical week if last week was atypical); and (iii) current intensity/vividness of phantom pain and sensations, during scanning day. |
| Philip and Frey (2014) | 8 unilateral upper limb amputees (traumatic), 8 age- and sex-matched | Phantom-limb pain (17) | 3 T, FSL 4.1.8 | TE: 30 ms TR: 2550 ms |
S1 and M1 (hand area) |
Correction for multiple comparisons | 0–1 measurements determined by visual analog scale |
| Gustin et al. (2010) | 11 patients (11 complete thoracic lesion due to trauma); 19 healthy controls | Below-level neuropathic pain (11) | 3 T, SPM5 | TE: 40 ms, TR: 3000 ms |
NA | Correction for multiple comparisons using FDR | Pain diary was completed for one week prior to scanning (0 cm = “no pain” to 10 cm = “maximum imaginable pain”) three times a day |
| Wrigley et al. (2009) | 20 patients (20 complete thoracic lesion); 21 age- and gender-matched healthy controls | Below-level neuropathic pain (10) | 3 T, SPM5 | TE: 40 ms, TR: 3000 ms |
NA | Correction for multiple comparisons using FDR | International Association for the Study of Pain SCI Pain Taxonomy. A pain diary was completed for one week prior to scanning (0 cm = “no pain” to 10 cm = “maximum imaginable pain”) three times a day |
DTI, diffusion tensor imaging; FDR, false-discovery rate; fMRI, functional magnetic resonance imaging; FSL, FMRIB software library; FWE, family-wise error, M1, primary motor cortex; NA, not applicable; SCI, spinal cord injury; SMA, supplementary motor area; SPM, statistical parameter mapping; S1, primary sensory cortex; S2, secondary sensory cortex; VBM, voxel-based morphometry.
Table 2.
Quality assessment of included studies.
| First author | Year | Design | Pathology | Scoring criteria for quality assessment |
Score % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
| Dettmers C. | 2001 | Cross-sectional | Amputation | Y | N | N | Y | N | Y | Y | N | N | N | 40 |
| Diers M. | 2010 | Cross-sectional | Amputation | N | N | N | Y | Y | Y | Y | Y | Y | Y | 70 |
| Foell J. | 2014 | Longitudinal | Amputation | Y | N | N | Y | Y | Y | N | Y | Y | Y | 70 |
| Lotze M. | 2001 | Cross-sectional | Amputation | N | N | N | Y | Y | Y | Y | N | Y | N | 50 |
| MacIver K. | 2008 | Longitudinal | Amputation | Y | Y | N | Y | Y | Y | N | Y | Y | Y | 80 |
| Makin T. | 2013 | Cross-sectional | Amputation | Y | N | N | Y | Y | Y | Y | Y | Y | N | 70 |
| Makin T. | 2015 | Cross-sectional | Amputation | Y | N | N | Y | Y | Y | Y | Y | Y | N | 70 |
| Philip & Frey | 2014 | Cross-sectional | Amputation | Y | N | N | Y | Y | Y | N | Y | Y | N | 70 |
| Gustin S. | 2010 | Cross-sectional | SCI | Y | N | N | Y | Y | Y | N | Y | Y | N | 60 |
| Wrigley P. | 2009 | Cross-sectional | SCI | Y | N | N | Y | Y | Y | Y | Y | Y | N | 70 |
| Overall totals % | 80 | 10 | 0 | 100 | 90 | 100 | 60 | 80 | 90 | 30 | ||||
-
1)Does the study have a clear defined research objective?
-
2)Does the study adequately describe the inclusion criteria?
-
3)Does the study adequately describe the exclusion criteria?
-
4)Does the study report on the population parameters/demographics?
-
5)Does the study report details on assessment of pain?
-
6)Does the study provide details of imaging protocol?
-
7)Does the study provide a proper control group?
-
8)Does the study apply proper statistical analysis? Correction for multiple comparisons?
-
9)Does the study adequately report on the strength of the results (e.g., ways of calculating effect sizes, reporting of confidence intervals/standard deviation)?
-
10)Do the authors report on the limitations of their study?
Y = yes, N = no, Y/N = applies partially.
Fig. 2.
Forest plot of mean age, time since deafferenation, and pain rating for each study, and the grand weighted average for each parameter. The results are displayed in mean ± standard deviation. Please note, Dettmers et al. (2001) did assess the presence of neuropathic pain, but do not report any pain intensities.
3.3. 1st level evidence: fMRI
According to our review criteria, five studies were identified as 1st level. The key findings of each study are summarized in Table 3. Three fMRI studies reported some form of support for maladaptive plasticity (Diers et al., 2010, Lotze et al., 2001, Wrigley et al., 2009). Two 1st level studies found no significant relationship between pain rating and cortical organization (Makin et al., 2013b, Makin et al., 2015b), as well as an association of pain with preserved functional activity in primary sensory (S1) and motor cortices (M1) (Makin et al., 2013b).
Table 3.
fMRI studies meeting the inclusion criteria and adequately designed to assess the relationship between cortical reorganization and neuropathic pain. n, number of subjects with pain; SCI, spinal cord injury; BOLD, blood oxygen level-dependent; M1, primary motor cortex; S1, primary sensory cortex.
| Publication | Type | Key task | n | Summary of key findings related to reorganization and pain | Quality scorea (/10) |
|---|---|---|---|---|---|
| Diers et al. (2010) | Amputation | Imagined movement (phantom hand) | 7 | Negative association between activation in M1 during mirror movements (i.e., contralateral to the hand seen in the mirror) and pain severity. | 7 |
| Lotze et al. (2001) | Amputation | Lip movement | 7 | Individuals with phantom limb pain have a medial shift of the lip into the deafferented hand area, enlarged representation of the mouth, and greater S1 and M1 BOLD activation during lip movement compared to amputees without neuropathic pain and healthy controls. | 5 |
| Makin et al., 2013a, Makin et al., 2013b | Amputation | Lip movements and executed phantom hand movements | 17 | No differences in activation related to lip movements between individuals with and without pain. BOLD activation in the M1 hand area significantly greater in individuals with phantom limb pain and healthy controls compared to amputees without pain; positively correlated with pain rating during executed movement of the phantom hand. | 7 |
| Makin et al., 2015a, Makin et al., 2015b | Amputation | Lip movements | 17 | Small shifts in lip representation contralateral to the missing hand towards, but not invading, the hand area. No statistical relationship between cortical reorganization and phantom sensations or pain. | 7 |
| Wrigley et al. (2009) | SCI | Brushing of the hand | 10 | Significant medial shifts (direction leg area) in location of BOLD activity in S1, correlated with the intensity of below-level neuropathic pain. | 7 |
n, number of subjects with pain; SCI, spinal cord injury; BOLD, blood oxygen level-dependent; M1, primary motor cortex; S1, primary sensory cortex.
Quality assessment criteria and single ratings are listed in supplementary Table 2.
3.4. 2nd level evidence
Summarized in Table 4, 2nd level fMRI studies all lacked one or more control conditions (i.e., healthy subjects and/or individuals without pain) (Dettmers et al., 2001, Foell et al., 2014, Gustin et al., 2010, MacIver et al., 2008, Philip and Frey, 2014). Two of these studies were designed longitudinally, and proposed support for the concept of maladaptive plasticity (Foell et al., 2014, MacIver et al., 2008). Findings from two other 2nd level studies extended the definition of maladaptive plasticity to other brain areas (Dettmers et al., 2001, Gustin et al., 2010). One 2nd level study also reported no associations between cortical activation and pain (Philip and Frey, 2014).
Table 4.
fMRI studies meeting the inclusion criteria and adequately designed to assess the relationship between cortical reorganization and neuropathic pain (second level evidence).
| Study | Type | Key task | n | Summary of findings related to reorganization and pain | Quality scorea (/10) |
|---|---|---|---|---|---|
| Dettmers et al. (2001) | Amputation | Anteflexion of stump | 8 | Increased BOLD activation in SMA in individuals with phantom limb pain. | 4 |
| Foell et al. (2014) | Amputation | Lip movement | 11 | Shift in S1 activity positively correlated with pain relief effect size in response to mirror therapy. | 7 |
| Gustin et al. (2010) | SCI | Imagined leg movement | 11 | Increased BOLD activation significantly correlated with increased pain in a variety of brain areas (not in S1/M1). | 6 |
| MacIver et al. (2008) | Amputation | Lip movement | 13 | Reduction in constant pain intensity significantly correlated with the reduction of activation in M1 hand area. | 8 |
| Philip and Frey (2014) | Amputation | Drawing task | 8 | No significant correlation between cortical activity and pain | 7 |
n, number of subjects with pain; SCI, spinal cord injury; BOLD, blood oxygen level-dependent; M1, primary motor cortex; SMA, supplementary motor area.
Quality assessment criteria and single ratings are listed in supplementary Table 2.
4. Discussion
The primary goal of this review was to determine how advanced functional neuroimaging has been adopted to examine the relationship between chronic pain, deafferentation and cortical reorganization. Based on a review of available literature, we conclude that:
1) There is 1st level evidence in support of the original operational definition of maladaptive plasticity (Diers et al., 2010, Flor et al., 1995, Lotze et al., 2001, Wrigley et al., 2009), accompanied by 2nd level evidence from longitudinal studies (Foell et al., 2014, MacIver et al., 2008).
2) The concept of maladaptive plasticity has been extended beyond the original operational definition (i.e., negative association,) (Diers et al., 2010), and now includes brain areas outside of primary sensorimotor cortices (Dettmers et al., 2001, Gustin et al., 2010).
Our review also revealed 1st level evidence challenging the concept of maladaptive plasticity, proposing the opposite relationship (i.e., preserved organization related to pain), as well as studies (1st and 2nd level) that found no significant association between chronic pain and cortical reorganization (Makin et al., 2015b, Philip and Frey, 2014). Taken together, conflicting and inconsistent findings from fMRI studies highlight an emerging controversy with regard to the strength of the relationship between chronic pain and cortical reorganization after deafferentation, and a need for further investigation using advanced neuroimaging techniques.
4.1. Functional reorganization: evidence of maladaptive plasticity
Although the concept of maladaptive plasticity is nearly 20 years old (Flor et al., 1995), relatively few studies have adopted quantitative fMRI to assess the relationship between pain and cortical reorganization after deafferentation. Nevertheless, consistent with the original definition of maladaptive plasticity, reorganization has been reported in primary sensorimotor cortices, characterized by shifts in cortical activity towards deafferented brain areas (e.g., hand and legs) (Lotze et al., 2006, Wrigley et al., 2009), which are positively related to pain (i.e., greater the shift, the more severe the pain) (Wrigley et al., 2009). Utilizing a mirror movement task, maladaptive plasticity has also been derived on the basis of reduced activation in individuals with phantom pain, and a negative correlation with pain severity — (Diers et al., 2010). In addition, other brain areas have been proposed to undergo maladaptive plasticity, including the supplementary motor area (Dettmers et al., 2001).
From a theoretical perspective, the most convincing evidence in support of the maladaptive plasticity model comes from longitudinal studies assessing pain-modulating interventions (Foell et al., 2014, MacIver et al., 2008). Conceptually, these studies offer the unique potential to examine the relationship between cortical reorganization and pain, as well as to evaluate how the relationship may change as result of an intervention. While consistent with the notion of maladaptive plasticity, both longitudinal studies consist of 2nd level evidence. Due to the lack of adequate control groups, no longitudinal study can conclude that reorganization is specific to reductions in pain. Thus, it remains possible that cortical organization is related to general changes in the brain, or may be a function of other factors (e.g., regression towards the mean) (Harvie and Moseley, 2014).
4.2. An alternative hypothesis: distinct effects of sensory loss and chronic pain on the brain
Interestingly, a number of recent studies have reported a lack of support for maladaptive plasticity, failing to replicate earlier results using a comparable motor task (i.e., lip movement) (Makin et al., 2013b, Makin et al., 2015b). While null findings may be explained by a variety of differences between fMRI studies, and thus not warrant reconsidering the concept of maladaptive plasticity in insolation, emerging evidence suggests an alternative and opposing hypothesis — deafferentation resulting in “disrupted functional cortical representations”, maintained by chronic pain (Makin et al., 2013b). Further questioning the concept of maladaptive plasticity, other factors have been shown to better account for cortical reorganization, including motor performance (Philip and Frey, 2014), and hand usage and dexterity (Makin et al., 2013a, Makin et al., 2013b).
4.3. Anatomical reorganization: what does it mean?
There are a number of emerging techniques to examine anatomical changes in the brain, including quantitative approaches to assess CNS microstructure (e.g., voxel based morphometry). Reorganization in humans has been historically considered in the context of underlying improved functional outcomes, with few studies reconciling the relationship between functional and structural alterations after deafferentation (Makin et al., 2013b, Makin et al., 2015b). The interpretation of anatomical changes in the brain related to deafferentation and pain is, at present, difficult. Indeed, structural changes in volume may indicate a number of different and meaningful physiological processes, including reorganization, but also degeneration and atrophy. In the absence of detailed functional information, resolving the role of anatomical reorganization in response to deafferentation may be problematic (May and Gaser, 2006). Interestingly, emerging anatomical data suggests that gray matter volume, similar to function, may be preserved by the presence of pain (Makin et al., 2013b). Other MR techniques, such as spectroscopy may also be useful to indicate changes in metabolic activity related to anatomical adaptations to pain and deafferentation.
4.4. Distinguishing deafferentation and pain effects on cortical reorganization
The goal of the current review was to focus exclusively on reorganization in individuals with chronic pain after deafferentation (i.e., SCI and amputation). Cortical reorganization has also been investigated using a variety of neuroimaging techniques in other chronic pain conditions, with many in favor of the concept of maladaptive plasticity (Flor, 2002, Flor et al., 1997, Gustin et al., 2012, Maihofner et al., 2003, Maihofner et al., 2007, Pleger et al., 2006). Others still have reported associations between maladaptive plasticity and specific pain symptoms (e.g., paraesthesia), but not pain severity per se (Maeda et al., 2014). The emerging controversy with regard to amputation and SCI may arise from difficulty distinguishing cortical reorganization resulting from deafferentation, and that specifically associated with chronic pain. Indeed, the complex interaction between deafferentation, pain, and cortical reorganization, combined with other factors that are difficult to assess or may be overlooked in the analysis (e.g., completeness of injury after SCI, other phantom sensations, usage of phantom limb) may introduce substantial variability between studies.
4.5. Areas of future research
There are several potential lines of future investigation. First, studies could be improved by consensus with regard to a standardized approach to assess reorganization. Lip movement is an obvious choice, which has already been employed across a number of investigations (Lotze et al., 2001, MacIver et al., 2008, Makin et al., 2013b, Makin et al., 2015a). However, empirical evidence should also be considered in terms of what methods are valid and reliable, as well as sensitive to subtle changes in function and structure. To our knowledge, no study to date has performed a test–rest reliability analysis of measures of cortical reorganization after deafferentation. The development of valid and reliable standardized tasks and methods of assessment across studies would facilitate pooling of results and a future meta-analysis. Adopting a standardized approach, additional cross-sectional studies are needed, further clarifying the direction of the relationship between pain and deafferentation, as well as exploring other confounding variables. An important aim of future cross-sectional studies should also be to include a larger, more representative sample of individuals with SCI and/or amputations. More specifically, individuals with incomplete injury SCI, as well as lower limb amputees should be included in future analyses. In terms of longitudinally designed studies, there is a considerable need to include patient populations without pain, as well as healthy controls, to determine the specificity of reversing reorganization to relieve pain. To improve the consistency and generalizability of findings, there is also a need to better standardize the assessment of pain across studies. Specifically, studies should consider how ‘maximum’ versus ‘average’ versus ‘present’ pain ratings influence cortical reorganization. Lastly, the reviewed studies document changes to the task-activated brain network (fMRI), but little is known about changes in the resting-state brain network due to deafferentation and pain. Results from studies of other pain conditions (e.g., lower back pain and diabetic neuropathic pain) indicate that the spatial or temporal properties of the resting-state networks may be altered in pain states (Cauda et al., 2009, Cauda et al., 2010, Tagliazucchi et al., 2010). Coinvestigating the resting-state brain networks in deafferentation-related pain might potentially reveal pathophysiologic correlates of maladaptive plasticity, which are not detectable employing task-related fMRI.
4.6. Limitations
The most notable limitation of this review is that we did not consider other techniques that have been used to examine cortical reorganization after SCI and amputation, such as electroencephalography (EEG), transcranial magnetic stimulation (TMS), and MEG. As such, we cannot make conclusions on the overall level of evidence, but only how advanced fMRI has contributed to the debate. However, it is interesting to note that the controversy with regard to the direction of the relationship between pain, deafferentation, and reorganization has also recently emerged using MEG (Blume et al., 2014). Different underlying principles of brain activation (i.e., BOLD versus electrical activity, stimulation of the motor cortex versus recording of the motor cortex) may render some functional imaging techniques more suitable than others to assess cortical reorganization. An additional limitation of our review, the quality assessment does not consider statistical approaches applied to assess imaging data (e.g., selection of statistical model, interpretation of correlation analyses). The measurement of cortical reorganization may depend considerably on how neuroimaging data is analyzed (Makin et al., 2015b).
4.7. Potential for publication and search bias
The limited number of studies reporting no association between chronic pain and cortical organization speaks to a high probability of publication, as well as potential for a search bias. We identified two studies that reported the presence and intensity of phantom pain in the methods, but did not plan, perform, and/or report findings from a “pain analysis” (i.e., examining relationship between pain intensity and imaging outcomes) (Bjorkman et al., 2012, Raffin et al., 2012). Since a considerable number of studies using a variety of neuroimaging techniques have addressed reorganization in cortical structures after deafferentation unrelated to pain (Chen et al., 2013, Cramer et al., 2005, Cruz et al., 2003, Curt et al., 2002, Lotze et al., 2006, Manduch et al., 2002, Schwenkreis et al., 2003, Schwenkreis et al., 2001), difficulty of publishing negative results (i.e., no pain specific differences) may contribute to a publication bias.
5. Conclusion
There is evidence supporting the concept of reorganization after SCI and limb amputation, and that the extent of reorganization may depend on the presence and intensity of chronic pain (Flor, 2003, Flor et al., 1995, Lotze et al., 2006). However, current findings from fMRI are inconsistent, even proposing the inverse to maladaptive plasticity — that is, reorganization related to sensory loss and preserved function associated with pain. There is an urgent need for additional studies appropriately designed (i.e., including requisite control groups, large and representative samples) to better address the most likely complex relationship between reorganization and pain after deafferentation. Future studies should also consider a standardized multimodal imaging approach to assess reorganization.
Author's contribution
Catherine R. Jutzeler contributed substantially to the data acquisition, analysis (i.e., quality assessment), and interpretation. Furthermore, she was involved in drafting the review article. Armin Curt made substantial contributions to data analysis (i.e., quality assessment) and participated in revising the review article critically for important intellectual content. John L.K. Kramer contributed substantially to data acquisition, analysis (i.e., quality assessment), and interpretation. Furthermore, he drafted the review article.
Acknowledgment
We would like to thank Prof. Dr. Volker Dietz for his insightful comments regarding the manuscript. The study was supported by the Swiss National Science Foundation (SNF) and the Clinical Research Priority Program “Neurorehab” of the University of Zurich (Grant No. 320030, 135558), Switzerland. John Kramer is supported by a Michael Smith Foundation for Health Research and Rick Hansen Institute Scholar award. Catherine Jutzeler was supported by an ICORD Trainee Travel grant.
Footnotes
The study was supported by the Swiss National Science Foundation (SNF) and the Clinical Research Priority Program “Neurorehab” of the University of Zurich, Switzerland. John Kramer is supported by a Michael Smith Foundation for Health Research and Rick Hansen Institute Scholar award. The authors report no conflict of interests.
References
- Bjorkman A., Weibull A., Olsrud J., Ehrsson H.H., Rosen B., Bjorkman-Burtscher I.M. Phantom digit somatotopy: a functional magnetic resonance imaging study in forearm amputees. Eur. J. Neurosci. 2012;36:2098–2106. doi: 10.1111/j.1460-9568.2012.08099.x. [DOI] [PubMed] [Google Scholar]
- Blume K.R., Dietrich C., Huonker R., Gotz T., Sens E., Friedel R., Hofmann G.O., Miltner W.H., Weiss T. Cortical reorganization after macroreplantation at the upper extremity: a magnetoencephalographic study. Brain. 2014;137:757–769. doi: 10.1093/brain/awt366. [DOI] [PubMed] [Google Scholar]
- Campbell P., Wynne-Jones G., Dunn K.M. The influence of informal social support on risk and prognosis in spinal pain: a systematic review. Eur. J. Pain. 2011;15(444) doi: 10.1016/j.ejpain.2010.09.011. (e441-414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., Sacco K., Duca S., Cocito D., D'Agata F., Geminiani G.C., Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., D'Agata F., Sacco K., Duca S., Cocito D., Paolasso I., Isoardo G., Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J. Neurol. Neurosurg. Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- Chen A., Yao J., Kuiken T., Dewald J.P. Cortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervation. NeuroImage Clin. 2013;3:498–506. doi: 10.1016/j.nicl.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S.C., Lastra L., Lacourse M.G., Cohen M.J. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005;128:2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- Cruz V.T., Nunes B., Reis A.M., Pereira J.R. Cortical remapping in amputees and dysmelic patients: a functional MRI study. NeuroRehabilitation. 2003;18:299–305. [PubMed] [Google Scholar]
- Curt A., Alkadhi H., Crelier G.R., Boendermaker S.H., Hepp-Reymond M.C., Kollias S.S. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain. 2002;125:2567–2578. doi: 10.1093/brain/awf250. [DOI] [PubMed] [Google Scholar]
- Dettmers C., Adler T., Rzanny R., van Schayck R., Gaser C., Weiss T., Miltner W.H., Bruckner L., Weiller C. Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neurosci. Lett. 2001;307:109–112. doi: 10.1016/s0304-3940(01)01953-x. [DOI] [PubMed] [Google Scholar]
- Diers M., Christmann C., Koeppe C., Ruf M., Flor H. Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. Pain. 2010;149:296–304. doi: 10.1016/j.pain.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Ding Y., Kastin A.J., Pan W. Neural plasticity after spinal cord injury. Curr. Pharm. Des. 2005;11:1441–1450. doi: 10.2174/1381612053507855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. The modification of cortical reorganization and chronic pain by sensory feedback. Appl. Psychophysiol. Biofeedback. 2002;27:215–227. doi: 10.1023/a:1016204029162. [DOI] [PubMed] [Google Scholar]
- Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J. Rehabil. Med. 2003;66–72 doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- Flor H., Elbert T., Knecht S., Wienbruch C., Pantev C., Birbaumer N., Larbig W., Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Flor H., Braun C., Elbert T., Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 1997;224:5–8. doi: 10.1016/s0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- Foell J., Bekrater-Bodmann R., Diers M., Flor H. Mirror therapy for phantom limb pain: brain changes and the role of body representation. Eur. J. Pain. 2014;18:729–739. doi: 10.1002/j.1532-2149.2013.00433.x. [DOI] [PubMed] [Google Scholar]
- Green J.B., Sora E., Bialy Y., Ricamato A., Thatcher R.W. Cortical motor reorganization after paraplegia: an EEG study. Neurology. 1999;53:736–743. doi: 10.1212/wnl.53.4.736. [DOI] [PubMed] [Google Scholar]
- Gustin S.M., Wrigley P.J., Henderson L.A., Siddall P.J. Brain circuitry underlying pain in response to imagined movement in people with spinal cord injury. Pain. 2010;148:438–445. doi: 10.1016/j.pain.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Gustin S.M., Peck C.C., Cheney L.B., Macey P.M., Murray G.M., Henderson L.A. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J. Neurosci. Off. J. Soc. Neurosci. 2012;32:14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie D., Moseley G.L. Exploring changes in the brain associated with recovery from phantom limb pain — the potential importance of telescoping. Eur. J. Pain. 2014;18:601–602. doi: 10.1002/j.1532-2149.2014.00458.x. [DOI] [PubMed] [Google Scholar]
- Jain N., Catania K.C., Kaas J.H. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- Jain N., Florence S.L., Qi H.X., Kaas J.H. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman C.M., Dijkstra P.U., Geertzen J.H., Elzinga A., van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87(1):33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- Lotze M., Flor H., Grodd W., Larbig W., Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124:2268–2277. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- Lotze M., Laubis-Herrmann U., Topka H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor. Neurol. Neurosci. 2006;24:97–107. [PubMed] [Google Scholar]
- MacIver K., Lloyd D.M., Kelly S., Roberts N., Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131:2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Kettner N., Holden J., Lee J., Kim J., Cina S., Malatesta C., Gerber J., McManus C., Im J., Libby A., Mezzacappa P., Morse L.R., Park K., Audette J., Tommerdahl M., Napadow V. Functional deficits in carpal tunnel syndrome reflect reorganization of primary somatosensory cortex. Brain. 2014;137:1741–1752. doi: 10.1093/brain/awu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihofner C., Handwerker H.O., Neundorfer B., Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–1715. doi: 10.1212/01.wnl.0000098939.02752.8e. [DOI] [PubMed] [Google Scholar]
- Maihofner C., Baron R., DeCol R., Binder A., Birklein F., Deuschl G., Handwerker H.O., Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- Makin T.R., Cramer A.O., Scholz J., Hahamy A., Henderson Slater D., Tracey I., Johansen-Berg H. Deprivation-related and use-dependent plasticity go hand in hand. eLife. 2013;2:e01273. doi: 10.7554/eLife.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., Scholz J., Filippini N., Henderson Slater D., Tracey I., Johansen-Berg H. Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 2013;4:1570. doi: 10.1038/ncomms2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., Filippini N., Duff E.P., Henderson Slater D., Tracey I., Johansen-Berg H. Network-level reorganisation of functional connectivity following arm amputation. NeuroImage. 2015;114:217–225. doi: 10.1016/j.neuroimage.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., Scholz J., Henderson Slater D., Johansen-Berg H., Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain. 2015;138:2140–2146. doi: 10.1093/brain/awv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduch M., Bezuhly M., Anastakis D.J., Crawley A.P., Mikulis D.J. Serial fMRI of adaptive changes in primary sensorimotor cortex following thumb reconstruction. Neurology. 2002;59:1278–1281. doi: 10.1212/wnl.59.8.1278. [DOI] [PubMed] [Google Scholar]
- May A., Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr. Opin. Neurol. 2006;19:407–411. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- Philip B.A., Frey S.H. Compensatory changes accompanying chronic forced use of the nondominant hand by unilateral amputees. J. Neurosci. Off. J. Soc. Neurosci. 2014;34:3622–3631. doi: 10.1523/JNEUROSCI.3770-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B., Ragert P., Schwenkreis P., Forster A.F., Wilimzig C., Dinse H., Nicolas V., Maier C., Tegenthoff M. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. NeuroImage. 2006;32:503–510. doi: 10.1016/j.neuroimage.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Poline J.B., Worsley K.J., Evans A.C., Friston K.J. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5(2):83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Raffin E., Mattout J., Reilly K.T., Giraux P. Disentangling motor execution from motor imagery with the phantom limb. Brain. 2012;135:582–595. doi: 10.1093/brain/awr337. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P., Witscher K., Janssen F., Pleger B., Dertwinkel R., Zenz M., Malin J.P., Tegenthoff M. Assessment of reorganization in the sensorimotor cortex after upper limb amputation. Clin. Neurophysiol. 2001;112:627–635. doi: 10.1016/s1388-2457(01)00486-2. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P., Pleger B., Cornelius B., Weyen U., Dertwinkel R., Zenz M., Malin J.P., Tegenthoff M. Reorganization in the ipsilateral motor cortex of patients with lower limb amputation. Neurosci. Lett. 2003;349:187–190. doi: 10.1016/s0304-3940(03)00838-3. [DOI] [PubMed] [Google Scholar]
- Sica R.E., Sanz O.P., Cohen L.G., Freyre J.D., Panizza M. Changes in the N1–P1 component of the somatosensory cortical evoked response in patients with partial limb amputation. Electromyogr. Clin. Neurophysiol. 1984;24:415–427. [PubMed] [Google Scholar]
- Tagliazucchi E., Balenzuela P., Fraiman D., Chialvo D.R. Brain resting state is disrupted in chronic back pain patients. Neurosci. Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley P.J., Press S.R., Gustin S.M., Macefield V.G., Gandevia S.C., Cousins M.J., Middleton J.W., Henderson L.A., Siddall P.J. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Yang T.T., Gallen C.C., Ramachandran V.S., Cobb S., Schwartz B.J., Bloom F.E. Noninvasive detection of cerebral plasticity in adult human somatosensory cortex. Neuroreport. 1994;5:701–704. doi: 10.1097/00001756-199402000-00010. [DOI] [PubMed] [Google Scholar]