Abstract
Background
Dysconnectivity in schizophrenia can be understood in terms of dysfunctional integration of a distributed network of brain regions. Here we propose a new methodology to analyze complex networks based on semi-metric behavior, whereby higher levels of semi-metricity may represent a higher level of redundancy and dispersed communication. It was hypothesized that individuals with (increased risk for) psychotic disorder would have more semi-metric paths compared to controls and that this would be associated with symptoms.
Methods
Resting-state functional MRI scans were obtained from 73 patients with psychotic disorder, 83 unaffected siblings and 72 controls. Semi-metric percentages (SMP) at the whole brain, hemispheric and lobar level were the dependent variables in a multilevel random regression analysis to investigate group differences. SMP was further examined in relation to symptomatology (i.e., psychotic/cognitive symptoms).
Results
At the whole brain and hemispheric level, patients had a significantly higher SMP compared to siblings and controls, with no difference between the latter. In the combined sibling and control group, individuals with high schizotypy had intermediate SMP values in the left hemisphere with respect to patients and individuals with low schizotypy. Exploratory analyses in patients revealed higher SMP in 12 out of 42 lobar divisions compared to controls, of which some were associated with worse PANSS symptomatology (i.e., positive symptoms, excitement and emotional distress) and worse cognitive performance on attention and emotion processing tasks. In the combined group of patients and controls, working memory, attention and social cognition were associated with higher SMP.
Discussion
The results are suggestive of more dispersed network communication in patients with psychotic disorder, with some evidence for trait-based network alterations in high-schizotypy individuals. Dispersed communication may contribute to the clinical phenotype in psychotic disorder. In addition, higher SMP may contribute to neuro- and social cognition, independent of psychosis risk.
Keywords: Semi-metric percentage, Psychotic disorder, Unaffected siblings, Graph theory, Functional brain network, Resting-state fMRI
Highlights
-
•
Higher SMP was observed at whole brain and hemispheric level in psychotic disorder.
-
•
In patients, lobar SMP was associated with psychotic and cognitive symptoms.
-
•
Trait-based SMP alterations were observed in high schizotypy individuals.
1. Introduction
Dysconnectivity in schizophrenia can be understood as dysfunctional integration of a distributed network of brain regions (Friston, 1999). Meta-analytic reviews of MRI studies of schizophrenia have revealed alterations in gray (Ellison-Wright et al., 2008, Glahn et al., 2008), and white matter organization (Ellison-Wright and Bullmore, 2009) as well as altered functional activation across cognitive tasks (Minzenberg et al., 2009). Furthermore, the resting-state functional MRI (rs-fMRI) literature has described dysconnectivity in schizophrenia in several brain regions, including prefrontal-temporal regions, default mode network (DMN) dysconnectivity and decreased frontoparietal connectivity (FPN) (Friston and Frith, 1995, Rotarska-Jagiela et al., 2010). Also, studies using graph theoretical analyses have shown that functional brain network organization in schizophrenia is typically less small world, less clustered, less efficiently wired and less dominated by hubs (Alexander-Bloch et al., 2010, Bassett and Bullmore, 2009, Bassett et al., 2008, Bullmore and Sporns, 2009, Liu et al., 2008, Lynall et al., 2010, van den Heuvel et al., 2010).
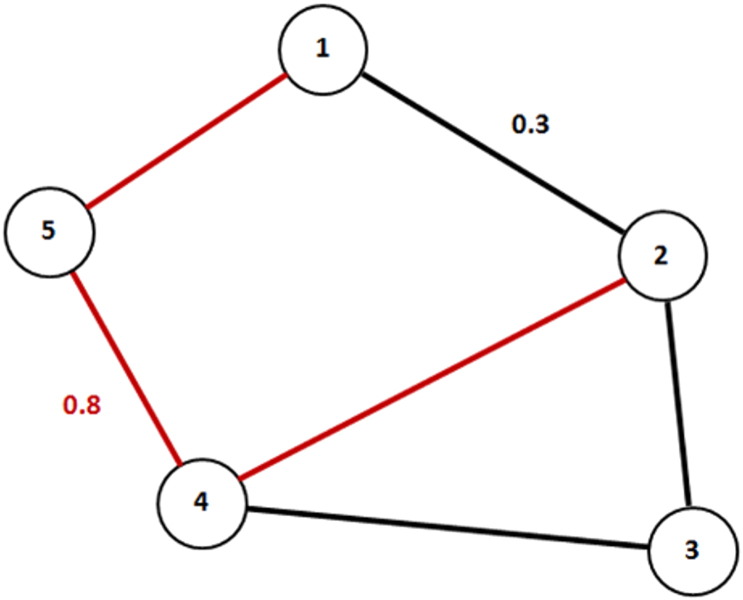
Here we propose a new graph theoretical methodology to analyze complex functional brain networks in psychotic disorder, based on semi-metric behavior. Generally, brain regions in a network are described as nodes and connections between nodes as edges (Bullmore and Sporns, 2009, Sporns et al., 2005). Weighted networks, such as fMRI connectivity networks are characterized by a high number of transitivity violations. A relation is transitive when a node A is related to a node B, and B is related to C, implying that A is also related to C (i.e., via indirect association). Transitivity violations occur when the sum of weights along an indirect path between two nodes (i.e., involvement of additional regions), is greater than the weight of the direct path between them. In this sense, the indirect path represents the shortest path between the two nodes (Fig. 1), which in the case of weighted graphs is a sequence of correlations that increase the overall correlation between the two nodes, compared to a more direct topological route. This type of network is called semi-metric (Simas et al., 2015). Higher levels of semi-metricity indicate more indirect paths, which may represent a higher level of redundancy (Simas et al., 2015), i.e., interactions between multiple network nodes (Leistritz et al., 2013).
Fig. 1.
Example of a semi-metric network. The red line represents the indirect path between node 1 and 2. The sum of weights along this indirect path (i.e., 0.8) is greater than the sum of weights of the direct path between node 1 and 2 (i.e., 0.3). Therefore, the indirect path is semi-metric.
To date, only one study has investigated the amount of semi-metric connections in a sample of adolescents with autism spectrum condition (ASC) and major depressive disorder (MDD). ASC was associated with more semi-metric connections, whereas MDD was characterized by a higher level of metric, constrained connections. It was suggested that constrained connections may be associated with rumination, which is characteristic in depression, whereas dispersed communication (the involvement of multiple regions) in ASC may be associated with a lack of central coherence often seen in this disorder (Belmonte et al., 2004). Thus, semi-metricity is thought to provide information about network communication/information processing, which potentially makes it a useful method to detect and classify a wide range of psychiatric and developmental disorders (Simas et al., 2015).
Psychotic disorder is characterized by a disruption in thought processes and an altered perception of reality. These symptoms may arise from aberrant communication between multiple brain regions (Calhoun et al., 2009, Repovs et al., 2011, Stephan et al., 2009). It is speculated that the interaction between multiple brain regions along a semi-metric path interferes with information processing and coordination of mental functions. Following this assumption, we expected that patients would have a higher number of semi-metric paths (i.e., more dispersed communication) than controls. Additionally, since there is evidence for rs-functional connectivity intermediate phenotypes (Chang et al., 2014, Fornito et al., 2013, Guo et al., 2014a, Guo et al., 2014b, Repovs and Barch, 2012, Su et al., 2013, Whitfield-Gabrieli and Ford, 2012, Yu et al., 2013b) it was hypothesized that this would also apply to semi-metric paths. As this is the first study that used semi-metricity to measure functional network alterations in patients with (increased risk for) psychotic disorder a whole brain, hypothesis-generating approach, was used.
Rs-fMRI studies have shown associations between functional connectivity (in, e.g., DMN, FPN) and symptoms of schizophrenia (Karbasforoushan and Woodward, 2012, van den Heuvel and Fornito, 2014). Only a few studies have investigated such relationships using graph theoretical approaches (Bassett et al., 2012, Shim et al., 2014, Skudlarski et al., 2010). Therefore, exploratory analyses were performed to investigate whether psychotic and cognitive symptoms would be associated with disease-related and risk-related semi-metricity alterations.
2. Methods
2.1. Participants
Data were collected from a longitudinal MRI study in Maastricht, the Netherlands. Recruitment and inclusion criteria have been described elsewhere (Habets et al., 2011). The final sample comprised 73 patients with psychotic disorder, 83 unaffected siblings and 72 controls, after excluding participants from the analyses based on: schizotypy (n = 3), movement (n = 8), scanner artifacts (n = 14), smoking cannabis prior to scanning (n = 1) and experimental issues (n = 20). The sample contained 46 families: 25 families with one patient and one sibling, three families with one patient and two siblings, one family with two patients, six families with two siblings, and two families with one patient and three siblings. In the control group, there were nine families with two siblings. In addition, 41 independent patients, 34 independent siblings, and 54 independent controls were included.
Diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV) criteria (American Psychiatric Association, 2000), assessed with the Comprehensive Assessment of Symptoms and History (CASH) interview (Andreasen et al., 1992). Patients were diagnosed with: schizophrenia (n = 47), schizoaffective disorder (n = 9), schizophreniform disorder (n = 4), brief psychotic disorder (n = 2), and psychotic disorder not otherwise specified (n = 11). Schizotypy was assessed with the Structured Interview for Schizotypy—revised (SIS-r) (Vollema and Ormel, 2000). Based on the CASH, 16 siblings and 10 controls had a history of MDD.
Before MRI acquisition, participants were screened for the following exclusion criteria: brain injury (unconsciousness > 1 h), meningitis or other neurological diseases that might have affected brain structure or function, cardiac arrhythmia requiring medical treatment, and severe claustrophobia. Additionally, participants with metal corpora aliena were excluded from the study, as were women with intrauterine device status and (suspected) pregnancy. The standing ethics committee approved the study, and all participants gave written informed consent in accordance with the committee's guidelines and with the Declaration of Helsinki (Nylenna and Riis, 1991).
2.2. Measures
Psychopathology assessments were done at the time of scanning using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) in all three groups. The five factor model by van der Gaag et al. (2006) was used (van der Gaag et al., 2006).
Educational level was defined as highest accomplished educational level. Handedness was assessed using the Annett Handedness Scale (Annett, 1970).
Attention/vigilance was assessed using a Continuous Performance Test (CPT-HQ) with WM load, also known as CPT-AX (Nuechterlein and Dawson, 1984) (longer reaction times reflecting worse performance). The WAIS-III (Wechsler, 1997) subtest Arithmetic was used to measure WM, which addresses both verbal comprehension and arithmetic skills. Two areas of social cognition that have been associated with psychotic symptoms were investigated, i.e., facial emotion processing and theory of mind (ToM) (de Achaval et al., 2010, Penn et al., 2008). Emotion processing was measured with the Degraded Facial Affect Recognition task (DFAR) using the overall proportion of correct answers (van 't Wout et al., 2004), whereas ToM was assessed using the raw scores of the hinting task (Versmissen et al., 2008). The hinting task assesses the mentalizing-capacity required to comprehend real intentions behind indirect speech. For the arithmetic, DFAR and hinting task, higher scores indicate better performance.
Substance use was measured with the Composite International Diagnostic Interview (CIDI) sections B, J and L (WHO, 1990). Cannabis (4% missing data) and other drug use (2% missing) were based on the lifetime number of instances of use. Cigarette smoking (7% missing) and alcohol use (9% missing) were based on respectively daily and weekly consumptions over the last 12 months.
In patients, antipsychotic (AP) medication use was determined by patient reports and verified with the treating consultant psychiatrist. Best estimate lifetime (cumulative) AP use was determined by multiplying the number of days of AP use with the corresponding haloperidol equivalents and summing these scores for all periods of AP use (including the exposure period between baseline assessment for the G.R.O.U.P. study and the moment of baseline MRI scanning), using the conversion formulas for AP dose equivalents described by Andreasen et al. (2010).
2.3. MRI acquisition
All functional and anatomical MRI images were acquired using the same 3 T Siemens scanner. The functional rs-data were acquired using an Echo-Planar Imaging (EPI) sequence: 200 volumes; 27 slices; TE: 30 ms; TR: 1500 ms; voxel size: 3.5 × 3.5 × 4.0 mm3; flip angle 90°; acquisition time: 5 min. During the scan, participants were instructed to lie with their eyes closed, think of nothing in particular, and not fall asleep. Additionally, anatomical scans had the following acquisition parameters: (1) Modified Driven Equilibrium Fourier Transform (MDEFT) sequence: 176 slices; voxel size: 1 mm isotropic; TE: 2.4 ms; TR: 7.92 ms; TI: 910 ms; flip angle: 15°; acquisition time: 12 min 51 s; (2) Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE; Alzheimer's Disease Neuroimaging Initiative) sequence: 192 slices; voxel size: 1 mm isotropic; TE: 2.6 ms; TR: 2250 ms; TI: 900 ms; flip angle 9°, acquisition time: 7 min 23 s. For both anatomical scans the matrix size was 256 × 256 and field of view was 256 × 256 mm2. Two sequences were used because of a scanner update during data collection. The MPRAGE and MDEFT are very similar, but to prevent systematic bias, the total proportion of MPRAGE scans (44%) was balanced between the groups.
2.4. Data preprocessing and analysis
Imaging data were pre-processed to account for head motion, as described by Patel et al. (2014) and Jo et al. (2013) using Analysis of Functional NeuroImages (AFNI, version 2011_12_21_1014) (Cox, 1996) and the Oxford Centre for Functional MRI of the Brain Software Library (FSL, version 5.0.4) (Jenkinson and Smith, 2001, Jenkinson et al., 2002). The first four volumes of each rs-data set were removed to eliminate the non-equilibrium magnetization effects. Preprocessing steps included slice-time correction, temporal despiking, high pass filtering (0.02 Hz), co-registration to structural scan, spatial normalization and spatial smoothing (6-mm full width at half maximum Gaussian kernel). Nuisance variables were removed through linear regression: six motion parameters, their first temporal derivatives, and cerebrospinal fluid (CSF) signal from ventricular regions of interest.
For each individual, a fully connected, non-negatively weighted (as suggested by Rubinov and Sporns (2010) (Rubinov and Sporns, 2010)), undirected brain network was derived from the mean time-series of each of the 90 anatomically parcellated regions (i.e. nodes) as specified in the automated anatomical labeling atlas (AAL) (Tzourio-Mazoyer et al., 2002). Time series averaged over all voxels in each of the regions were computed and constituted the set of regional mean time series used for wavelet correlation analysis. Connectivity graphs were constructed based on correlations between wavelet coefficients of the second scale, corresponding to a 0.08–0.167 Hz frequency band. Scale 2 was selected since it represents the frequency band where the BOLD signal is strongly correlated with the underlying physiology; the remaining scales were not subjected to investigation as they may represent uncorrelated noise from non-biological sources (Achard et al., 2006). Although previous functional connectivity studies have focused on low frequency bands (< 0.1 Hz), recent studies have indicated the importance of higher frequencies (> 0.1 Hz) (Chen and Glover, 2014, Hyder and Rothman, 2010, Yuan et al., 2014).
Data were further analyzed using Matlab (The MathWorks, Natick, MA, USA). Output was, per participant, depicted in an association matrix in which the semi-metric ratio of the 90 regions was displayed (see supplemental information for theoretical background). Semi-metric ratio is defined by dividing the direct distance between two nodes by the indirect distance between two nodes.
where is the shortest (direct or indirect) distance between i and j in distance graph D calculated by Johnson's algorithm (Johnson, 1954). sij is positive and > 1 for semi-metric edges (see Rocha, 2002 for more details).
Subsequently, we calculated the average percentage of semi-metric paths for each individual association matrix:
where sij represents the semi-metric ratio and |E| the total number of direct connections in the network. It could be speculated that the synchronous co-activation between two regions and all regions in the semi-metric path represents the level of dispersed communication in a functional brain network.
These analyses were done for the whole brain as well as for both hemispheres (left/right) and lobar divisions. Lobar divisions included frontal, parietal, occipital, temporal, limbic and basal ganglia (Table S1). This lobar structure was used to localize global differences of semi-metricity within the brain network and to observe their inter-regional relationships. Semi-metric percentage (SMP) was assessed within and between lobar divisions (confined to nodes within the lobar division but including all paths). This resulted in a total of 21 (inter-/intra-) lobar division SMP values per hemisphere (Table S2).
2.5. Group differences in semi-metric percentage
For every participant, the whole brain, hemispheric and lobar SMPs were transported to STATA version 12 (StataCorp., 2011). Multilevel random regression models were fitted using the XTREG command (Goldstein, 1987) given hierarchical clustering occasioned by the fact that participants were clustered in families, compromising statistical independence of the observations. SMP (whole brain/hemispheres/lobar divisions) was the dependent variable and group was the independent variable. Group was entered as linear and dummy variables (controls = 0, siblings = 1, patients = 2). Analyses were adjusted for the a-priori hypothesized confounders: age, sex, handedness and educational level. In addition, between-group analyses at the whole brain and hemispheric level were performed with correction for the subject-level confounders tobacco, alcohol, cannabis and other drugs. Although not previously investigated, these confounders may affect SMP since studies have shown that these substances have an influence on functional connectivity (Ding and Lee, 2013, Ma et al., 2011, Roberts and Garavan, 2010, Tomasi et al., 2010, Volkow et al., 2008). The patient population included 26 patients with a diagnosis other than schizophrenia. Planned sensitivity analyses were conducted by excluding these individuals from the analyses. Furthermore, to examine whether participants with higher schizotypy scores would be more similar to patients with respect to SMP, analyses were repeated including a low and high schizotypy group (these groups were created using the XTILE command in STATA). Schizotypy was measured in the combined group of siblings and controls and the mean score on the following SIS-r items was entered in the analyses: referential thinking, suspiciousness, magical ideation, illusions, psychotic phenomena, derealization/depersonalization, social isolation, introversion, sensitivity, restricted affect, disturbances in associative and goal-directed thinking, poverty of speech and eccentric behavior.
2.6. Exploratory analyses
In case of significant group differences at the whole brain level and hemispheric level, (inter-/intra-) lobar level analyses were conducted. Thus, the lobar-specific group comparisons and associated clinical correlates were described as exploratory post-hoc results and not subjected to multiple comparisons.
The associations between SMPs (independent variable) and psychotic symptoms/(social) cognitive performance (dependent variable) were examined with multilevel regression analyses, using the lobar divisions that revealed significant between-group effects. In patients, the association between SMP and psychotic symptoms was corrected for age, sex, lifetime AP exposure and illness duration. Associations with (social) cognitive performance were investigated in the combined group (of either patients, siblings and controls or patients and controls depending on the group differences that were found). To examine whether the association between SMP and (social) cognitive performance would be conditional on group, interactions were tested between group and SMP. In case of significant interactions, stratified effect sizes for SMP for each group were calculated by combination of effects from the model containing the interactions using the STATA MARGINS routine. Analyses with (social) cognitive performance were corrected for group, age, sex, handedness and educational level.
In addition, associations between AP medication and SMP at whole brain and hemispheric level were analyzed in patients only, with AP medication as independent variable and age, sex and illness duration as confounders.
3. Results
3.1. Descriptive analyses
The study comprised a relatively stable patient group as reflected by the low PANSS scores. Patients had lower scores on the arithmetic and hinting task compared to controls and siblings, indicating worse WM and ToM. Patients had a longer reaction time on the CPT-HQ compared to controls, reflecting a worse span of attention/vigilance. No differences in performance were observed for the DFAR task (emotion processing) (Table 1). Sixty-four patients were receiving AP medication (second generation: n = 60; first generation: n = 4). Furthermore, twelve patients used antidepressants, three used benzodiazepines, five used anticonvulsants and one used lithium. Two siblings and two controls used antidepressants, and one control used benzodiazepines. Lifetime AP use was not associated with SMP (whole brain: B < 0.01, p = 0.903, hemispheres; left: B < 0.01, p = 0.973, right: B < 0.01, p = 0.617).
Table 1.
Demographics of the participants.
| Patients (n = 73) Mean (SD) |
Siblings (n = 83) Mean (SD) |
Controls (n = 72) Mean (SD) |
|
|---|---|---|---|
| Age at scan | 27.8 (6.6) | 29.6 (9.1) | 30.0 (10.8) |
| Sex n (%) male | 49 (65%) | 45 (54%) | 26 (36%) |
| Handedness | 72.1 (63.9) | 80.1 (53.8) | 73.5 (61.2) |
| Level of education | 4.2 (2.0) | 5.2 (1.9) | 5.4 (1.8) |
| Cannabis usea | 51.7 (47.6) | 18.1 (36.0) | 8.4 (22.8) |
| Cigarettes useb | 11.4 (11.0) | 2.6 (6.2) | 1.9 (6.1) |
| Alcohol usec | 6.7 (13.0) | 10.1 (17.7) | 5.1 (7.2) |
| Other drug used | 44.4 (87.5) | 6.4 (33.0) | 2.4 (12.8) |
| PANSS positive | 9.7 (4.1) | 7.4 (1.5) | 7.3 (1.2) |
| PANSS negative | 11.9 (6.1) | 8.5 (2.2) | 8.2 (1.0) |
| PANSS disorganization | 12.0 (3.3) | 10.4 (1.0) | 10.2 (1.2) |
| PANSS excitement | 9.9 (2.9) | 8.6 (1.4) | 8.3 (1.1) |
| PANSS emotional distress | 12.7 (5.2) | 9.9 (2.7) | 9.3 (2.1) |
| SIS-r positive subscale | 0.6 (0.4) | 0.5 (0.5) | |
| CPT-HQ reaction time | 442.3 (91.8) | 414.9 (76.6) | 412.3 (82.7) |
| WAIS-III arithmetic | 12.5 (4.2) | 15.3 (3.7) | 15.5 (4.1) |
| DFAR | 71.2 (10.4) | 71.8 (8.4) | 73.0 (8.6) |
| Hinting task | 18.0 (2.9) | 19.2 (1.3) | 19.3 (1.1) |
| Age of onset (years) | 21.4 (6.8) | ||
| Illness duration (years) | 6.4 (3.7) | ||
| Lifetime exposure to APe | 7022.9 (6711.3) | ||
| Current dosage of AP medication (mg)f | 5.3 (4.8) |
Abbreviations: SD = standard deviation, PANSS = Positive and Negative Syndrome Scale; SIS-r = Structured Interview for Schizotypy—revised; CPT-HQ = Continuous Performance Test; WAIS = Wechsler Adult Intelligence Scale; DFAR = Degraded Facial Affect Recognition; AP = anti-psychotics.
Lifetime number of instances of cannabis use.
Number of daily consumptions over the last 12 months.
Number of weekly consumptions over the last 12 months.
Lifetime number of times of hard drug use.
Lifetime number of days of AP use.
In terms of standard haloperidal equivalents.
3.2. Between-group differences at the whole brain and hemispheric level
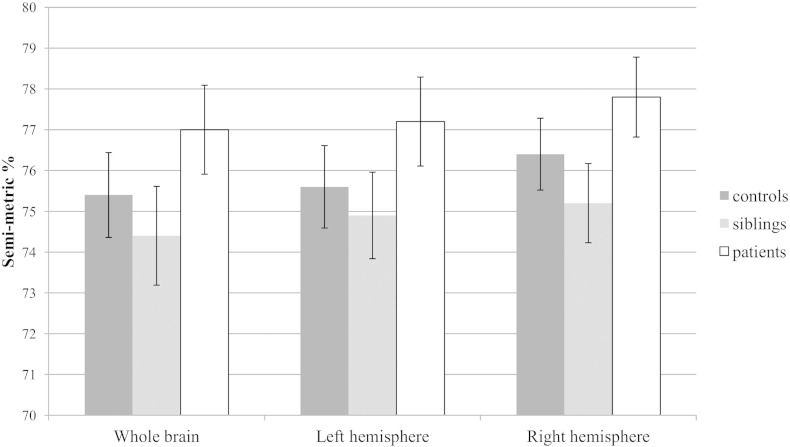
At the whole brain level, patients showed a significantly higher SMP compared to siblings and controls, whereas siblings and controls did not differ on SMP. Similarly, in both hemispheres patients revealed a significantly higher SMP compared to siblings and controls, with no difference between the latter (Table 2 and Fig. 2).
Table 2.
Associations between genetic risk for psychotic disorder (group) and semi-metric percentage at whole brain and hemispheric level.
| Semi-metric percentage N = 228 |
Group differences in semi-metric percentage |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients (n = 73) |
Siblings (n = 83) |
Controls (n = 72) |
P vs. C |
S vs. C |
P vs. S |
||||
| Mean (SD) | Mean (SD) | Mean (SD) | B | p | B | p | B | p | |
| Whole brain | 77.0 (3.8) | 74.4 (4.6) | 75.4 (4.6) | 1.97 | 0.010⁎ | − 0.68 | 0.307 | 2.54 | 0.000⁎ |
| Left hemisphere | 77.2 (4.2) | 74.9 (4.9) | 75.6 (5.1) | 1.77 | 0.028⁎ | − 0.36 | 0.625 | 2.13 | 0.005⁎ |
| Right hemisphere | 77.8 (4.2) | 75.2 (5.0) | 76.4 (4.6) | 1.97 | 0.012⁎ | − 0.67 | 0.354 | 2.63 | 0.000⁎ |
The B-values represent the regression coefficients from multilevel random regression analysis in Stata. Abbreviations SD = standard deviation; P = Patients; C = controls; p-values refer to between group differences; the asterisks (*) represent significant group differences.
Fig. 2.
Mean semi-metric percentage with 95% confidence interval for whole brain and both hemispheres.
Repeating the analyses correcting for additional confounders (tobacco, alcohol, cannabis and other drugs) did not affect the pattern of results (Table S3). As siblings were not significantly different from patients and controls and did not have intermediate SMP values, they were excluded from the exploratory (lobar) analyses presented below.
3.3. Between group differences at the lobar level
To test for patient-control differences at a (inter-/intra-) lobar level 21 statistical tests per hemisphere were conducted. In the left hemisphere, group differences were observed within five lobar divisions: parietal, temporal, frontal–occipital, limbic–parietal and limbic–basal ganglia. In the right hemisphere, seven lobar divisions revealed significant group differences: the temporal, frontal–limbic, frontal–basal ganglia, frontal–temporal, limbic–temporal, occipital–parietal and occipital–basal ganglia lobar divisions. Patients had a higher SMP compared to controls in all the above mentioned lobar divisions (Table 3).
Table 3.
Significant associations between genetic risk for psychotic disorder (group) and semi-metric percentage at a lobar level. The B-values represent the regression coefficients from multilevel random regression analysis in Stata.
| Semi-metric percentage N = 145 |
Group differences in semi-metric percentage |
|||
|---|---|---|---|---|
| Patients (n = 73) Mean (SD) |
Controls (n = 72) Mean (SD) |
P vs C |
||
| B | P | |||
| Lobar divisions, left hemisphere | ||||
| Parietal | 36.6 (13.8) | 28.8 (15.2) | 6.88 | 0.008⁎ |
| Temporal | 72.8 (11.4) | 66.3 (12.6) | 5.88 | 0.006⁎ |
| Frontal–occipital | 82.4 (6.0) | 80.3 (6.2) | 2.38 | 0.028⁎ |
| Limbic–parietal | 86.3 (8.0) | 83.8 (9.2) | 3.11 | 0.044⁎ |
| Limbic–basal ganglia | 79.6 (7.9) | 76.9 (9.4) | 3.68 | 0.018⁎ |
| Lobar divisions, right hemisphere | ||||
| Temporal | 62.5 (13.4) | 55.7 (13.9) | 7.32 | 0.002⁎ |
| Frontal–limbic | 83.7 (4.8) | 81.9 (6.4) | 2.28 | 0.024⁎ |
| Frontal–basal ganglia | 82.2 (8.0) | 79.7 (7.8) | 3.07 | 0.039⁎ |
| Frontal–temporal | 81.5 (7.6) | 79.7 (6.8) | 3.34 | 0.007⁎ |
| Limbic–temporal | 76.3 (8.2) | 73.5 (9.8) | 3.18 | 0.048⁎ |
| Occipital–parietal | 74.3 (8.7) | 72.4 (8.5) | 3.30 | 0.030⁎ |
| Occipital–basal ganglia | 85.4 (9.9) | 81.5 (10.7) | 4.97 | 0.006⁎ |
Abbreviations SD = standard deviation; P = Patients; C = controls; p-values refer to between group differences; the asterisks (*) represent lobar divisions with significant group differences.
3.4. Association between lobar semi-metric percentage and PANSS symptoms
The 12 lobar divisions that revealed significant group differences were selected to examine the association between SMP and psychopathology (i.e., positive symptoms, negative symptoms, disorganization symptoms, excitement and emotional distress) and yielded 7 significant results. In the patient group, a significant positive association was found between both right occipital–parietal and right occipital–basal ganglia SMP and positive symptoms, excitement and emotional distress. Furthermore, a significant positive association was found between right frontal–basal ganglia SMP and excitement in the patient group (Table 4).
Table 4.
Associations between SMP and psychotic symptoms in patients.
| Positive symptoms |
Negative symptoms |
Disorganization symptoms |
Excitement |
Emotional distress |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | p | B | p | B | P | B | p | B | p | |
| Lobar division, left hemisphere | ||||||||||
| Parietal | 0.01 | 0.835 | 0.07 | 0.243 | 0.05 | 0.152 | 0.05 | 0.122 | 0.04 | 0.446 |
| Temporal | 0.04 | 0.404 | 0.00 | 0.963 | − 0.01 | 0.698 | − 0.01 | 0.710 | − 0.04 | 0.491 |
| Frontal–occipital | 0.12 | 0.238 | 0.25 | 0.085 | 0.09 | 0.303 | 0.14 | 0.059 | 0.11 | 0.419 |
| Limbic–parietal | − 0.03 | 0.693 | − 0.02 | 0.889 | − 0.11 | 0.086 | − 0.01 | 0.922 | − 0.12 | 0.216 |
| Limbic–basal ganglia | 0.08 | 0.273 | 0.03 | 0.757 | − 0.02 | 0.688 | − 0.02 | 0.736 | 0.06 | 0.485 |
| Lobar division, right hemisphere | ||||||||||
| Temporal | 0.05 | 0.213 | − 0.03 | 0.568 | − 0.02 | 0.551 | − 0.00 | 0.973 | − 0.02 | 0.753 |
| Frontal–limbic | 0.17 | 0.113 | 0.27 | 0.081 | 0.09 | 0.277 | 0.12 | 0.114 | 0.04 | 0.761 |
| Frontal–basal ganglia | 0.09 | 0.171 | 0.16 | 0.071 | 0.02 | 0.693 | 0.10 | 0.036⁎ | 0.04 | 0.629 |
| Frontal–temporal | − 0.04 | 0.550 | 0.06 | 0.540 | − 0.00 | 0.983 | 0.02 | 0.672 | 0.04 | 0.659 |
| Limbic–temporal | 0.03 | 0.685 | 0.07 | 0.478 | 0.04 | 0.415 | 0.03 | 0.490 | 0.12 | 0.147 |
| Occipital–parietal | 0.13 | 0.034⁎ | 0.14 | 0.114 | 0.09 | 0.079 | 0.11 | 0.010⁎ | 0.25 | 0.001⁎ |
| Occipital–basal ganglia | 0.17 | 0.008⁎ | 0.09 | 0.340 | 0.07 | 0.182 | 0.13 | 0.005⁎ | 0.17 | 0.041⁎ |
The B-values represent the regression coefficients from multilevel random regression analysis in Stata; p-values refer to between group differences; the asterisks (*) represent areas which are significant.
3.5. Association between lobar semi-metric percentage and cognitive symptoms
The 12 lobar divisions that showed significant group differences were used to examine the association between SMP and four (social) cognitive performance measures, which resulted in 8 significant tests.
3.5.1. Neurocognition
In the total group without siblings, there was a significant negative association between WM performance and left temporal SMP and a significant positive association between attention and left limbic–basal ganglia SMP. Furthermore, a significant group × right occipital–basal ganglia SMP interaction was found in the model of WM. Stratified analyses revealed that, in controls, higher SMP was significantly associated with better WM performance (B = 0.09, p = 0.025), whereas this was not found in the patient group (B = − 0.02, p = 0.528). Also, there was a significant group × right temporal SMP interaction in the model of attention, indicating that the association between SMP and attention varied with group. Stratified analyses revealed that, in patients, higher SMP was significantly associated with worse attention (B = 1.91, p = 0.009), which was not found in controls (B = − 0.33, p = 0.643).
3.5.2. Social cognition
With regard to social cognition, there were significant negative associations between emotion processing and right frontal–limbic SMP, and between ToM and left temporal and right frontal–temporal SMP (Table 5). In addition, significant group × right frontal–basal ganglia SMP interaction was found in the model of emotion processing. Stratified analyses revealed that, in patients, higher SMP was associated with worse emotion processing (B = − 0.41, p = 0.005), which was not found in controls (B = 0.02, p = 0.892). There were no significant group × SMP interactions in the model of ToM (Table 5).
Table 5.
Associations between SMP and cognitive performance in the total group and SMP x group interactions on cognitive performance.
| Main effect |
Interaction |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic |
Attention |
Emotion processing |
Theory of mind |
Arithmetic |
Attention |
Emotion processing |
Theory of mind |
|||||||||
| B | p | B | p | B | p | B | p | χ2 | p | χ2 | p | χ2 | p | χ2 | p | |
| Lobar division, left hemisphere | ||||||||||||||||
| Parietal | 0.03 | 0.218 | − 0.49 | 0.344 | − 0.04 | 0.520 | 0.01 | 0.672 | 0.06 | 0.804 | 3.12 | 0.077 | 2.02 | 0.155 | 0.05 | 0.826 |
| Temporal | − 0.06 | 0.023⁎ | − 0.61 | 0.294 | 0.09 | 0.158 | − 0.04 | 0.011⁎ | 0.72 | 0.396 | 1.82 | 0.177 | 0.01 | 0.935 | 0.86 | 0.355 |
| Frontal–occipital | − 0.03 | 0.545 | − 0.81 | 0.515 | 0.15 | 0.265 | − 0.02 | 0.428 | 0.08 | 0.776 | 0.15 | 0.702 | 1.32 | 0.251 | 0.19 | 0.661 |
| Limbic–parietal | − 0.01 | 0.814 | − 0.09 | 0.923 | 0.03 | 0.769 | − 0.01 | 0.520 | 0.07 | 0.787 | 3.85 | 0.051 | 3.44 | 0.064 | 0.57 | 0.451 |
| Limbic–basal ganglia | 0.00 | 0.910 | 2.05 | 0.012⁎ | 0.17 | 0.070 | 0.00 | 0.907 | 2.13 | 0.144 | 0.43 | 0.512 | 0.00 | 0.946 | 0.40 | 0.529 |
| Lobar division, right hemisphere | ||||||||||||||||
| Temporal | − 0.01 | 0.796 | 0.75 | 0.151 | − 0.06 | 0.288 | − 0.02 | 0.185 | 0.27 | 0.606 | 4.95 | 0.026⁎ | 0.90 | 0.343 | 1.14 | 0.286 |
| Frontal–limbic | 0.00 | 0.952 | 0.73 | 0.564 | − 0.35 | 0.013⁎ | 0.00 | 0.918 | 0.72 | 0.396 | 0.32 | 0.572 | 0.30 | 0.586 | 0.24 | 0.623 |
| Frontal–basal ganglia | − 0.04 | 0.359 | − 0.13 | 0.888 | − 0.19 | 0.071 | − 0.01 | 0.498 | 0.67 | 0.414 | 0.07 | 0.791 | 4.43 | 0.035⁎ | 0.00 | 0.953 |
| Frontal–temporal | 0.00 | 0.973 | 0.41 | 0.698 | − 0.01 | 0.926 | − 0.05 | 0.032⁎ | 0.04 | 0.841 | 0.21 | 0.644 | 0.00 | 0.981 | 0.27 | 0.604 |
| Limbic–temporal | 0.00 | 0.898 | 0.21 | 0.790 | − 0.09 | 0.300 | − 0.02 | 0.309 | 0.45 | 0.500 | 0.77 | 0.381 | 0.83 | 0.363 | 0.20 | 0.654 |
| Occipital–parietal | − 0.02 | 0.617 | − 0.36 | 0.667 | − 0.04 | 0.680 | − 0.13 | 0.532 | 0.33 | 0.566 | 2.09 | 0.148 | 0.00 | 0.978 | 0.81 | 0.368 |
| Occipital–basal ganglia | 0.04 | 0.225 | − 0.28 | 0.707 | − 0.08 | 0.351 | − 0.14 | 0.424 | 3.97 | 0.046⁎ | 0.04 | 0.837 | 0.07 | 0.789 | 0.25 | 0.619 |
The B-values represent the regression coefficients from multilevel random regression analysis in Stata; p values refer to between group differences; The χ2 and corresponding p-values represent the results of the Wald test; the asterisks (*) represent areas which are significant.
3.6. Exclusion of patients with a diagnosis other than schizophrenia
Repeating the analyses excluding patients with a diagnosis other than schizophrenia did not change the SMP pattern at whole brain and hemispheric level (Table S4). At the lobar level small differences were noticeable. That is, in the left hemisphere group differences in two lobar divisions became apparent (occipital, limbic–occipital), whereas the group difference for the limbic–parietal lobar division disappeared. In the right hemisphere, in three additional lobar divisions group differences were observed (parietal, limbic–basal ganglia and parietal–basal ganglia). For all lobar divisions, patients revealed a higher SMP compared to controls (Table S5).
3.7. Sensitivity analyses based on schizotypy scores in the combined sibling-control group
Comparing patients with psychotic disorder with the combined group of siblings and controls, divided into a high and low schizotypy group, did not change the pattern of results at the whole brain and right hemispheric level (significant higher SMP for patients compared to the other groups). However, for the left hemisphere, there was a significant difference between the low schizotypy group and patients with psychotic disorder, with the high schizotypy group showing intermediate SMP values that were not significantly different from either group (patients or low schizotypy) (Table S6).
4. Discussion
The present study described a methodology (i.e., semi-metricity) which, for the first time, was applied in a family study on functional network connectivity in psychotic disorder. Rs-fMRI data were used to construct fully-connected, weighted functional brain networks in individuals with psychotic disorder, their unaffected siblings and controls. Individuals with psychotic disorder had a higher SMP compared to siblings and controls, at a whole brain and hemispheric level. Exploratory analyses revealed that patients had a higher SMP compared to controls in specific lobar divisions. In addition, higher SMP in specific lobar divisions was associated with clinical symptoms (i.e., positive symptoms, excitement and emotional distress), worse attention and emotion processing in the patient group and worse cognitive performance on WM, attention and social cognition tasks in the combined group of patients and controls.
4.1. Semi-metricity in individuals with psychotic disorder at a whole brain/hemispheric level
The group differences in SMP in the present study support dysconnectivity theories of psychotic disorder (Bullmore et al., 1997, Friston, 2002). That is, previous rs-fMRI studies have shown dysconnectivity between brain regions in patients with psychotic disorder, e.g., frontal–frontal/fronto-temporal (Andreasen et al., 1998, Friston and Frith, 1995, Rotarska-Jagiela et al., 2010), temporal–temporal/limbic–temporal (Garrity et al., 2007), and limbic–basal ganglia (Ongur et al., 2010) dysconnectivity. In addition, studies of brain networks using graph theoretical analyses in patients with schizophrenia have revealed alterations in topological properties of the functional brain network (Alexander-Bloch et al., 2010, Bullmore and Sporns, 2009, Liu et al., 2008, Ma et al., 2012), such as reduced local information processing (clustering coefficient and local efficiency) (Alexander-Bloch et al., 2010, Liu et al., 2008, Lynall et al., 2010) and increased global efficiency (Alexander-Bloch et al., 2010, Lynall et al., 2010). These findings are also in agreement with electroencephalographic research showing reduced clustering coefficient and path length in first-episode patients, interpreted as evidence of a subtle randomization of network topology (Rubinov et al., 2009).
SMP is a topological property, which may provide insight into the information flow across brain regions, which can be either dispersed, involving more regions than would otherwise be the case (indirect connections) or constrained which is characterized by a higher than average number of direct connections between regions (Simas et al., 2015). In the present study, the higher SMP observed in the patient group suggests that the functional brain network is characterized by dispersed connections i.e., involvement of an excessive number of regions. It could be speculated that these regions may not be accustomed to processing the information that is passing through them. Hence, it could be hypothesized that the change in SMP contributes to psychopathology in the sense that dispersed information flow may hamper the discrimination between relevant and non-relevant information and/or the efficiency in complex information processing. Thus, inadequate filtering associated with dispersion could contribute to the formation of symptoms in psychotic disorder, such as thought disorder, hallucinations or cognitive problems.
Furthermore, involvement of a large number of brain regions may impede the ability for specialized processing to occur within densely interconnected groups of brain regions, which is in agreement with the notion of reduced functional segregation in patients with schizophrenia, and with decreased short-range connections and increased long-range connections relative to controls (Alexander-Bloch et al., 2010, Fornito et al., 2012, Lynall et al., 2010, Rubinov and Sporns, 2010). Thus, the present study extends previous graph-theoretical results and provides evidence that disturbances in topological measures are not only apparent in undirected unweighted representations of the functional brain, but are also noticeable in undirected, weighted representations, which is in agreement with other studies examining weighted networks (i.e., Bassett et al., 2012, Rubinov et al., 2009, Yu et al., 2013a).
The siblings did not exhibit the similar tendency of higher SMP that was observed in the patient group. This suggests that the functional brain network does not represent trait-related SMP changes at a whole brain and hemispheric level. Of note, SIS-R schizotypy scores did not differ between siblings and controls, which may have precluded detection of a possible intermediate phenotype at the whole brain/hemispheric level, but does not necessarily imply absence of lobar SMP differences. Based on average schizotypy scores measured in a general population sample (M = 2.2, SD = 2.2 for positive dimensions and M = 2.9, SD = 2.8 for negative dimensions of the SIS-r) (Konings et al., 2006), it is concluded that in the present study siblings had lower schizotypy scores and may represent a more ‘healthy’ group (M = 0.6, SD = 0.4 for positive dimensions and M = 0.3, SD = 0.3 for negative dimensions of the SIS-r). Alternatively, questionnaires with overt psychotic-like contents may be more susceptible to a defensive response style in the relatives of patients with schizophrenia (Kendler et al., 1997). Since siblings and controls did not differ on schizotypy scores, sensitivity analyses were conducted in which these two groups were combined and divided into high and low schizotypy. Results of the patient–schizotypy group comparisons were similar at a whole brain and right hemispheric level (higher SMP in patients compared to both groups) as compared to the original group comparisons. However, in the left hemisphere, the high schizotypy SMP values were intermediate to those of patients and the low schizotypy group, suggesting some disease liability on the level of SMP.
4.2. Lobar semi-metric percentages
At the lobar level, higher SMP was observed in the patient group in 12 out of 42 lobar divisions (Table 3). Regions within these lobar divisions, e.g., hippocampus (limbic lobar division), middle temporal gyrus (temporal lobar division), medial prefrontal cortex (frontal lobar division), ventral striatum (basal ganglia lobar division) have previously been implicated in memory formation, affective flattening, emotional processing and cognitive control in patients with psychotic disorder (Khadka et al., 2013, Meda et al., 2014, van Buuren et al., 2012). The present results suggest that higher (inter-/intra-) lobar SMP is associated with the manifestation of psychotic disorder.
4.3. Lobar SMP in relation to psychotic symptoms and cognitive performance
Some graph theoretical studies have investigated the association between symptom severity and small-world characteristics (Bassett et al., 2012, Skudlarski et al., 2010) and, for example, reported that higher levels of negative and cognitive symptoms were associated with a reduced clustering coefficient and increased path length (Shim et al., 2014). The current study is the first in applying semi-metric analyses to investigate such associations. In the right hemisphere, associations were found between occipital–parietal/basal ganglia SMP and positive symptoms, excitement and emotional distress as well as an association between frontal–basal ganglia SMP and PANSS excitement scores. These results may indicate that more dispersed network communication between specific brain areas may be associated with psychopathology, with an important role for the occipital lobe and basal ganglia. Structural and functional abnormalities of the basal ganglia have repeatedly been associated with psychotic symptoms (Mehler-Wex et al., 2006, Ring and Serra-Mestres, 2002), whereas much less is known about the association between occipital abnormalities and psychotic symptoms. Nevertheless, several structural MRI studies have shown that patients with schizophrenia show significant cortical thinning and gray matter density reductions within the occipital cortex (Narr et al., 2005, Onitsuka et al., 2007), which may affect SMP. That is, cortical thinning influences the Blood Oxygenation Level Dependent (BOLD) response, which in turn may affect semi-metricity measures. Furthermore, task-related fMRI studies have reported reduced activity in the occipital cortex in patients with early-onset schizophrenia, suggesting problems with regard to visual processing (White et al., 2012). The current results, although exploratory, showed that the mechanism of dispersed communication may contribute to explain the pathophysiology of psychotic symptoms in which both basal ganglia and occipital cortex are involved.
There were significant associations between SMP and performance on both neuro- and social cognitive tasks in the combined group of patients and controls. First, higher SMP in the left-hemispheric temporal lobar division was related to worse WM performance. This is in agreement with literature that suggests that the left hemisphere (including both frontal and temporal regions) is implicated in verbal memory (Nagel et al., 2013, Petersson et al., 2006). This finding suggests that higher SMP may interfere with manipulating new information through the process of dispersed communication, precluding further cognitive processing or adequate response selection (Baddeley, 1992). In addition, higher SMP within the right occipital–parietal lobar division was associated with significant better performance in the control group. Occipital–parietal regions have been implicated in spatial working memory performance (Smith and Jonides, 1997). Although the working memory task assessed in the present study measured WM associated with verbal comprehension and mental arithmetic, the findings suggest that controls use additional brain circuits (i.e., higher amount of semi-metric paths between brain areas) possibly contributing to their enhanced cognitive performance.
With regard to social cognition, higher SMP between right frontal–limbic and right frontal–temporal/left temporal lobar divisions was associated with respectively poor emotion processing and ToM in the combined group of patients and controls. Regions within these lobar divisions have previously been implicated in social cognitive functioning (Adolphs, 2003, Gallese et al., 2004). These results suggest that higher SMP values within these specific areas are associated with poor social cognitive functioning, irrespective of group. In addition, higher SMP in the right frontal–basal ganglia lobar division was associated with poor emotion processing specifically in patients. Previous task-based fMRI research has shown that activity within right hemispheric structures (e.g., inferior/middle frontal gyrus, temporoparietal junction) was associated with social cognitive functions in patients with schizophrenia (de Achaval et al., 2012). In theory, dispersed and inadequate filtering in the right hemisphere may lead to compromised inferring of beliefs and emotions of others, which could lead to misattribution of intentions, social withdrawal and isolation (Dodell-Feder et al., 2014).
With regard to attention processing, higher left limbic–basal ganglia SMP was associated with worse performance in the combined group of participants, whereas higher SMP within the right temporal lobar division was associated with worse attention performance in the patients only. Both the basal ganglia and the limbic system have previously been implicated in attentional processes in respectively patients with ADHD (Qiu et al., 2009) and healthy volunteers (Mohanty et al., 2008), which corroborates our finding of non-specificity. In addition, a higher than average activated number of regions in the temporal lobe may compromise information processing by a decreased ability to distinguish signal information from noise. This concurs with a task-based fMRI study on schizophrenia, showing patients experienced increased distraction by novel stimuli (i.e., noise), and that activity in right temporal and parietal regions was associated with an inability to extract the (ir)relevance of novel stimuli for subsequent behavior (Laurens et al., 2005).
These results suggest that in several lobar divisions the interaction between group and SMP in the models of attention/WM and social cognition is associated with human cognitive processes that are conditional on the clinical phenotype.
4.4. Conclusion
The present study provided evidence for higher SMP at whole brain, hemispheric and lobar level in patients with psychotic disorder, associated with specific symptoms (i.e., positive symptoms, excitement, emotional distress), a lower span of attention and difficulties with the processing of emotions. Together with the extant literature, this suggests that psychotic disorder is characterized by large-scale changes in information flow across the brain. There was no conclusive evidence for a SMP intermediate phenotype, although some trait-based SMP alterations were seen in high schizotypy individuals. SMP was associated with WM, attention and social cognition independent of psychosis risk. As this is the first study examining SMP in psychotic disorder, replication is required.
4.5. Methodological considerations
The large and representative sample and the inclusion of a genetic risk group were strengths of the study. Despite heterogeneity in the sample, SMP appeared sensitive enough to detect group differences and symptom-based associations.
As there was a SMP difference at the whole brain and hemispheric level between patients and controls, the subsequent exploratory analyses were not corrected for multiple comparisons. Nevertheless, for every analysis (lobar SMP differences within hemispheres, association between lobar SMP and psychotic/cognitive symptoms) the number of lobar divisions that revealed significant group differences was higher than what would be expected based on chance.
Semi-metricity assumes that indirect paths are functionally relevant in functional networks; i.e., that it is possible for information to travel along them. In this sense, the correlation coefficient contains the information about the interaction between two regions. However, interpretation of indirect paths may be difficult since there is no guarantee of a structural link where a functional connectivity value may be high (Rubinov and Sporns, 2010). Thus, to better understand the representation of an indirect path in a functional network, future studies may benefit from including information on structural connectivity.
In the present study it is speculated that semi-metricity provides information about network communication/diffusion of information processing. It remains unclear how paths representing functional connectivity relate to information processing during cognition, or at rest.
We used the AAL template, although alternative templates (e.g., the FMRIB Software Library's Harvard-Oxford atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases)) are available. Despite the preservation of organizational aspects across templates, research has shown that characteristics of network measures may be dependent on the brain atlas that is used, limiting the direct comparison of study results (de Reus and van den Heuvel, 2013). Therefore, for the purpose of replication it is important to use a similar atlas.
A priori planned sensitivity analyses were done excluding the patients with a diagnosis other than schizophrenia. Although a few additional lobar divisions revealed group differences, the whole brain and hemispheric SMP pattern remained the same.
To test whether the use of two scan sequences (i.e., MPRAGE and MDEFT) would have influenced the results, analyses were repeated with scan sequence as covariate. This did not change the results.
AP medication, cannabis, alcohol, tobacco and other drugs may have an effect on functional network organization. However, the present results indicated that AP medication and additional confounders do not have an effect on whole-brain/hemispheric SMP.
Financial disclosures
Jim van Os is or has been an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker from Lilly, BMS, Lundbeck, Organon, Janssen, GlaxoSmithKline, AstraZeneca, Pfizer and Servier. Machteld Marcelis has received financial compensation as an independent symposium speaker from Lilly and Janssen. All other authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
We thank all participants and contributing staff of the participating mental health care centers. We thank Truda Driesen and Inge Crolla for their coordinating roles in the data collection, as well as the G.R.O.U.P. investigators: Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, René Kahn, Carin Meijer, Inez Myin-Germeys, Jim van Os and Durk Wiersma.
This work was sponsored by the Geestkracht program of the Dutch Health Research Council (ZON-MW, grant number 10-000-1002) and the European Community's Seventh Framework Programme under Grant Agreement No. HEALTH-F2-2009-241909 (European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Consortium). Both funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.10.003.
Appendix A. Supplementary data
Supplementary material.
References
- Achard S., Salvador R., Whitcher B., Suckling J., Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A.F., Gogtay N., Meunier D., Birn R., Clasen L., Lalonde F., Lenroot R., Giedd J., Bullmore E.T. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front. Syst. Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andreasen N.C., Flaum M., Arndt S. The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Arch. Gen. Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Paradiso S., O'Leary D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br. J. Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E.T. Human brain networks in health and disease. Curr. Opin. Neurol. 2009;22:340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E., Verchinski B.A., Mattay V.S., Weinberger D.R., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Nelson B.G., Mueller B.A., Camchong J., Lim K.O. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59:2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte M.K., Allen G., Beckel-Mitchener A., Boulanger L.M., Carper R.A., Webb S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Frangou S., Murray R.M. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr. Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Eichele T., Pearlson G. Functional brain networks in schizophrenia: a review. Front. Hum. Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Shen H., Wang L., Liu Z., Xin W., Hu D., Miao D. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 2014;1562:87–99. doi: 10.1016/j.brainres.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Chen J.E., Glover G.H. BOLD fractional contribution to resting-state functional connectivity above 0.1 Hz. Neuroimage. 2014;107C:207–218. doi: 10.1016/j.neuroimage.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Achaval D., Costanzo E.Y., Villarreal M., Jauregui I.O., Chiodi A., Castro M.N., Fahrer R.D., Leiguarda R.C., Chu E.M., Guinjoan S.M. Emotion processing and theory of mind in schizophrenia patients and their unaffected first-degree relatives. Neuropsychologia. 2010;48:1209–1215. doi: 10.1016/j.neuropsychologia.2009.12.019. [DOI] [PubMed] [Google Scholar]
- de Achaval D., Villarreal M.F., Costanzo E.Y., Douer J., Castro M.N., Mora M.C., Nemeroff C.B., Chu E., Bar K.J., Guinjoan S.M. Decreased activity in right-hemisphere structures involved in social cognition in siblings discordant for schizophrenia. Schizophr. Res. 2012;134:171–179. doi: 10.1016/j.schres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- de Reus M.A., van den Heuvel M.P. The parcellation-based connectome: limitations and extensions. Neuroimage. 2013;80:397–404. doi: 10.1016/j.neuroimage.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Ding X., Lee S.W. Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Delisi L.E., Hooker C.I. The relationship between default mode network connectivity and social functioning in individuals at familial high-risk for schizophrenia. Schizophr. Res. 2014;156:87–95. doi: 10.1016/j.schres.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Glahn D.C., Laird A.R., Thelen S.M., Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am. J. Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Pantelis C., Bullmore E.T. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Goodby E., Dean A., Ooi C., Nathan P.J., Lennox B.R., Jones P.B., Suckling J., Bullmore E.T. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Schizophrenia and the disconnection hypothesis. Acta Psychiatr. Scand. Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1:66–71. [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Gallese V., Keysers C., Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glahn D.C., Laird A.R., Ellison-Wright I., Thelen S.M., Robinson J.L., Lancaster J.L., Bullmore E., Fox P.T. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H. Griffin; London: 1987. Multilevel Models in Educational and Social Research. [Google Scholar]
- Guo W., Jiang J., Xiao C., Zhang Z., Zhang J., Yu L., Liu J., Liu G. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr. Res. 2014;152:170–175. doi: 10.1016/j.schres.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Yao D., Jiang J., Su Q., Zhang Z., Zhang J., Yu L., Zhai J., Xiao C. Decreased default-mode network homogeneity in unaffected siblings of schizophrenia patients at rest. Psychiatry Res. 2014;224:218–224. doi: 10.1016/j.pscychresns.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Habets P., Marcelis M., Gronenschild E., Drukker M., van Os J., Genetic R., Outcome of P. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol. Psychiatry. 2011;69:487–494. doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Hyder F., Rothman D.L. Neuronal correlate of BOLD signal fluctuations at rest: Err on the side of the baseline. PNAS. 2010;107 doi: 10.1073/pnas.1005135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jo H.J., Gotts S.J., Reynolds R.C., Bandettini P.A., Martin A., Cox R.W., Saad Z.S. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J. Appl. Math. 2013;2013 doi: 10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.M. Optimal two- and three-stage production schedules with setup times included. Nav. Res. Logist. Q. 1954;1:8. [Google Scholar]
- Karbasforoushan H., Woodward N.D. Resting-state networks in schizophrenia. Curr. Top. Med. Chem. 2012;12:2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski-Shuman L., O'Neill F.A., Straub R.E., MacLean C.J., Walsh D. Resemblance of psychotic symptoms and syndromes in affected sibling pairs from the Irish study of high-density schizophrenia families: evidence for possible etiologic heterogeneity. Am. J. Psychiatry. 1997;154:191–198. doi: 10.1176/ajp.154.2.191. [DOI] [PubMed] [Google Scholar]
- Khadka S., Meda S.A., Stevens M.C., Glahn D.C., Calhoun V.D., Sweeney J.A., Tamminga C.A., Keshavan M.S., O'Neil K., Schretlen D., Pearlson G.D. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol. Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings M., Bak M., Hanssen M., van Os J., Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr. Scand. 2006;114:55–61. doi: 10.1111/j.1600-0447.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- Laurens K.R., Kiehl K.A., Ngan E.T., Liddle P.F. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophr. Res. 2005;75:159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Leistritz L., Weiss T., Bar K.J., De VicoFallani F., Babiloni F., Witte H., Lehmann T. Network redundancy analysis of effective brain networks: a comparison of healthy controls and patients with major depression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liang M., Zhou Y., He Y., Hao Y., Song M., Yu C., Liu H., Liu Z., Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Lynall M.E., Bassett D.S., Kerwin R., McKenna P.J., Kitzbichler M., Muller U., Bullmore E. Functional connectivity and brain networks in schizophrenia. J. Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Liu Y., Fu X.M., Li N., Wang C.X., Zhang H., Qian R.B., Xu H.S., Hu X., Zhang D.R. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Calhoun V.D., Eichele T., Du W., Adali T. Modulations of functional connectivity in the healthy and schizophrenia groups during task and rest. Neuroimage. 2012;62:1694–1704. doi: 10.1016/j.neuroimage.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S.A., Ruano G., Windemuth A., O'Neil K., Berwise C., Dunn S.M., Boccaccio L.E., Narayanan B., Kocherla M., Sprooten E., Keshavan M.S., Tamminga C.A., Sweeney J.A., Clementz B.A., Calhoun V.D., Pearlson G.D. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler-Wex C., Riederer P., Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson's disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox. Res. 2006;10:167–179. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Gitelman D.R., Small D.M., Mesulam M.M. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb. Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel B.J., Herting M.M., Maxwell E.C., Bruno R., Fair D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013;82:58–68. doi: 10.1016/j.bandc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr K.L., Toga A.W., Szeszko P., Thompson P.M., Woods R.P., Robinson D., Sevy S., Wang Y., Schrock K., Bilder R.M. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol. Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Dawson M.E. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr. Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Nylenna M., Riis P. Identification of patients in medical publications: need for informed consent. BMJ. 1991;302:1182. doi: 10.1136/bmj.302.6786.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D., Lundy M., Greenhouse I., Shinn A.K., Menon V., Cohen B.M., Renshaw P.F. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitsuka T., McCarley R.W., Kuroki N., Dickey C.C., Kubicki M., Demeo S.S., Frumin M., Kikinis R., Jolesz F.A., Shenton M.E. Occipital lobe gray matter volume in male patients with chronic schizophrenia: a quantitative MRI study. Schizophr. Res. 2007;92:197–206. doi: 10.1016/j.schres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.X., Kundu P., Rubinov M., Jones P.S., Vertes P.E., Ersche K.D., Suckling J., Bullmore E.T. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 2014;95:287–304. doi: 10.1016/j.neuroimage.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D.L., Sanna L.J., Roberts D.L. Social cognition in schizophrenia: an overview. Schizophr. Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson K.M., Gisselgard J., Gretzer M., Ingvar M. Interaction between a verbal working memory network and the medial temporal lobe. Neuroimage. 2006;33:1207–1217. doi: 10.1016/j.neuroimage.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Qiu A., Crocetti D., Adler M., Mahone E.M., Denckla M.B., Miller M.I., Mostofsky S.H. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am. J. Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Barch D.M. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front. Hum. Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain network connectivity in individuals with schizophrenia and their siblings. Biol. Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring H.A., Serra-Mestres J. Neuropsychiatry of the basal ganglia. J. Neurol. Neurosurg. Psychiatry. 2002;72:12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G.M., Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage. 2010;52:429–435. doi: 10.1016/j.neuroimage.2010.04.192. [DOI] [PubMed] [Google Scholar]
- Rocha L.M. Semi-metric behavior in document networks and its application to recommendation systems. In: Loia V., editor. Soft Computing Agents: A New Perspective for Dynamic Information Systems. IOS Press; 2002. pp. 137–163. [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knochel V., Uhlhaas P.J., Vogeley K., Linden D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Knock S.A., Stam C.J., Micheloyannis S., Harris A.W., Williams L.M., Breakspear M. Small-world properties of nonlinear brain activity in schizophrenia. Hum. Brain Mapp. 2009;30:403–416. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M., Kim D.W., Lee S.H., Im C.H. Disruptions in small-world cortical functional connectivity network during an auditory oddball paradigm task in patients with schizophrenia. Schizophr. Res. 2014;156:197–203. doi: 10.1016/j.schres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Simas T., Chattopadhyay S., Hagan C., Kundu P., Patel A., Holt R., Floris D., Graham J., Ooi C., Tait R., Spencer M., Baron-Cohen S., Sahakian B., Bullmore E., Goodyer I., Suckling J. Semi-metric topology of the human connectome: sensitivity and specificity to autism and major depressive disorder. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P., Jagannathan K., Anderson K., Stevens M.C., Calhoun V.D., Skudlarska B.A., Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Working memory: a view from neuroimaging. Cogn. Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Sporns O., Tononi G., Kotter R. The human connectome: a structural description of the human brain. PLoS Comput. Biol. 2005;1(e42):0245–0251. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. StataCorp LP; College Station, TX: 2011. Stata Statistical Software: Release 12. [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.W., Lan T.H., Hsu T.W., Biswal B.B., Tsai P.J., Lin W.C., Lin C.P. Reduced neuro-integration from the dorsolateral prefrontal cortex to the whole brain and executive dysfunction in schizophrenia patients and their relatives. Schizophr. Res. 2013;148:50–58. doi: 10.1016/j.schres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D., Wang R., Carrillo J.H., Maloney T., Alia-Klein N., Woicik P.A., Telang F., Goldstein R.Z. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Buuren M., Vink M., Kahn R.S. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr. Res. 2012;142:237–243. doi: 10.1016/j.schres.2012.09.017. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Fornito A. Brain networks in schizophrenia. Neuropsychol. Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Mandl R.C., Stam C.J., Kahn R.S., Hulshoff Pol H.E. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J. Neurosci. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag M., Hoffman T., Remijsen M., Hijman R., de Haan L., van Meijel B., van Harten P.N., Valmaggia L., de Hert M., Cuijpers A., Wiersma D. The five-factor model of the positive and negative syndrome scale II: a ten-fold cross-validation of a revised model. Schizophr. Res. 2006;85:280–287. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- van 't Wout M., Aleman A., Kessels R.P., Laroi F., Kahn R.S. Emotional processing in a non-clinical psychosis-prone sample. Schizophr. Res. 2004;68:271–281. doi: 10.1016/j.schres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Versmissen D., Janssen I., Myin-Germeys I., Mengelers R., Campo J.A., van Os J., Krabbendam L. Evidence for a relationship between mentalising deficits and paranoia over the psychosis continuum. Schizophr. Res. 2008;99:103–110. doi: 10.1016/j.schres.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Ma Y., Zhu W., Fowler J.S., Li J., Rao M., Mueller K., Pradhan K., Wong C., Wang G.J. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollema M.G., Ormel J. The reliability of the structured interview for schizotypy—revised. Schizophr. Bull. 2000;26:619–629. doi: 10.1093/oxfordjournals.schbul.a033482. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonia, Texas: 1997. Wechsler Adult Intelligence Scale — Third Revision. [Google Scholar]
- White T., Moeller S., Schmidt M., Pardo J.V., Olman C. Evidence for intact local connectivity but disrupted regional function in the occipital lobe in children and adolescents with schizophrenia. Hum. Brain Mapp. 2012;33:1803–1811. doi: 10.1002/hbm.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 1990. Composite International Diagnostic Interview (CIDI) [Google Scholar]
- Yu Q., Sui J., Kiehl K.A., Pearlson G., Calhoun V.D. State-related functional integration and functional segregation brain networks in schizophrenia. Schizophr. Res. 2013;150:450–458. doi: 10.1016/j.schres.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Shen H., Zhang H., Zeng L.L., Xue Z., Hu D. Functional connectivity-based signatures of schizophrenia revealed by multiclass pattern analysis of resting-state fMRI from schizophrenic patients and their healthy siblings. Biomed. Eng. Online. 2013;12:10. doi: 10.1186/1475-925X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B.K., Wang J., Zang Y.F., Liu D.Q. Amplitude differences in high-frequency fMRI signals between eyes open and eyes closed resting states. Front. Hum. Neurosci. 2014;8:503. doi: 10.3389/fnhum.2014.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.