Abstract
Background
Management of language difficulties is an important aspect of clinical care for glioma patients, and accurately identifying the possible language deficits in patients based on lesion location would be beneficial to clinicians. To that end, we examined the relationship between lesion presence and language performance on tests of receptive language and expressive language using a highly specific voxel-based lesion–symptom mapping (VLSM) approach in glioma patients.
Methods
98 adults with primary glioma, who were pre-surgical candidates, were administered seven neurocognitive tests within the domains of receptive language and expressive language. The association between language performance and lesion presence was examined using VLSM. Statistical parametric maps were created for each test, and composite maps for both receptive language and expressive language were created to display the significant voxels common to all tests within these language domains.
Results
We identified clusters of voxels with a significant relationship between lesion presence and language performance. All tasks were associated with several white matter pathways. The receptive language tasks were additionally all associated with regions primarily within the lateral temporal lobe and medial temporal lobe. In contrast, the expressive language tasks shared little overlap, despite each task being independently associated with large anatomic areas.
Conclusions
Our findings identify the key anatomic structures involved in language functioning in adult glioma patients using an innovative lesion analysis technique and suggest that expressive language abilities may be more task-dependent and distributed than receptive language abilities.
Keywords: Glioma, Neurocognitive functioning, Language, Lesion–symptom mapping, Language, MRI
Highlights
-
•
Examined the association between lesion location and language in glioma patients.
-
•
Utilized a highly specific voxel-based lesion–symptom mapping (VLSM) approach.
-
•
Receptive language tasks were all associated with temporal and subcortical regions.
-
•
Expressive language tasks showed little overlap across associated brain regions.
-
•
Findings suggest expressive language is a more task-dependent, distributed ability.
1. Introduction
Language difficulties are of particular concern to brain tumor patients and greatly affect their overall quality of life. For example, difficulties with language functioning have been demonstrated to result in reduced involvement in daily activities including social activities, educational endeavors, occupational roles, and leisure activities, and have been shown to negatively impact patients' self-confidence and relationships with significant others (Christoffersen and Wells, 1998, Dalemans et al., 2008, Darrigrand et al., 2011, Thomas et al., 1995). As formal neurocognitive testing has documented cognitive deficits in up to 90% of treated brain tumor patients (Zucchella et al., 2013), monitoring changes in the functioning of such a vital ability as language is a critical component of the clinical care for brain tumor patients. Of particular benefit to clinicians would be a tool to identify the language deficits that patients may currently or soon experience based on tumor location and MR imaging results. To that end, the current study explores the relationship between lesion location and performance on tests of receptive and expressive language in adult glioma patients.
To date, both functional neuroimaging and lesion-deficit approaches have been used to analyze the relationship between generalized lesion location and cognition in patients with cerebral infarction and other vascular pathologies, but few studies have been performed with adult glioma patients. A particular method termed “voxel-based lesion–symptom mapping” (VLSM) (Bates et al., 2003) presents many benefits over other neuroimaging and lesion-deficit methods. VLSM is a highly specific voxel-by-voxel analytic method used to identify the brain regions associated with significant differences in task performance. In particular, statistical analysis is performed on every voxel of the brain, comparing cognitive test performance for patients with lesion present in that voxel to the performance of patients without lesion present in that voxel. One significant advantage of VLSM is that it enables the identification of all brain regions associated with differences in cognitive test performance rather than focusing on pre-determined regions of interest, while allowing cognitive data to be treated as continuous rather than dichotomous measures of performance (Bates et al., 2003, Tyler et al., 2005). Additionally, because VLSM produces a statistical value for each voxel, highly reliable inferences can be made regarding neural networks over that of both other lesion-deficit and traditional neuroimaging techniques (Tyler et al., 2005). Lastly, in contrast to functional magnetic resonance imaging (fMRI) or diffusion tensor imaging (DTI) of the brain, VLSM is able to identify the brain regions critical for cognitive performance extending into both white and grey matter, making it especially valuable for studying glioma patients, in which lesions can be found extending into both white and gray matter and often affect cognition through interference with information processing across brain regions (Kinno et al., 2011). The use of VLSM with glioma patients therefore allows us to better understand, with a high level of sensitivity, the particular brain regions that are utilized and impaired in this patient population.
In the current study, VLSM analysis was utilized to examine the relationship between lesion location and performance on neurocognitive tests of expressive and receptive language in adult glioma patients who were pre-surgical candidates. Expressive language skills reflect communication abilities, and receptive language skills reflect the ability to understanding language (Bernal and Ardila, 2014). A greater understanding of the specific associations between lesion location and language functioning will provide guidance to healthcare providers in the treatment of brain tumor patients with language difficulties, which are especially disruptive to patients' quality of life and independence (Boele et al., 2014, Bosma et al., 2007, Dalemans et al., 2008, Darrigrand et al., 2011, Klein et al., 2001).
2. Materials and methods
2.1. Participants
All patients participating in the present study signed institutional review board-approved informed consent to have imaging and neurocognitive data stored as part of our institution's neuro-oncology database. Neurocognitive and imaging data acquisition was performed as part of clinical care and in compliance with all applicable Health Insurance Portability and Accountability Act (HIPAA) regulations. A total of 98 patients who met the following criteria were selected: (1) patients had pathology-confirmed, supratentorial, intraaxial glioma; (2) patients had received neurocognitive testing of language abilities using standardized procedures; (3) brain MRI and language testing had been performed close in time (9.3 days on average) with no change in treatments or tumor progression between these procedures; and (4) patients were right-handed.
The study sample of 98 glioma patients ranged from 18 to 80 years of age (M = 46.9, SD = 14.6) and was comprised of 60% males and 40% females. Years of education ranged from 11 to 21 (M = 15.8, SD = 2.4). Tumor grade at the time of the language assessment comprised WHO grade 2 (28%), grade 3 (26%), and grade 4 (46%), with 29% of patients having experienced prior tumor progression. With regard to treatment history, 63% of the sample had received prior surgery and 40% had received prior radiochemotherapy. The demographics, disease, and treatment characteristics, as described in this paragraph, were statistically controlled for in the analyses.
2.2. Neurocognitive assessment of language functioning
Neurocognitive testing of language functioning was conducted by a licensed neuropsychologist or a trained doctoral student under the supervision of a licensed neuropsychologist. Participants were candidates for surgery and received a neurocognitive language evaluation as part of routine pre-surgical clinical practice to identify any potential language concerns that should be considered during surgery. Seven neurocognitive tests assessing expressive language and receptive language were administered in the present study. These tests are described in Table 1. Standardized administration as well as standardized scoring procedures using normative data was followed as per the instructions outlined in the test manuals (Goodglass et al., 2000, Tombaugh et al., 1999).
Table 1.
Overview of neurocognitive tests and test results grouped by type of language functioning.
| Type of language functioning | Test | Description | Range of possible scores | M | SD |
|---|---|---|---|---|---|
| Receptive language | BDAE-3 Commands | Follow orally presented commands | 0–100 (Percent) | 93.72 | 12.66 |
| BDAE-3 Complex Ideational Material | Provide yes/no responses to syntactically complex questions | 0–100 (percent) | 87.53 | 15.96 | |
| BDAE-3 Reading Comprehension — Sentences and Paragraphs | Silently read sentences/paragraphs and select the response that correctly completes each | 0–100 (percent) | 88.95 | 17.71 | |
| Expressive language | Phonemic Fluency | Generate as many words as possible beginning with a specified letter (F, A, S) in 1 min | 1–80 (T score) | 35.61 | 12.69 |
| Category Fluency | Generate as many animal names as possible in 1 min | 1–80 (T score) | 38.02 | 14.75 | |
| Boston Naming Test | Name line-drawn objects | 1–80 (T score) | 34.56 | 19.36 | |
| BDAE-3 Responsive Naming | Name objects based on orally presented descriptions | 0–100 (percent) | 93.74 | 16.69 |
Abbreviation: BDAE-3, Boston Diagnostic Aphasia Examination-Third Edition.
2.3. Brain imaging
Data were collected on either a 1.5 T or 3 T MR imaging scanner. Pre-contrast T1, T2-weighted FLAIR, T2-weighted fast spin echo (FSE), diffusion-weighted MRI, perfusion MRI, and post-contrast T1-weighted MR images were obtained as part of a standard clinical protocol, but only T2-weighted FSE images were used for the current study with repetition time (TR) and echo time (TE) ranging from 2717–6670 ms to 56–123 ms, respectively, with number of averages = 1–2, matrix size 256 × 256 to 512 × 512, field-of-view (FOV) from 20 to 24 cm2, and slice thickness between 3 and 5 mm with 0–1 mm interslice gap. The T2-weighted images for each patient were registered to a 1.0 mm isotropic brain atlas (Montreal Neurological Institute (MNI) 152) by using a mutual information algorithm and a 12-degree of freedom transformation by using the Functional MR Imaging of the Brain Software Library (http://www.fmrib.ox.ac.uk/fsl/), followed by visual inspection to ensure adequate alignment.
Segmentation of the T2-weighted images involved isolation of the resection cavities and T2-hyperintense regions, which include both areas of vasogenic edema and non-enhancing tumor, excluding areas of obvious cerebrospinal fluid, blood products, and central necrosis. The volume was segmented via a semi-automated procedure consisting of (1) manually defining an area containing the relative region of tumor occurrence, (2) empirically determining thresholds on T2-weighted images within these regions, and (3) manually editing the resulting masks to exclude any non-tumor tissue. ROIs were completed via custom scripts in the Analysis of Functional NeuroImages (AFNI) program (National Institute of Mental Health; http://afni.nimh.nih.gov/afni). Initial segmentation was performed by a graduate research assistant (R.J.H.) and final segmented volumes were verified by an imaging scientist (B.M.E.).
2.4. Voxel-based lesion–symptom mapping analysis
Neurocognitive test and brain imaging data were analyzed using VLSM (Bates et al., 2003). Voxelwise comparisons were performed in all voxels with a minimum of 10 patients with segmented brain tumors for each neurocognitive test. Statistical parametric maps (SPM) were created using a voxelwise generalized linear model (GLM) with normal error distribution in MATLAB that included seven covariates (prior surgery, prior radiochemotherapy, tumor grade, tumor recurrence, age, gender, and education) along with the binary designation defining whether the patient had tumor at a particular voxel. Essentially, for each voxel, a generalized linear model was used to test for differences in language performance between patients with and without lesion in that voxel, while accounting for demographic and treatment-related covariates.
In order to account for multiple comparisons performed for each image voxel, a cluster-based thresholding technique based on random permutations was implemented as outlined previously (Bullmore et al., 1999). Briefly, the minimum cluster size retained for each neurocognitive test was determined by: 1) randomly re-assigning the patient task scores to a different patient's tumor, 2) performing the VLSM technique based on the GLM test at each voxel using the re-assigned values, 3) retaining all voxels with a p-value less than 0.05, and 4) recording the maximum cluster size defined as the number of contiguous statistically significant voxels. Steps 1–4 were repeated 1,000 times resulting in a unique distribution of maximum cluster sizes for each neurocognitive task. The 95th percentiles of these distributions were used to define the minimum allowable cluster size for the final respective statistical parameter maps.
SPMs were generated for each of the seven neurocognitive tests described in Table 1. List-wise deletion was used to handle missing data for each neurocognitive test. Following VLSM analysis of each neurocognitive test, the tests were categorized as measuring receptive language or expressive language. A composite map for receptive language was created from the intersection of the significant voxels from the three receptive language tests, and a composite map for expressive language was created from the intersection of significant voxels from the four expressive language tests. Additionally, the Dice Similarity Coefficient was calculated for each pair of tasks in order to understand the inherent similarity between clusters arising from each task as: , where s is the Dice Coefficient, A is the cluster outline for task A, B is the cluster for task B, and A ∩ B is the intersection of the two clusters.
The Johns Hopkins (JHU) White Matter Tractography Atlas and the Wake Forest (WFU) PickAtlas in MNI space were used as the anatomical templates for determining the size of the overlap of clusters in various brain regions. More specifically, the JHU atlas was used for defining the overlap in white matter regions, while the WFU atlas was utilized for the rest of the brain. All voxels that were erroneously located in the atlas' ventricular regions due to mass effect were eliminated.
3. Results
3.1. Lesion location
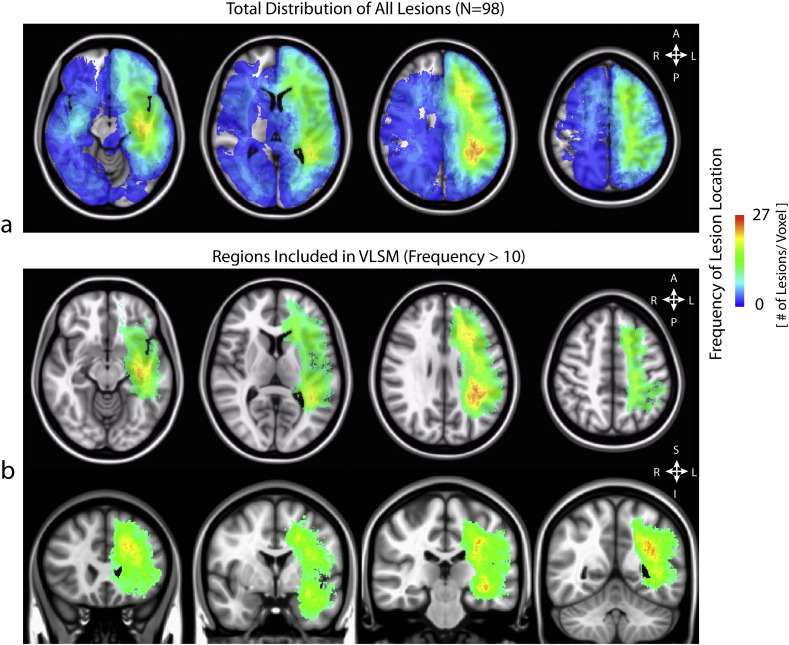
Lesion overlap maps, as shown in Fig. 1, were created to present an overview of the tumor locations for the study sample. The maps display all lesions for the patient sample, as well as the voxels that were eligible for inclusion in the VLSM analyses based on a minimum of 10 patients per voxel. These maps demonstrate that the majority of patients in the current study had lesions localized to the left hemisphere, primarily within the frontal, parietal, and temporal lobes, including the insula.
Fig. 1.
(a) Lesion overlap map for the patient sample (b) Lesion overlap map showing the voxels meeting the inclusion criterion for the VLSM analysis, with a minimum of 10 patients per voxel.
3.2. Neurocognitive performance
Table 1 displays the participants' performance on each of the seven neurocognitive tests. Means and standard deviations for each neurocognitive test of language functioning across all 98 participants are reported, in addition to a description of each test and the possible normative ranges for the test scores. For all tests, participants performed below expectation as compared to normative data of healthy controls.
3.3. VLSM findings
VLSM analysis was utilized to examine the association between language test performance and presence of lesion on a voxel-by-voxel basis, as described in Section 2.4. SPMs displaying significant clusters of voxels (p < .05) were created for each neurocognitive test. Composite maps for receptive language and expressive language were also created to display the significant voxels common to all tests within that domain. These SPMs and composite maps are shown in Fig. 2, Fig. 3, and the similarity between these significant clusters for all tasks is shown in Table 2. In all cases, lesion presence was associated with worsened performance on the corresponding neurocognitive test.
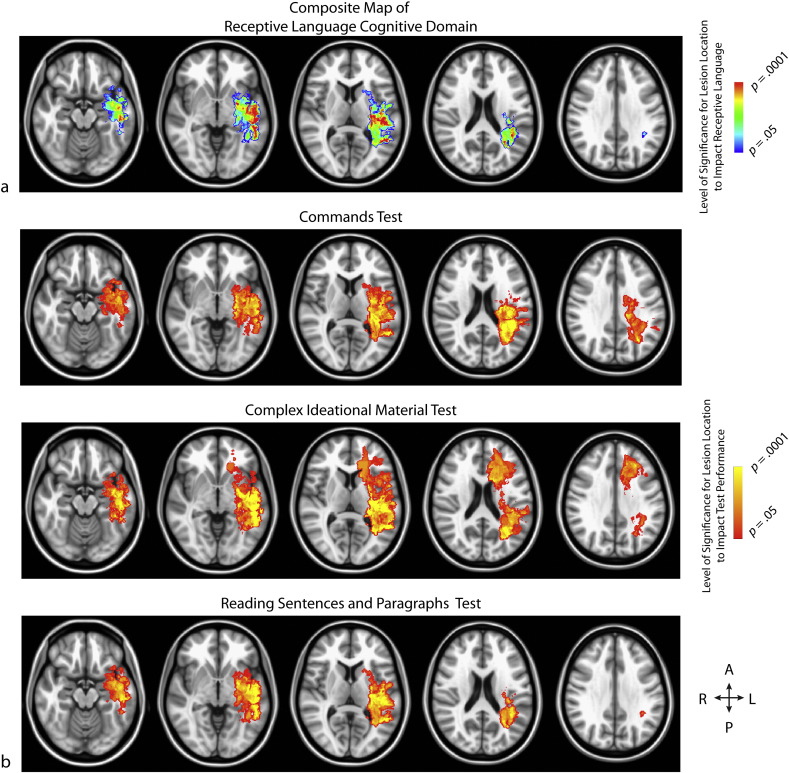
Fig. 2.
(a) Composite map of receptive language, comprised of the intersecting areas from the statistical parametric maps for each neurocognitive test within this domain. p-values for each voxel are reflective of the average t-statistic values from the tests within this domain (b) The rows, from top to bottom, demonstrate the significant voxels from the Phonemic Fluency, Category Fluency, Boston Naming, and Responsive Naming tests, respectively.
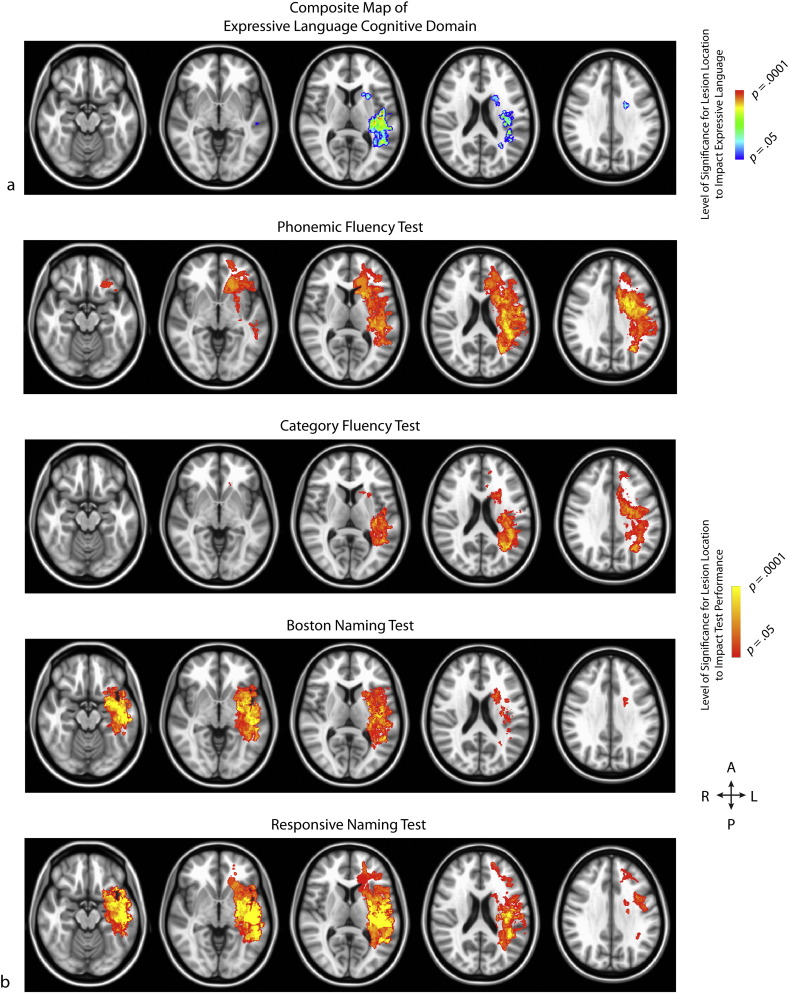
Fig. 3.
(a) Composite map of expressive language, comprised of the intersecting areas from the statistical parametric maps for each neurocognitive test within this domain. p-values for each voxel are reflective of the average t-statistic values from the tests within this domain (b) The rows, from top to bottom, demonstrate the significant voxels from the Commands, Complex Ideational Material, and Reading Sentences and Paragraphs tests, respectively.
Table 2.
Dice similarity coefficients for each pair of tasks.
| Task | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 | 1.00 | 0.56 | 0.61 | 0.38 | 0.41 | 0.56 | 0.62 |
| 2 | 1.00 | 0.57 | 0.47 | 0.32 | 0.53 | 0.68 | |
| 3 | 1.00 | 0.23 | 0.23 | 0.73 | 0.68 | ||
| 4 | 1.00 | 0.51 | 0.22 | 0.42 | |||
| 5 | 1.00 | 0.14 | 0.23 | ||||
| 6 | 1.00 | 0.71 | |||||
| 7 | 1.00 |
1 = BDAE-3 Commands.
2 = BDAE-3 Complex Ideational Material.
3 = BDAE-3 Reading Comprehension — Sentences and Paragraphs.
4 = Phonemic Fluency.
5 = Category Fluency.
6 = Boston Naming Test.
7 = BDAE-3 Responsive Naming.
Within receptive language, the three neurocognitive tests used to assess this type of language functioning showed only modest similarity (Table 2; mean Dice Coefficient = 0.58; range = 0.56–0.61), but were associated with a relatively large intersecting volume of approximately 37 cm3. Specifically, these tests were all associated with lesions in the left middle and superior temporal gyri including Wernicke's area, left internal capsule and posterior thalamic radiation, left external capsule, left inferior and superior longitudinal fasciculi, and left Heschl's gyrus in primary auditory cortex (Nagarajan et al., 1999) (see Fig. 2).
In contrast, the neuroanatomical regions associated with the four neurocognitive tests within the area of expressive language shared little overlap, averaging a Dice coefficient of 0.37 (Table 2; range = 0.14–0.71). The 6.6 cm3 of intersecting volume comprised regions of the left retrolenticular limb of the internal capsule, left superior longitudinal fasciculus, left rolandic operculum, left insula, and left middle and superior temporal gyri (see Fig. 3). Individually, however, performance on each of the four expressive language tests was associated with much larger clusters: Phonemic Fluency with approximately 114.8 cm3, Category Fluency with 52.5 cm3, Boston Naming Test with 63.7 cm3, and Responsive Naming with 104.1 cm3. These unshared regions included greater areas of the left temporal lobe, left middle and superior frontal gyri, and left inferior parietal lobule. Examination of the individual tests demonstrated that the lack of overlapping regions across expressive language tests was not influenced by any one test in particular, but rather that as a whole the tests shared little overlap.
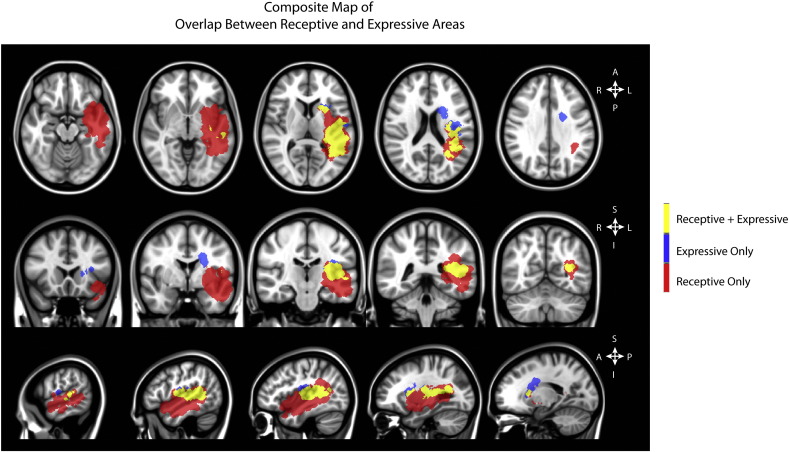
Lastly, regions of overlap between receptive and expressive language regions were evaluated (Fig. 4). Results suggest lesions extending through tissue connecting Broca's and Wernicke's areas appear to significantly influence both receptive and expressive language function. In particular, lesions within the external capsule along with the insula and extending into the posterior speech regions including Wernicke's area, significantly decreases both receptive and expressive language function.
Fig. 4.
Localization of regions shown to significantly influence receptive (red) and expressive (blue) language function, as well as overlap between these areas (yellow).
4. Discussion
The current study investigated the neural basis of language functioning in adult glioma patients using a highly sensitive voxel-by-voxel analytic method coupled with standardized neurocognitive tests of language functioning. The present study utilized voxel-based lesion-symptom mapping (VLSM), a modern technique that has been used primarily with patients with cerebral infarction, in order to examine the relationships between lesion location and language performance in adult glioma patients. Impaired performance across both receptive and expressive language tests was associated with damage to several left-lateralized cortical and subcortical regions.
4.1. Associations between lesion location and type of language functioning
The associations between neurocognitive test performance and respective regions of interest were largely similar across all of the receptive language tests. For the receptive language tests, the observed associations with left middle and superior temporal gyri including Wernicke's area were consistent with numerous studies (Bates et al., 2003, Binder et al., 1997, Haglund et al., 1994, Lesser et al., 1986), as was the association with primary auditory cortex as this region plays a role in reading and listening (Bookheimer et al., 2000, Nagarajan et al., 1999). Importantly, the present study also identified several voxels within white matter that were associated with receptive language, particularly near the insula and the inferior and superior longitudinal fasciculi. This suggests that damage to these areas plays a significant role in the receptive language deficits in glioma patients, possibly through interference of connecting frontal–striatal circuits and pathways to temporal and parietal regions (Cummings, 1995, Friederici, 2009, Glasser and Rilling, 2008).
Unlike receptive language, the tests of expressive language were associated with largely independent brain regions, suggesting that expressive language abilities may be quite task dependent and thus much more distributed within the brain. This finding is understandable when considering the cognitive steps involved in language. Receptive language develops earlier in childhood as it is thought to be a simpler ability (Paul, 1996), and when asked to perform a task such as following oral commands (Commands test) or answering orally presented questions (Complex Ideational Material test), many steps in processing the oral prompt are likely similar. In contrast, the processing of an expressive language task involving the generation of words from memory that begin with certain sounds (Phonemic Fluency test) is likely quite different than that of a task involving the naming of line-drawn objects (Boston Naming Test).
Additionally, the brain regions that were common to all of the expressive language tests were largely within the temporal lobe and notably did not include Broca's area. This finding suggests that the etiology of expressive language deficits in glioma patients may be more commonly due to difficulty with category-specific semantic organization and semantic knowledge which are temporally mediated (Bartha et al., 2003), rather than difficulty with strategic word retrieval due to Broca's area involvement (Keller et al., 2009). Also, the white matter regions found to be associated with expressive language in the current study have been recently hypothesized to play a significant role in expressive language abilities (Kolb and Whishaw, 2009). In particular, expressive language deficits may occur due to dysfunction of the insula, which has been shown in some studies to be related to apraxia of speech (Dronkers, 1996, Oh et al., 2014), the superior temporal gyrus which can cause impairment in sentence comprehension and production of sentences due to syntactic and lexical processing difficulties (Friederici et al., 2009, Friederici et al., 2003), and the superior longitudinal fasciculus which can cause impairment in utterances (i.e., smallest unit of speech) and repetition (Berthier et al., 2012, Moritz-Gasser and Duffau, 2013).
4.2. Study limitations
While it may be hypothesized that regions of the left hemisphere would primarily be identified in the current investigation of language abilities, a confounding factor in the present study is that the majority of participants had tumors localized to the left hemisphere. For this particular study, a number of patients with right hemisphere lesions were present, and data from these patients were utilized in the GLM to compare the test performance of participants with and without lesions in each voxel. However, right hemisphere lesion areas were not specifically examined in the VLSM analysis because the group size in each of the right hemisphere voxels totaled less than our criterion of ten. As a result, from the present study, it is not possible to draw conclusions about the involvement of right hemispheric structures for expressive and receptive language in adult glioma patients. It is therefore recommended that future VLSM studies examine whether bilateral correlates of language may be found with samples that have a more diverse distribution of lesion locations, as several neuroimaging and lesion-deficit studies have identified bilateral representation of language (Baddeley, 2003, Price, 2000).
Additionally, the retrospective design of the present study may have resulted in a selection bias in the sample. Because neurocognitive testing was performed for clinical purposes, it is possible that patients without cognitive concerns or patients with severely debilitating disease were not represented in the sample due to being poor candidates for testing. Also, heterogeneity with respect to MRI acquisition parameters (e.g. field strength, matrix size, etc.) may have introduced slight errors in lesion boundaries for individual patients, which may have translated into slight differences in the resulting statistical parameter maps. However, regions of abnormal T2 signal intensity were masked based on relative contrast to normal tissue, confirmed by imaging experts, and therefore were not likely to be influenced by subtle differences in acquisition parameters. Similarly, image registration of anatomically distorted brains into standard stereotactic atlas space may have introduced subtle errors in lesion localization. Despite our best effort to align images manually (when necessary) and verification of alignment by more than 2 independent observers, this lack of accuracy is a potential limitation.
Furthermore, the sample that was utilized in the particular study was heterogeneous with regard to treatment history and medical demographics. These heterogeneous demographics may affect language localization and language performance differently in the participant sample; for example, surgery, radiation, and chemotherapy can result in neuroplastic changes for the patients who have received these treatments. While these characteristics were controlled for in the present study and the current sample is representative of the patients who may present with language concerns within a large neuro-oncology clinic, analysis of more homogeneous populations is also warranted. Future studies may also benefit from examining whether corticosteroid use has a significant impact on the identified brain–behavior associations; although corticosteroid use was not a covariate in the present study, the majority of the participants had been treated with corticosteroids.
It is important to point out that the aphasia classification used in the current study for expressive and receptive language function is rather antiquated. Newer and more accurate classifications of these functions are also available. Additionally, use of VLSM is a rather outdated approach for mapping language function, particularly when trying to estimate function in individual patients. Similar, and potentially more localized, information may be obtained for individual patients using advanced functional imaging techniques including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), electroencephalography (EEG), or magnetoencephalography (MEG). However, it is important to point out that the VLSM method is useful to determine the relationship between tumor location and loss of function, whereas other techniques are more useful for identifying regions of retained function.
There are a few possible explanations for the relatively high Dice coefficients found in the current study, particularly when compared with fMRI experiments. Importantly, the VLSM analysis in the current study includes both cortical gray matter and subcortical white matter structures. FMRI, alternatively, involves cortical activation due to hemodynamic characteristics near cortical regions and is not particularly sensitive to subcortical white matter regions that may also be involved in functional tasks, limiting the total number of regions that may have potential overlap in function. Additionally, broad damage to white matter regions due to infiltrative and/or destructive tumor may result in loss of function in a multitude of tasks, as the neurological tasks used in the current study rely on many processing components and may not be particularly localized.
Lastly, VLSM analyses have primarily been performed with patients with cerebral infarction. An advantage of this patient population is that the site of infarcts can often be precisely identified, resulting in very specific, localized findings from VLSM analyses. In contrast, gliomas involve substantially larger lesion areas than that of cerebral infarction and can have ill-defined boundaries.
5. Conclusion
The current study examined the critical brain regions involved in receptive language and expressive language using standardized neurocognitive testing and VLSM analysis in adult glioma patients. The findings of the present study demonstrated that language performance was associated with lesion location, which provides support for patient-specific selection of tests for language assessment (Wefel et al., 2011), using tumor location as a factor. In addition, the findings of the current study suggest that the location of hyperintense regions on MR images can be utilized by healthcare providers at patients' clinical visits as an objective predictor of the language deficits that glioma patients may experience. When healthcare providers encounter patients with lesions in the brain regions identified in the present study, they can intervene earlier by making accurate and timely referrals for neurocognitive evaluation and language rehabilitation, which may lead to improved quality of life for patients with language difficulties.
Acknowledgments
The authors would like to thank the following for their funding of this research: National Institutes of Health/National Cancer Institute (R21CA167354 to B.M.E.); UCLA Institute for Molecular Medicine Seed Grant to B.M.E.; UCLA Jonsson Comprehensive Cancer Center Seed Grant to B.M.E.; UCLA Radiology Exploratory Research Grant to B.M.E.; University of California Cancer Research Coordinating Committee Grant to B.M.E.; American College of Radiology Imaging Network Young Investigator Initiative Grant to B.M.E.; National Brain Tumor Society Research Grant to B.M.E.; Siemens Healthcare Research Grant to B.M.E.; Art of the Brain to P.B. and T.F.C.; Ziering Family Foundation in memory of Sigi Ziering to T.F.C.; Singleton Family Foundation to T.F.C.; and Clarence Klein Fund for Neuro-Oncology to T.F.C.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.10.010.
Contributor Information
Pia Banerjee, Email: PBanerjee@mednet.ucla.edu.
Kevin Leu, Email: KLeu@mednet.ucla.edu.
Robert J. Harris, Email: RHarris@mednet.ucla.edu.
Timothy F. Cloughesy, Email: TCloughesy@mednet.ucla.edu.
Albert Lai, Email: AlbertLai@mednet.ucla.edu.
Phioanh L. Nghiemphu, Email: PNghiemphu@mednet.ucla.edu.
Whitney B. Pope, Email: WPope@mednet.ucla.edu.
Susan Y. Bookheimer, Email: SBook@ucla.edu.
Benjamin M. Ellingson, Email: BEllingson@mednet.ucla.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Supplemental Fig. 1.
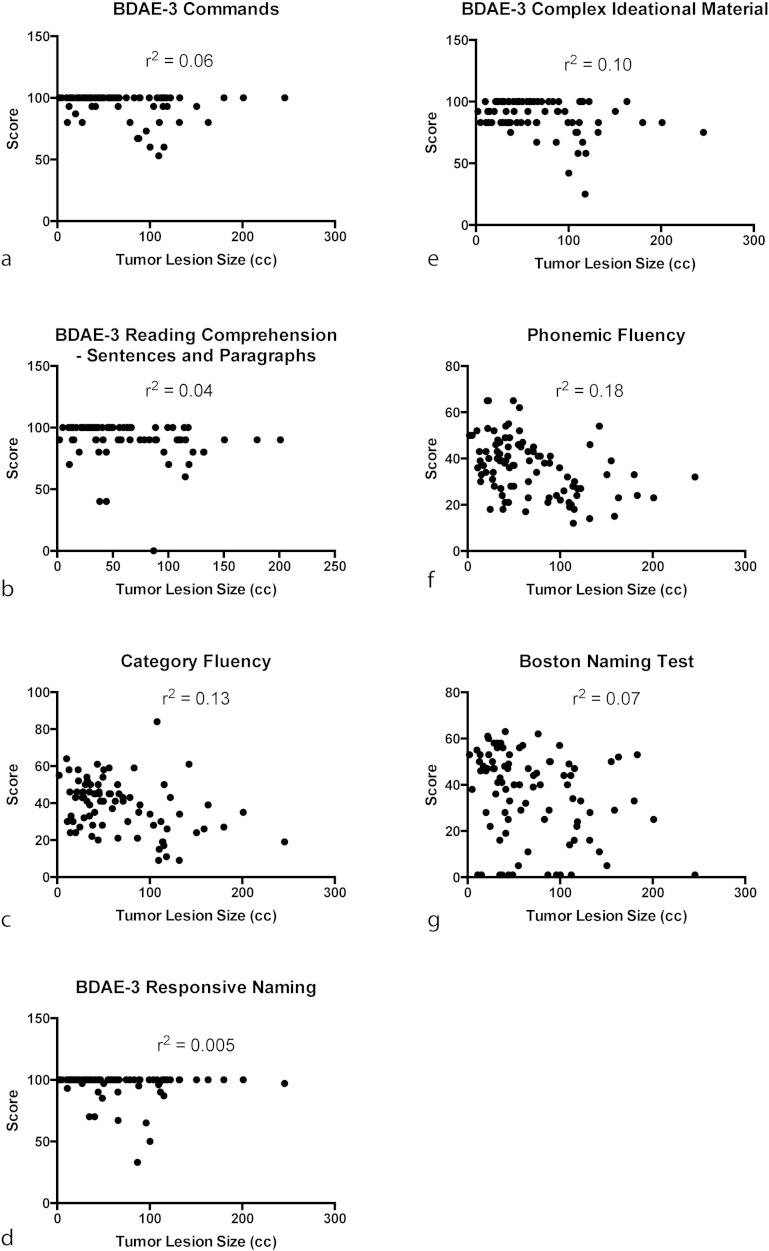
Relationships between (a) BDAE-3 Commands and lesion size (R2 = 0.06), (b) BDAE-3 Reading Comprehension for Sentences and Paragraphs and lesion size (R2 = 0.04), (c) Category Fluency and lesion size (R2 = 0.13), (d) BDAE-3 Responsive Naming and lesion size (R2 = 0.005), (e) BDAE-3 Complex Ideational Material and lesion size (R2 = 0.10), (f) Phonemic Fluency and lesion size (R2 = 0.18), and (e) Boston Naming Test and lesion size (R2 = 0.07).
References
- Baddeley A. Working memory and language: an overview. J. Commun. Disord. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Bartha L., Brenneis C., Schocke M., Trinka E., Köylü B., Trieb T., Kremser C., Jaschke W., Bauer G., Poewe W. Medial temporal lobe activation during semantic language processing: fMRI findings in healthy left-and right-handers. Cogn. Brain Res. 2003;17:339–346. doi: 10.1016/s0926-6410(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., Dronkers N.F. Voxel-based lesion–symptom mapping. Nat. Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bernal B., Ardila A. Bilateral representation of language: a critical review and analysis of some unusual cases. J. Neurolinguistics. 2014;28:63–80. [Google Scholar]
- Berthier M.L., Lambon Ralph M.A., Pujol J., Green C. Arcuate fasciculus variability and repetition: the left sometimes can be right. Cortex. 2012;48:133–143. doi: 10.1016/j.cortex.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Cox R.W., Rao S.M., Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J. Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele F.W., Zant M., Heine E.C., Aaronson N.K., Taphoorn M.J., Reijneveld J.C., Postma T.J., Heimans J.J., Klein M. The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neuro Oncol. Pract. 2014;1:40–46. doi: 10.1093/nop/npu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S., Zeffiro T.A., Blaxton T.A., Gaillard W., Theodore W.H. Activation of language cortex with automatic speech tasks. Neurology. 2000;55:1151–1157. doi: 10.1212/wnl.55.8.1151. [DOI] [PubMed] [Google Scholar]
- Bosma I., Vos M.J., Heimans J.J., Taphoorn M.J., Aaronson N.K., Postma T.J., van der Ploeg H.M., Muller M., Vandertop W.P., Slotman B.J. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9:53–62. doi: 10.1215/15228517-2006-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., Brammer M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Christoffersen E.P., Wells D.L. Expressive aphasia in glioblastoma multiforme patients: an application of content methodology. Can. Oncol. Nurs. J. 1998;8:121–127. doi: 10.5737/1181912x82121127. [DOI] [PubMed] [Google Scholar]
- Cummings J.L. Anatomic and behavioral aspects of frontal–subcortical circuits. Ann. N. Y. Acad. Sci. 1995;769:1–14. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Dalemans R.J., De Witte L.P., Wade D.T., Van den Heuvel W.J. A description of social participation in working-age persons with aphasia: a review of the literature. Aphasiology. 2008;22:1071–1091. [Google Scholar]
- Darrigrand B., Dutheil S., Michelet V., Rereau S., Rousseaux M., Mazaux J.-M. Communication impairment and activity limitation in stroke patients with severe aphasia. Disabil. Rehabil. 2011;33:1169–1178. doi: 10.3109/09638288.2010.524271. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Pathways to language: fiber tracts in the human brain. Trends Cogn. Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Rueschemeyer S.-A., Hahne A., Fiebach C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Makuuchi M., Bahlmann J. The role of the posterior superior temporal cortex in sentence comprehension. Neuroreport. 2009;20:563–568. doi: 10.1097/WNR.0b013e3283297dee. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain's language pathways. Cereb. Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Goodglass H., Kaplan E., Barressi B. 3rd edition. Pearson; San Antonio, Texas: 2000. The Boston Diagnostic Aphasia Examination. (BDAE-3) [Google Scholar]
- Haglund M.M., Berger M.S., Shamseldin M., Lettich E., Ojemann G.A. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- Keller S.S., Crow T., Foundas A., Amunts K., Roberts N. Broca's area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kinno R., Muragaki Y., Sakai K.L. Gray or white? The contribution of gray matter in a glioma to language deficits. In: Chen C.C., editor. Advances in the Biology, Imaging and Therapies for Glioblastoma. InTech; Rijeka: 2011. pp. 107–122. [Google Scholar]
- Klein M., Taphoorn M.J., Heimans J.J., van der Ploeg H.M., Vandertop W.P., Smit E.F., Leenstra S., Tulleken C.A., Boogerd W., Belderbos J.S. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J. Clin. Oncol. 2001;19:4037–4047. doi: 10.1200/JCO.2001.19.20.4037. [DOI] [PubMed] [Google Scholar]
- Kolb B., Whishaw I.Q. Worth Publishers; New York: 2009. Language. Fundamentals of Human Neuropsychology; pp. 524–556. [Google Scholar]
- Lesser R., Lüders H., Morris H., Dinner D., Klem G., Hahn J., Harrison M. Electrical stimulation of Wernicke's area interferes with comprehension. Neurology. 1986;36:658–663. doi: 10.1212/wnl.36.5.658. [DOI] [PubMed] [Google Scholar]
- Moritz-Gasser S., Duffau H. The anatomo-functional connectivity of word repetition: Insights provided by awake brain tumor surgery. Front. Hum. Neurosci. 2013;7:1–4. doi: 10.3389/fnhum.2013.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S., Mahncke H., Salz T., Tallal P., Roberts T., Merzenich M.M. Cortical auditory signal processing in poor readers. Proc. Natl. Acad. Sci. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh A., Duerden E.G., Pang E.W. The role of the insula in speech and language processing. Brain Lang. 2014;135:96–103. doi: 10.1016/j.bandl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. Clinical implications of the natural history of slow expressive language development. Am. J. Speech Lang. Pathol. 1996;5:5–21. [Google Scholar]
- Price C.J. The anatomy of language: contributions from functional neuroimaging. J. Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R., O'Connor A.M., Ashley S. Speech and language disorders in patients with high grade glioma and its influence on prognosis. J. Neuro-Oncol. 1995;23:265–270. doi: 10.1007/BF01059960. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- Tyler L.K., Marslen-Wilson W., Stamatakis E.A. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia. 2005;43:771–778. doi: 10.1016/j.neuropsychologia.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Wefel J.S., Vardy J., Ahles T., Schagen S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Zucchella C., Bartolo M., Di Lorenzo C., Villani V., Pace A. Cognitive impairment in primary brain tumors outpatients: a prospective cross-sectional survey. J. Neuro-Oncol. 2013;112:455–460. doi: 10.1007/s11060-013-1076-8. [DOI] [PubMed] [Google Scholar]