Abstract
Aims
Fibrate medications weakly stimulate the nuclear receptor peroxisome proliferator-activated receptor-α (PPAR-α) and are currently employed clinically in patients with dyslipidaemia. The potent and selective agonist of PPAR-α LY518674 is known to substantially increase apolipoprotein A-I (apoA-I) turnover without major impact on steady-state levels of apoA-I or high-density lipoprotein-cholesterol (HDL-C). We sought to determine whether therapy with a PPAR-α agonist impacts cholesterol efflux capacity, a marker of HDL function.
Methods and results
Cholesterol efflux capacity was measured at baseline and after 8 weeks of therapy in a randomized, placebo-controlled trial involving participants with metabolic syndrome treated with either LY518674 100 μg daily (n = 13) or placebo (n = 15). Efflux capacity assessment was quantified using a previously validated ex vivo assay that measures the ability of apolipoprotein-B depleted plasma to mobilize cholesterol from macrophages. LY518674 led to a 15.7% increase from baseline (95% CI 3.3–28.1%; P = 0.02, P vs. placebo = 0.01) in efflux capacity. The change in apoA-I production rate in the active treatment arm was strongly linked to change in cholesterol efflux capacity (r = 0.67, P = 0.01).
Conclusions
Potent stimulation of PPAR-α leads to accelerated turnover of apoA-I and an increase in cholesterol efflux capacity in metabolic syndrome patients despite no change in HDL-C or apoA-I levels. This finding reinforces the notion that changes in HDL-C levels may poorly predict impact on functionality and thus has implications for ongoing pharmacologic efforts to enhance apoA-I metabolism.
Keywords: Cholesterol efflux capacity, HDL-cholesterol, Lipid metabolism, PPAR-α
Translational perspective.
The complexities of high-density lipoprotein cholesterol (HDL-C) metabolism have led to interest in moving beyond static assessments of HDL-C levels to assessing functionality. One such measure, cholesterol efflux capacity, measures a key step in the reverse cholesterol transport pathway and is associated with both the prevalence and incidence of coronary disease. Here, we demonstrate that treatment of metabolic syndrome patients with a peroxisome proliferator-activated receptor-α agonist is associated with improvement in cholesterol efflux capacity despite no change in HDL-C levels. This increase was closely linked to an increase in the rate of apolipoprotein A-I production rate. This finding may inform ongoing efforts to evaluate and develop efficacious therapeutics targeting HDL metabolism.
Introduction
Therapeutic targeting of high-density lipoprotein cholesterol (HDL-C) metabolism has proven challenging with multiple clinical trial setbacks in recent years involving niacin or cholesteryl ester transfer protein inhibition. Steady-state assessment of circulating HDL concentrations may incompletely reflect in vivo functionality. Cholesterol efflux capacity quantifies the ability of HDL lipoproteins to mobilize cholesterol from macrophages, a critical first step in the anti-atherogenic reverse cholesterol transport pathway. This metric has been shown to be inversely related to both atherosclerotic burden and, more recently, incident cardiovascular events in multiple cohorts independent of circulating levels of HDL-cholesterol.1,2
Peroxisome proliferator-activated receptors (PPARs) are a family of nuclear receptors that modulate both lipid and glucose metabolism. Fibrate therapies serve as weak activators of PPAR-α and are in frequent clinical use in patients with elevated triglycerides. Subsequent efforts have led to more potent and specific PPAR-α ligands, including LY518674. Previous studies with LY518674 in patients with atherogenic dyslipidaemia or the metabolic syndrome has noted decreased triglycerides but increased LDL-C levels and minimal impact on HDL-C or apoA-I levels.3,4 However, a balanced >30% increase in both the production and catabolic rate was noted, reflective of enhanced apoA-I turnover.
Prior efforts to document a change in cholesterol efflux capacity with LY518674 using murine bone marrow-derived macrophages did not show a significant impact. The present study reassessed efflux capacity with a more recently validated assay using the J774 macrophage cell line that may be better suited to clinical samples.
Materials and methods
The study population was derived from a previously described randomized controlled trial that investigated the impact of LY518674 on HDL metabolism (ClinicalTrials.gov NCT00327002).4 All subjects had low HDL-C levels as well as at least two additional components of the metabolic syndrome. Exclusion criteria included treatment with fibrates, thiazolinediones, ezetemibe, or niacin (>250 mg/day) as well as a history of cardiovascular disease or diabetes. Participants were randomized in a double-blind fashion to receive LY518674, 100 μg daily, or placebo for 8 weeks. Apolipoprotein kinetics were measured using a deuterated leucine tracer to quantify rate of apoA-I production (i.e. the amount of newly synthesized apoA-I entering plasma).4
Cholesterol efflux capacity was assessed using an assay that quantifies the ability of apolipoprotein B-depleted plasma to accept 3H-radiolabeled cholesterol from J774 macrophages ex vivo as previously reported.1 Efflux capacity assays were performed in duplicate in a paired fashion on 24-well plates.
Paired t-tests were used to assess the effect of LY518674 and placebo on HDL metabolic parameters. These changes were compared across treatment arms via an analysis of covariance, which included the patient's baseline value and the treatment group as covariates.
Results
The study population included 28 patients, with 13 randomized to LY518674 and 15 to placebo. Baseline characteristics were well-balanced across randomization groups as previously reported. The cohort included 14 males (50%) with a mean age of 49 years, body mass index of 37 kg/m2, and blood pressure of 130/78 mmHg. Baseline laboratory values, expressed as mean ± SD, were total cholesterol 189 ± 43 mg/dL, HDL-C 37 ± 6 mg/dL, LDL-C 117 ± 36 mg/dL, and apoA-I 108 ± 15 mg/dL. Median (IQR) triglyceride value was 167 (104–211) mg/dL.
Cholesterol efflux capacity at baseline was similar across the two treatment arms with mean ± SD of 1.08 ± 0.15 and 1.07 ± 0.22 for the LY518674 and placebo groups, respectively. Minimal relationship (r = 0.17; P = 0.38) was noted between this efflux assessment and previously reported total efflux capacity, likely reflective of differences in assay technique.
Eight weeks of therapy with LY518674 were associated with a 15.7% (95% CI 3.3–28.1%) increase from baseline in cholesterol efflux capacity and a 31.1% (95% CI 15.3–46.9%) increase from baseline in the production rate of apoA-I despite no change in HDL-C or apoA-I levels (Table 1). Neither change in HDL-C (r = 0.17; P = 0.66) nor change in apoA-I (r = 0.17; P = 0.66) was predictive of change in efflux capacity with PPAR-α agonist treatment. However, change in apoA-I production rate strongly predicted increased cholesterol efflux capacity (r = 0.67; P = 0.01) as displayed in Figure 1.
Table 1.
Percent Changes in high-density lipoprotein metabolism-related phenotypes after 8 weeks of therapy with either LY518674 or placebo
| LY518674 | Placebo | P-value | |
|---|---|---|---|
| HDL-C | −0.8 (−13.2 to 11.7) P = 0.96 |
−3.2 (−7.5 to 1.2) P = 0.08 |
0.31 |
| ApoA-I | 0.7 (−9.6 to 8.2) P = 0.81 |
5.6 (2.0 to 9.3) P = 0.01 |
0.26 |
| Apolipoprotein A-I production rate | 31.1 (15.3 to 46.9) P = 0.001 |
−0.4 (−6.5 to 5.7) P = 0.80 |
0.0001 |
| Cholesterol efflux capacity | 15.7 (3.3 to 28.1) P = 0.02 |
−0.2 (−5.1 to 4.8) P = 0.87 |
0.01 |
Values represent mean % change (95% CI) for each parameter. P-values are presented for the significance of both change from baseline and difference between treatment arms.
Figure 1.
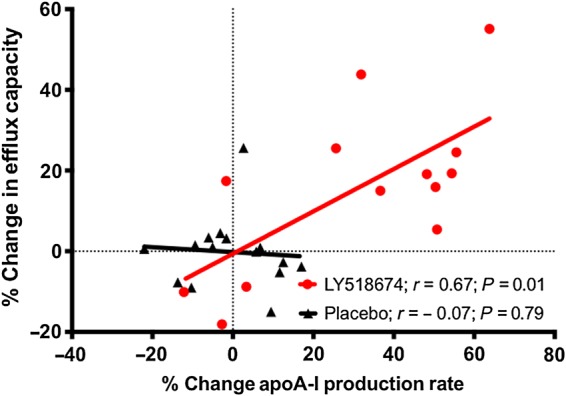
Relationship between change in apoA-I production rate and change in cholesterol efflux capacity after 8 weeks of therapy with either LY518674 or placebo.
Discussion
Potent and specific stimulation of the PPAR-α receptor leads to an increase in cholesterol efflux capacity, an effect that is associated with the enhanced production of apoA-1, in patients with metabolic syndrome. This finding likely reflects an increase in hepatic generation of lipid poor apoA-I particles that serve as efficient acceptors of cholesterol. Our results are well aligned with a murine study that noted enhanced macrophage reverse cholesterol transport after treatment with potent PPAR-α activation.5 Although the clinical development of novel PPAR agonists with an acceptable safety profile has proven challenging, several molecules remain in clinical development.
The discrepancy between the current findings and previously reported cholesterol efflux values likely reflects differences in design of the cell-based assay. The prior study quantified efflux to whole plasma from bone marrow-derived murine macrophages loaded with acetylated LDL and treated with a liver X receptor agonist. Our present assay implemented apolipoprotein-B depleted serum using a validated precipitation step that thus eliminates confounding from VLDL, LDL, and lipoprotein(a) particles.1 With regard to the donor cell line, we used cAMP-stimulated J774 macrophage cells that quantify total efflux mediated by several relevant pathways including ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1), scavenger receptor B1, and aqueous diffusion. Importantly, a pooled plasma control was used to normalize values obtained across assays which improves reproducibility. In this study, average coefficient of variation across duplicates was 5.9%; average change in efflux capacity with placebo treatment was −0.2% with a high degree of correlation (r = 0.89) between baseline and on-treatment values, again confirming longitudinal stability.
The current findings represent another example of discordance between changes in HDL-C levels and functionality with pharmacologic therapy. For example, the addition of niacin to statin therapy had no impact on efflux capacity despite resulting in a 29% increase in HDL-C.6 The cholesteryl ester protein inhibitor dalcetrapib increased HDL-C levels by 34% with only a 10% increase in total efflux capacity.7 These assessments may prove fruitful in offering additional mechanistic understanding to the multiple HDL and apoA-I centric therapeutic candidates currently in development.
Funding
This work was supported by a grant from Eli Lilly and Company, an NIH Public Health Services Research grant (M01-RR00040 to D.J.R.), and an ACCF/Merck Research Fellowship in Cardiovascular Disease to A.V.K.
Conflict of interest: A.V.K. has received consulting fees from Merck, Sharp, and Dohme, Inc. J.S.M. has received research funding from Eli Lilly and Company. G.R. is employee and stockholder of Eli Lilly and Company. D.J.R. has received research funding from Eli Lilly and Company, consulting fees from Eli Lilly and Company, and Merck, Sharp, and Dohme, Inc, is a founder of VascularStrategies, a company which performs specialized assays including cholesterol efflux capacity via contracted service agreements, and is an inventor on a pending patent for the measurement of in vivo reverse cholesterol transport in humans.
References
- 1.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, Gomez EV, Russo JM. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA 2007;297:1362–1373. [DOI] [PubMed] [Google Scholar]
- 4.Millar JS, Duffy D, Gadi R, Bloedon LT, Dunbar RL, Wolfe ML, Movva R, Shah A, Fuki IV, McCoy M, Harris CJ, Wang MD, Howey DC, Rader DJ. Potent and selective PPAR-alpha agonist LY518674 upregulates both ApoA-I production and catabolism in human subjects with the metabolic syndrome. Arterioscler Thromb Vasc Biol 2009;29:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakaya K, Tohyama J, Naik SU, Tanigawa H, MacPhee C, Billheimer JT, Rader DJ. Peroxisome proliferator-activated receptor-α activation promotes macrophage reverse cholesterol transport through a liver X receptor-dependent pathway. Arterioscler Thromb Vasc Biol 2011;31:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khera AV, Patel PJ, Reilly MP, Rader DJ. The addition of niacin to statin therapy improves high-density lipoprotein cholesterol levels but not metrics of functionality. J Am Coll Cardiol 2013;62:1909–1910. [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, Ditmarsch M, Kallend D, Niesor EJ, Suchankova G, Upmanyu R, Anzures-Cabrera J, Lehnert V, Pauly-Evers M, Holme I, Štásek J, van Hessen MW, Jones P; dal-ACUTE Investigators. The effect of cholesteryl ester transfer protein inhibition on lipids, lipoproteins, and markers of HDL function after an acute coronary syndrome: the dal-ACUTE randomized trial. Eur Heart J 2014;35:1792–1800. [DOI] [PubMed] [Google Scholar]


