Abstract
Background:
Occult hepatitis B infection (OBI) is a major public health problem worldwide, which harbors potential risk of hepatitis B virus (HBV) transmission through blood transfusion and transplantation. OBI is characterized by the presence of HBV-DNA in the blood or liver tissue without detectable hepatitis B surface antigen (HBsAg) in the serum. An important cause of OBI is the occurrence of mutations in the HBV genome, especially in the S region.
Objectives:
The study aims to analyze mutations in S and pre-core/core regions of HBV-DNA in hemodialysis patients.
Patients and Methods:
Sera of 216 hemodialysis patients were tested for HBsAg and hepatitis B core antibody (HBcAb) by ELISA. Sera of patients that tested negative for HBsAg were evaluated by PCR for the detection of HBV-DNA in the S and pre-core/core regions. In total, six PCR products were sequenced, aligned, and compared with the HBV reference sequence. Amino acid deletion and nucleotide substitution were considered mutations in S and pre-core/core regions of HBV-DNA.
Results:
Among 216 patients, 203 (93.98%) and 175 (81.01%) sera samples tested negative for HBsAg and HBcAb, respectively. Among all HBsAg-negative samples, six (2.9%) tested positive for HBV-DNA, including four (1.97%) for S and two (0.98%) for pre-core regions. All four (1.97%) samples that tested positive for the S region belonged to HBV-subtype awy. The amino acid sequence of all four samples showed the YMDD motif in position 204 (rtM204). There were three amino acid substitutions in the S region (T127P, P153L, and F170S) and one substitution in the RT region (Y135S). Moreover, two (0.98%) pre-core/core positive patients had an unexpected stop codon in position 1896.
Conclusions:
This study indicates that 2.9% of hemodialysis patients had OBI, which is considered as a major public health problem worldwide. Moreover, we observed three mutations in S region, including T127P, P153L, and F170S, which caused OBI. This study is first to report a mutation analysis of HBV in hemodialysis patients in southwestern Iran. These results indicate that current screening tests based on HBsAg detection are not reliable for detection of HBV infection in dialysis patients.
Keywords: Hepatitis B, Chronic, Hemodialysis Solutions, Mutation
1. Background
Hepatitis B virus (HBV), a member of the Hepadnaviridae family with partial double-stranded DNA, causes hepatitis B infection, which is as a major public health problem worldwide. Approximately 400 million people have been infected with the hepatitis B virus globally and are recognized as healthy carriers (1). Transmission methods of HBV include blood transfusion, saliva, semen, blood products, and tattooing (2, 3). Hepatitis B surface antigen (HBsAg) is an important diagnostic marker of HBV infection, but remains undetected in some patient sera (4). HBV replicates by reverse transcription of an intermediate RNA lacking proofreading activity, which results in the emergence of mutations in the HBV genome. Studies show that point mutations in the HBV genome reduce the secretion or synthesis of HBsAg or produce defective mutants. Moreover, deletion or nucleotide substitution is responsible for the occurrence of occult hepatitis B infection (OBI) (5).
Occult hepatitis B infection is characterized by the presence of HBV-DNA in the serum, without detectable HBsAg (6). The level of DNA in sera usually is < 104 copies/mL and can be detected by a sensitive PCR assay (7). Several reasons have been proposed for the presence of HBV-DNA in HBsAg negative patients, including integration of HBV-DNA into the host chromosome (8); genetic variations in HBV that suppress the expression of S gene (9); interaction of HBsAg with immune complexes, where HBsAg may be hidden (10); existence of a window after acute HBV infection; co-infection with hepatitis C virus; immunosuppression; or other host factors (11, 12). Conditions that weaken the immune system, such as AIDS or chemotherapy, can reactivate HBV in OBI patients and subsequently result in active or fulminant hepatitis B infection. Risk factors associated with hemodialysis patients include shared dialysis equipment, invasive procedures, and low response to HBV vaccination. Moreover, OBI causes the progression of conditions such as liver fibrosis, hepatocellular carcinoma (HCC), and fulminant hepatitis (13, 14). Investigation of these mutations will be helpful in predicting the progression of liver disease in hemodialysis patients with OBI.
2. Objectives
This study aims analyze mutations in the S and pre-core/core regions of HBV in dialysis patients with OBI in Ahvaz city, Khuzestan province, Iran.
3. Patients and Methods
In total, 216 hemodialysis patients, including 85 women and 131 men, were selected from the dialysis units of 4 hospitals in Ahvaz city from January 2012 to April 2012. Blood samples were collected and their sera were tested for HBsAg by ELISA (RPC/ Russia), according to the manufacturer’s instruction. In total, 13 sera samples collected from eight males and five females tested positive for HBsAg (RPC Diagnostic systems, Nizhny Novgorod, Russia) and were excluded from this study. The remaining 203 sera samples were tested for the presence of HBV-DNA and HBcIgG ELISA kit (DIA.PRO, Milan, Italy).
3.1. Genome Extraction
Genome extraction was conducted for 203 HBsAg-negative samples by High Pure Viral Nucleic Acid Kit (Roche/Germany) according to the manufacturer’s instruction.
3.1.1. The Nested-PCR for S Region
The following primers were used for detection and mutation analysis of the S region (High Pure Viral Nucleic Acid Kit (Roche, Indianapolis, USA): outer primers, SA1: 5′-ATCGCTGGATGTGTCTGCGG-3′ ′, and SA2: 5′-GGCAACGGGGTAAAGGTTCA-3′ (position 369–388 and 1136–1155); and inner primers, SB1: 5′-TTAGGGTTTAAATGTATACCC-3′ (position 822 - 842), and SB2: 5′-CATCTTCTTGTTGGTTCTTCTG-3′ (position 427 - 448) (15). First round PCR was performed with a 25 µL total volume, containing 7 µL of HBV-DNA as template, 2.5 µL 10x reaction buffer, 0.75 µL MgCl2 (50 mM), 0.5 µL forward/reverse primer, 1 µL dNTP (10 mM), 0.2 Taq DNA Polymerase (Cinna Gen, Tehran, Iran) (5 u/µL), and 13.55 µL of double-distilled water. The thermal cycler was programmed for the first round as follows: 35 cycles with initial denaturation at 94°C for 5 minutes, 94° C for 1 minute, 54°C for 1 minute, 72°C for 1.5 minutes, and final extension at 72°C for 10 minutes. The second round was conducted with the same components except for the DNA template; 5 µL of first round product was used as the template for second round. The second round was programed as follows: 30 cycles with initial denaturation at 94°C for 5 min, 94° C for 1 minute, 45°C for 1 minute, 72°C for 1 minute, and final extension at 72°C for 10 minutes. The 416 bp PCR product was subjected to electrophoresis on a 2% agarose gel supplemented with DNA safe Stain agarose and DNA Safe stain (Cinna Gen, Tehran, Iran) and visualized using a UV-trans illuminator.
3.1.2. Nested PCR for Pre-Core/Core Region
The following primers were used for detection of pre-core/core mutations UV-trans illuminator (Kiagen, Tehran, Iran): outer primers, PC16: 5′-GTTGCATGGAGACCACCGTGAAC-3′ (nt. 1605–1627) and PC17: 5′-CTTCTGCGACGCGGCGATGGAGA-3′ (nt. 2410–2432); and inner primers, PC70: 5-′CATAAGAGGACTCTTGGACT-3′ (nt. 1655 - 1674) and PC74: 5′-GGCGAGGGAGTTCTTCTTC-3′ (nt. 2378 - 2396) (15). First round PCR was performed with 25 µL volume, containing 7 µL of HBV-DNA as template, 2.5 µL 10x reaction buffer, 0.75 µL MgCl2 (50 mM), 0.5 µL forward/reverse primer, 1 µL dNTP (10 mM),0.2 Cinna Gen Taq DNA Polymerase (5 u/µL), and 13.55 µL of double-distilled water. The reaction mixture was subjected to thermal cycler (TC-512, Techne, Staffordshire, UK) with the following program: 35 cycles with initial denaturation at 94°C for 5 minutes, 94°C for 1 minute, 60°C for 1 minute, 72°C for 2 minutes, and final extension at 72°C for 10 minutes. The first round of PCR product was used as the template for the second round. The amount of PCR components was the same as the first round except for the DNA template, which was 5 µL in the second round. The thermal cycler was programmed as follows: initial denaturation at 94°C for 5 minutes, 30 cycles consisting of 94°C for 1 minute, 44°C for 1 minute, 72°C for 1.5 minutes, and final extension at 72°C for 10 minutes. The 730 bp PCR product was subjected to electrophoresis on a 1% agarose gel supplemented with DNA safe Stain (CinnaGen) and visualized using a UV-trans illuminator.
3.2. TA Cloning
The TA cloning was performed for positive PCR products of S and pre-core/core regions. The purified PCR products of both S and pre-core/core regions were cloned separately with InsTAclone PCR Cloning Kit (Thermo Scientific/USA) according to the manufacturer’s instruction. The recombinant vectors were transformed into the Escherichia coli TOP 10 strain. After blue-white screening, two or three colonies were selected and tested by colony PCR. Plasmid extraction was performed for the detection of positive PCR colonies by using AccuPrep Plasmid MiniPrep DNA Extraction Kit (Bioneer/Korea) based on the protocol and sequenced using a universal primer (M13-20).
3.3. Mutation Analysis of HBV S and Pre-Core/Core S Regions
Sequencing of both nested PCR products and TA cloning products of S and pre-core/core regions was performed by Bioneer (Korea). The results were analyzed by MEGA 5.2, Gene Runner, and web-based tools such as NCBI, Hepatitis B Virus Database, and Geno2pheno. The reference sequence from GeneBank with accession number NC-003977.1 was used to compare and detect the presence of mutations in isolated HBV samples from hemodialysis patients.
4. Results
This study involved 203 hemodialysis patients who tested negative for HBsAg, including 123 (60.6%) males and 80 (39.4) females with an average age of 51 ± 15.37 years. PCR results revealed that out of 203 samples, 175 (81.01%) were negative for hepatitis B core antibody (HBcAb), six (2.9%) were positive for HBV-DNA including four (1.97%) for S (Figure 1) and two (0.98%) for pre-core/core regions (Figure 2) and were recognized as OBI (Table 1). Out of six positive HBV-DNA samples, four (1.97%) samples were positive for the S region. All positive PCR samples were negative for both HBeAb and HBeAg. PCR analysis of the HBV-DNA of the S region showed that all four samples had “YMDD” motif in position 204 (rtM204), which revealed Lamivudine sensitivity. Moreover, sequencing of HBV-DNA of the S region of all four (1.97%) samples showed arginine (R) in position 122 and lysine (K) in position 160, indicating the presence of HBV subtype awy and genotype D.
Figure 1. PCR Results of the HBV S Region With a 416 bp PCR Product.
L: 100 bp DNA ladder, NC: negative control, PC: positive control, 1 to 17: patient’s samples.
Figure 2. PCR results of HBV pre-core/core region with a 730 bp PCR product.
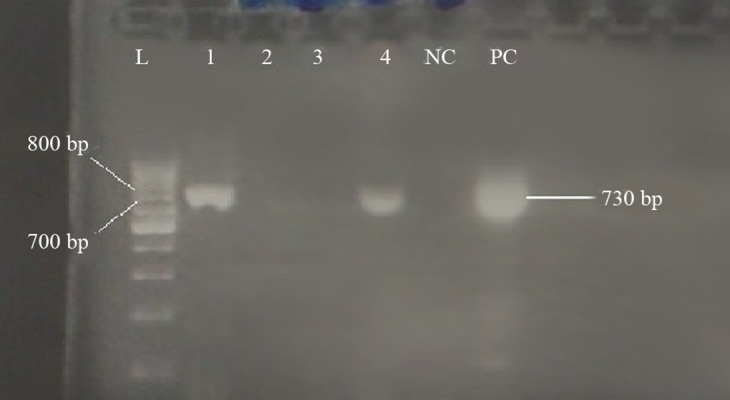
L: 100 bp DNA ladder, NC: negative control, PC: positive control, 1 to 4: patient’s sera.
Table 1. Demographic Data of Hemodialysis Patients With and Without Occult Hepatitis B Infection. Data for 64 Hemodialysis Patients Were Unavailable a.
| Variable | HbsAg/PCR + | HbsAg/PCR - |
|---|---|---|
| Gender | 2 F and 4 M | 51 F and 82 M |
| Age, y | 59 ± 19.5 (38 - 77) | 51 ± 15.37 (10 - 82) |
| Receiving blood product | 3/6 | 95/133 |
| Tattooing | 2/6 | 30/133 |
| Surgery | 4/6 | 103/133 |
| HBV pre-core/core region positive | 2/6 | - |
| HBV S region positive | 5/6 | - |
| ALT, IU/L | 4.08±6.45 | 9.8 ± 10 |
| AST, IU/L | 9.5±6.95 | 16.3 ± 12.5 |
| Total Bilirubin, mg/dL | 0.43±0.1 | 0.63 ± 0.18 |
| Direct Bilirubin, mg/dL | 0.16±0.08 | 0.33 ± 0.13 |
| Alkaline Phosphatase, IU/L | 81±236.03 | 364.37 ± 212.2 |
a Abbreviations: F, Female; M, Male.
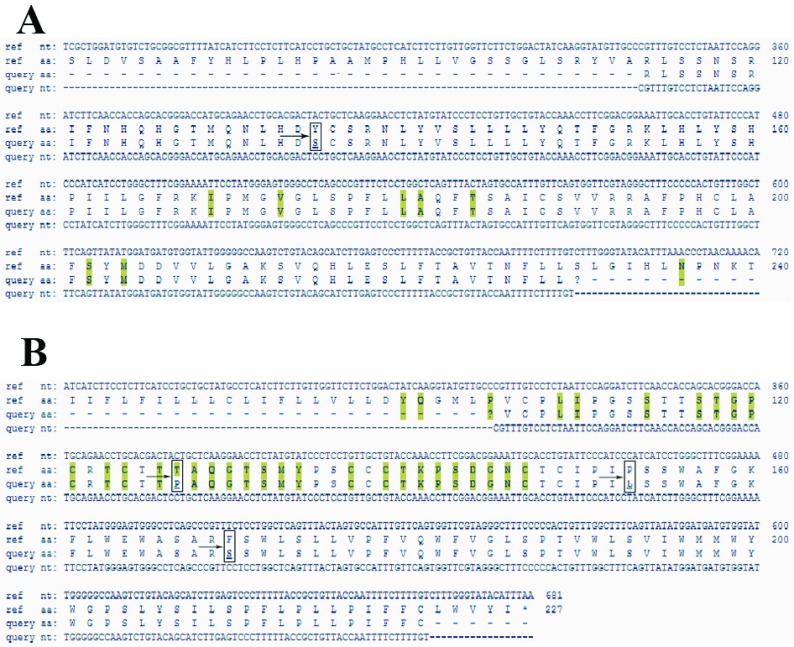
In this study, we observed a substitution in the RT region (Y135S) and three amino-acid substitutions in the S region (T127P, P153L, F170S); T127P was an escape mutant that produce different surface antigen (Figure 3). There was 100% homology among all four samples in the S region. One (0.4%) of the six OBI patients was HBcAb positive, which is known as seropositive occult hepatitis B infection. Analysis of the pre-core/core regions showed nucleic acid replacement in position G1896A, which results in an unwanted stop codon ( Figure 4).
Figure 3. Alignment of HBV RT and S Regions.
Highlighted regions show hot spots for mutation. A: arrow indicates mutation in RT region in position Y135S. B: arrows indicate mutations in the S region in positions T127P, P153L, and F170S. Analysis was conducted by the Geno2pheno online tool.
Figure 4. Alignment of the HBV Pre-Core Region.
The Arrow Indicates A Mutation in the Sample, Which Created an Unwanted Stop Codon
5. Discussion
Occult hepatitis B infection is a major health concern globally, and a major cause of chronic liver diseases, cirrhosis, and infections transmitted by blood transfusion (16). Moreover, OBI diagnosis is possible by analyzing low levels of the harbored HBV-DNA (less than 200 IU/mL) by PCR, when HBsAg is absent (17, 18). Several studies have shown that OBI increases the risk of HBV transmission through blood transfusion in hemodialysis patients (19, 20); however, the prevention of OBI transmission in hemodialysis patients remains a challenge (21). There is notable variance in the prevalence of OBI in hemodialysis patients, ranging from 3.11% to 58%, worldwide (3, 22, 23). This study reported the presence of OBI in 2.9% of hemodialysis patients. Studies show that antibody against a determinant (amino acid 120 - 150) of the surface antigen is more important for immunity against HBV infection, and mutations in this region can create escape mutants and cause OBI (24-26). For example, Jeantet et al. found mutations in the S region of HBV genome (27). Minuk et al. reported that out of 239 HBsAg negative patients, nine (3.8%) were HBV-DNA positive and seven of the nine (78%) showed mutations in the S region (G145R) (28). In another study, Mu et al., reported that out of 46 HBsAg negative children, five children had OBI and a sequence analysis of the S region showed mutation in position C139S, which makes HBV vaccine inactive (29).
Substitution of amino acid 145 is a frequent mutation in the S region, which leads to the production of different surface antigen and escape mutants. Moreover, positions such as 144, 143, 142, 141, 137, 133, 120, 124, 126, and 129 can be affected by mutations (24). Awerkiew et al. determined three different S gene mutations (L109R, C137W, G145R) in a patient with occult hepatitis B, which led to false negative results for HBsAg but positive for HBsAb (30). Nainan et al. reported that the T127R mutation is an escape mutant in the S region (24). We observed a substitution in the 127 position, but in our study, T was replaced with P instead of R, which is not a frequent substitution in this position. This result is similar to that reported by Sayan et al, who also reported a T to P substitution at position 127 (26). Hou et al. reported 32 amino acid substitutions between positions 100 and 160 of the MHR (major hydrophobic region) of HBsAg, which were observed in OBI patients (20, 31).
In our study, we detected a new substitution in position 153 (P153L), which can create escape mutant when placed in MHR (20). Van Hemert et al. have identified a deletion mutation in RNA splicing stage that delete nucleotides between positions 2986 and 202, which blocks 274 surface protein gene expression without affecting polymerase, core, and X-protein functions (32). Mutations in the pre-core region can create a stop codon that prevents production of the pre-core protein and HBeAg, which generally occurs in HBV genotype D (33). Moreover, Bremer et al. found an occult HBV with a stop mutation in the pre-core region of blood donors, which led to the loss of HBeAg expression (34). In our study, an unexpected stop codon was found in position 1896 of the pre-core region, which prevented the production of HBeAg in six OBI patients. All these patients tested negative for HBeAg and HBeAb. All four positive samples were genotype D, which was in accordance with other studies reported in Iran (1).
PCR revealed that two (0.98%) of the occult hepatitis B patients tested negative for the S region, while they tested positive for the HBV pre-core/core region. In a similar study, Wilson et al. reported the absence of positive PCR results for the pre-core region in six patients, who exhibited positive PCR results for S region (35). The negative PCR result in the pre-core region is because of a mutation in the 1896 position, which has a significant relationship with viral load (36). This study demonstrated a new substitution in position 153 of the HBV genome, which creates an escape mutant HBV in OBI patients. Hence, whole genome sequencing is important and useful for recognizing other mutations in other regions of the HBV genome to detect new escape mutants in OBI patients in southwestern Iran.
Acknowledgments
This project was conducted as a research project with registration No 91128 at the Ahvaz Jundishapur university of medical sciences, Ahvaz, Iran. We thank the chief and personnel of health research institute, infectious, and tropical diseases research center of Ahvaz Jundishapur university of medical sciences, Ahvaz, Iran for approval and financial support.
Footnotes
Authors’ Contributions:Manoochehr Makvandi: study design, funding support and manuscript correction. Alireza Samarbafzadeh: Study design. Nasrin Rastegarvand: doing experiment, Data collection, writing manuscript. Niloofar Neisi: sample collection. Mojtaba Rasti: doing experiment, writing manuscript. Amir Pouremamali: sample collection. Ali Teimoori: Bioanformatic consultor. Abdolnabi Shabani: Data collection and doing experiment. Manoochehr Makvandi: manuscript correction.
Funding/Support:This study was supported by infectious and tropical disease research center in ahvaz university of medical sciences.
References
- 1.Hamidi-Fard M, Makvandi M, Samarbaf-Zadeh A, Hajiani E, Shayesteh A, Masjedizadeh A. Mutation analysis of hepatitis B virus reverse transcriptase region among untreated chronically infected patients in Ahvaz city (South-West of Iran). Indian J Med Microbiol. 2013;31(4):360–5. doi: 10.4103/0255-0857.118882. [DOI] [PubMed] [Google Scholar]
- 2.Ramezani A, Banifazl M, Mamishi S, Sofian M, Eslamifar A, Aghakhani A. The influence of human leukocyte antigen and IL-10 gene polymorphisms on hepatitis B virus outcome. Hepat Mon. 2012;12(5):320–5. doi: 10.5812/hepatmon.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arababadi MK, Nasiri Ahmadabadi B, Yousefi Daredor H, Kennedy D. Epidemiology of occult hepatitis B infection among thalassemic, hemophilia, and hemodialysis patients. Hepat Mon. 2012;12(5):315–9. doi: 10.5812/hepatmon.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shavakhi A, Norinayer B, Esteghamat FS, Seghatoleslami M, Khodadustan M, Somi MH, et al. Occult hepatitis B among Iranian hepatitis C patients. J Res Med Sci. 2009;14(1):13–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SJ, Zhao YX, Fang Y, Xu WZ, Ma YX, Song ZW, et al. Viral deletions among healthy young Chinese adults with occult hepatitis B virus infection. Virus Res. 2012;163(1):197–201. doi: 10.1016/j.virusres.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat. 2002;9(4):243–57. doi: 10.1046/j.1365-2893.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- 7.Shahmoradi S, Yahyapour Y, Mahmoodi M, Alavian SM, Fazeli Z, Jazayeri SM. High prevalence of occult hepatitis B virus infection in children born to HBsAg-positive mothers despite prophylaxis with hepatitis B vaccination and HBIG. J Hepatol. 2012;57(3):515–21. doi: 10.1016/j.jhep.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman AJ. Effect of hepatitis B virus mutants on efficacy of vaccination. Lancet. 2000;355(9213):1382–4. doi: 10.1016/S0140-6736(00)02132-2. [DOI] [PubMed] [Google Scholar]
- 9.Ackerman Z, Wands JR, Gazitt Y, Brechot C, Kew MC, Shouval D. Enhancement of HBsAg detection in serum of patients with chronic liver disease following removal of circulating immune complexes. J Hepatol. 1994;20(3):398–404. doi: 10.1016/s0168-8278(94)80015-4. [DOI] [PubMed] [Google Scholar]
- 10.Chemin I, Zoulim F, Merle P, Arkhis A, Chevallier M, Kay A, et al. High incidence of hepatitis B infections among chronic hepatitis cases of unknown aetiology. J Hepatol. 2001;34(3):447–54. doi: 10.1016/s0168-8278(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 11.Hamkar R, Aghakhani A, Soufian S, Banifazl M, Ghavami N, Nadri M, et al. Surface gene mutations of hepatitis B virus among high-risk patients with occult hepatitis B virus infection. Diagn Microbiol Infect Dis. 2010;66(3):285–91. doi: 10.1016/j.diagmicrobio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Jafarzadeh A, Kazem iArababadi M, Mirzaee M, Pourazar A. Occult hepatitis B virus infection among blood donors with antibodies to hepatitis B core antigen. Acta Med Iran. 2008;46(1):27–32. [Google Scholar]
- 13.Aghakhani A, Banifazl M, Velayati AA, Eslamifar A, Ramezani A. Occult hepatitis B virus infection in hemodialysis patients: a concept for consideration. Ther Apher Dial. 2012;16(4):328–33. doi: 10.1111/j.1744-9987.2012.01072.x. [DOI] [PubMed] [Google Scholar]
- 14.Arababadi MK, Hassanshahi G, Pourfathollah AA, Zarandi ER, Kennedy D. Post-transfusion occult hepatitis B (OBI): a global challenge for blood recipients and health authorities. Hepat Mon. 2011;11(9):714–8. doi: 10.5812/kowsar.1735143X.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar GT, Kazim SN, Kumar M, Hissar S, Chauhan R, Basir SF, et al. Hepatitis B virus genotypes and hepatitis B surface antigen mutations in family contacts of hepatitis B virus infected patients with occult hepatitis B virus infection. J Gastroenterol Hepatol. 2009;24(4):588–98. doi: 10.1111/j.1440-1746.2008.05727.x. [DOI] [PubMed] [Google Scholar]
- 16.Arababadi MK, Mohammadzadeh A, Pourfathollah AA, Kennedy D. Polymorphisms within Fas gene are not associated with occult hepatitis B virus infection: Polymorphisms within Fas gene in occult HBV infection. Hepat Mon. 2011;11(1):23–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Bruni R, Prosperi M, Marcantonio C, Amadori A, Villano U, Tritarelli E, et al. A computational approach to identify point mutations associated with occult hepatitis B: significant mutations affect coding regions but not regulative elements of HBV. Virol J. 2011;8:394. doi: 10.1186/1743-422X-8-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torbenson M, Thomas DL. Occult hepatitis B. Lanc Infect Dis. 2002;2(8):479–86. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 19.Aghakhani A, Banifazl M, Kalantar E, Eslamifar A, Ahmadi F, Razeghi E, et al. Occult hepatitis B virus infection in hemodialysis patients with isolated hepatitis B core antibody: a multicenter study. Ther Apher Dial. 2010;14(3):349–53. doi: 10.1111/j.1744-9987.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 20.Besharat S, Katoonizadeh A, Moradi A. Potential mutations associated with occult hepatitis B virus status. Hepat Mon. 2014;14(5):e23686. doi: 10.5812/hepatmon.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyvani H, Agah S, Kabir A, Alavian SM. Prevalence and risk factors of isolated anti-HBc antibody and occult hepatitis B infection in hemodialysis patients: a nationwide study. Ann Hepatol. 2013;12(2):213–9. [PubMed] [Google Scholar]
- 22.Abu El Makarem MA, Abdel Hamid M, Abdel Aleem A, Ali A, Shatat M, Sayed D, et al. Prevalence of occult hepatitis B virus infection in hemodialysis patients from egypt with or without hepatitis C virus infection. Hepat Mon. 2012;12(4):253–8. doi: 10.5812/hepatmon.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alavian SM. Occult hepatitis B virus infection among hemodialysis patients. Hepat Mon. 2012;12(4):242–3. doi: 10.5812/hepatmon.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nainan OV, Khristova ML, Byun K, Xia G, Taylor PE, Stevens CE, et al. Genetic variation of hepatitis B surface antigen coding region among infants with chronic hepatitis B virus infection. J Med Virol. 2002;68(3):319–27. doi: 10.1002/jmv.10206. [DOI] [PubMed] [Google Scholar]
- 25.Gerlich WH. Breakthrough of hepatitis B virus escape mutants after vaccination and virus reactivation. J Clin Virol. 2006;36 Suppl 1:S18–22. doi: 10.1016/s1386-6532(06)80004-1. [DOI] [PubMed] [Google Scholar]
- 26.Sayan M, Senturk O, Akhan SC, Hulagu S, Cekmen MB. Monitoring of hepatitis B virus surface antigen escape mutations and concomitantly nucleos(t)ide analog resistance mutations in Turkish patients with chronic hepatitis B. Int J Infect Dis. 2010;14 Suppl 3:e136–41. doi: 10.1016/j.ijid.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 27.Jeantet D, Chemin I, Mandrand B, Tran A, Zoulim F, Merle P, et al. Cloning and expression of surface antigens from occult chronic hepatitis B virus infections and their recognition by commercial detection assays. J Med Virol. 2004;73(4):508–15. doi: 10.1002/jmv.20119. [DOI] [PubMed] [Google Scholar]
- 28.Minuk GY, Sun DF, Greenberg R, Zhang M, Hawkins K, Uhanova J, et al. Occult hepatitis B virus infection in a North American adult hemodialysis patient population. Hepatology. 2004;40(5):1072–7. doi: 10.1002/hep.20435. [DOI] [PubMed] [Google Scholar]
- 29.Mu SC, Lin YM, Jow GM, Chen BF. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol. 2009;50(2):264–72. doi: 10.1016/j.jhep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Awerkiew S, Daumer M, Reiser M, Wend UC, Pfister H, Kaiser R, et al. Reactivation of an occult hepatitis B virus escape mutant in an anti-HBs positive, anti-HBc negative lymphoma patient. J Clin Virol. 2007;38(1):83–6. doi: 10.1016/j.jcv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Wang Z, Cheng J, Lin Y, Lau GK, Sun J, et al. Prevalence of naturally occurring surface gene variants of hepatitis B virus in nonimmunized surface antigen-negative Chinese carriers. Hepatology. 2001;34(5):1027–34. doi: 10.1053/jhep.2001.28708. [DOI] [PubMed] [Google Scholar]
- 32.van Hemert FJ, Zaaijer HL, Berkhout B, Lukashov VV. Occult hepatitis B infection: an evolutionary scenario. Virol J. 2008;5:146. doi: 10.1186/1743-422X-5-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaaijer HL, van Hemert FJ, Koppelman MH, Lukashov VV. Independent evolution of overlapping polymerase and surface protein genes of hepatitis B virus. J Gen Virol. 2007;88(Pt 8):2137–43. doi: 10.1099/vir.0.82906-0. [DOI] [PubMed] [Google Scholar]
- 34.Bremer CM, Saniewski M, Wend UC, Torres P, Lelie N, Gerlich WH, et al. Transient occult hepatitis B virus infection in a blood donor with high viremia. Transfusion. 2009;49(8):1621–9. doi: 10.1111/j.1537-2995.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 35.Rios-Ocampo WA, Cortes-Mancera F, Olarte JC, Soto A, Navas MC. Occult hepatitis B virus infection among blood donors in Colombia. Virol J. 2014;11:206. doi: 10.1186/s12985-014-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghabeshi S, Sharifi Z, Hosseini SM, Mahmoodian Shooshtari M. Correlation between viral load of HBV in chronic hepatitis B patients and precore and Basal core promoter mutations. Hepat Mon. 2013;13(2):e23686. doi: 10.5812/hepatmon.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]