Abstract
Background:
Pregabalin as a new anticonvulsant has been used in different pain treatments.
Objectives:
The aim of this study was to investigate the role of N-methyl-D-aspartate (NMDA) ligands in antinociceptive effect of pregabalin in mice using tail flick.
Materials and Methods:
NMDA (15 and 30 mg/kg) as an agonist or MK801 (0.02 and 0.05 mg/kg) as an antagonist were injected intraperitoneally either alone or 15 minutes before antinociceptive dose of pregabalin (100 mg/kg). Then the latency times and %MPE were measured in the tail flick assay during 75 minutes.
Results:
NMDA and MK801 had no effects alone. NMDA pretreatment significantly decreased the latency times of pregabalin till 75th minutes. In NMDA pretreated groups, %MPE30 unlike %MPE75 decreased significantly compared to those of pregabalin. MK801 delayed the latency times in pretreated groups, but %MPE30 and %MPE75 did not change significantly compared to pregabalin alone.
Conclusions:
Our findings support the role of NMDARs in pregabalin antinociception, because the NMDAR agonist, unlike the antagonist, decreased the antinociceptive effect of pregabalin, even if tail flick is not an adequate pain assessment method in this regard.
Keywords: Leucylarginine, Pregabalin, MK801, NMDA, Tail Flick, N-Methyl-D-Aspartate (NMDA) Receptor
1. Background
Pain is defined as an unpleasant sensation classified into types of nociceptive or transient, inflammatory, neuropathic and functional (1).
Gabapentinoids, the new anticonvulsants, introduced as adjuvant or analgesics in the treatment of different pain syndromes. Both analgesic and anticonvulsant effects of gabapentin and pregabalin were recognized by coupling to alpha-2-delta-1 subunit of voltage- gated Ca-channels, which decrease calcium influx and consequently decrease the release of neurotransmitters such as norepinephrine, serotonin, dopamine and glutamine (2, 3). More than a few clinical studies documented pregabalin efficacy in peripheral neuropathy, fibromyalgia and other chronic pain states (2, 4, 5). Although it is not considered clinically an analgesic in relief of acute pain (4), several studies demonstrated its analgesic effect on acute postoperative pain (6-8). Also in experimental animal models of transient pain, pregabalin demonstrated an adequate antinociceptive effect (9-13), suggesting its use in acute pain treatment alone or as adjuvant (2-4).
N-methyl-D-aspartate receptors (NMDARs) are known to be involved in pain associated with peripheral tissue or nerve injury (14-17). NMDARs reported to be present on peripheral unmyelinated sensory afferent fibers (18). They are recognized on both the enteric nervous system and the peripheral nervous system (19). Activation of NMDARs attributed to increased excitability of sensory nerves and decreased threshold of nociceptor, which is defined as peripheral sensitization component of pain sensation (1, 3, 15, 17, 18). In a recent study on capsaicin-induced hyperalgesia, activation of NMDARs located on peripheral afferent nerves was observed to evoke a nociceptive response (20). Overall, the role of NMDARs in peripheral sensitization and location of these receptors on afferent somatic nerves is (15) established and suggests a probable mechanism of analgesics in pain treatment.
2. Objectives
The antinociceptive effect of pregabalin has been shown in our previous studies, in tail flick and hot plate (11, 12). To clarify the mechanisms of antinociceptive effect of pregabalin, we investigated the role of NMDARs in tail flick as a transient model of pain using MK801 and NMDA as NMDAR ligands.
3. Materials and Methods
3.1. Animals
Ninety male Swiss albino mice, weighing 25 - 35 grams were used. The animals were kept four or five per cage at a controlled temperature (22 ± 2°C) on a 12-hour light-dark cycle with free access to food and water. In line with similar studies, the number of mice for each group set as six (9-13, 21-30). However, the number of animals calculated as 5 to 7 animals per group considering the sample size calculation for animal studies (if α = 0.01, β = 0.9, the constant C is 14.88, SD = 8 to 10 and expected difference or d = 20%) using the following equation.
| (1) |
The experiments were performed on the light cycle between 8 and 12 a.m. All animals were used for only one procedure before being humanely killed under anesthesia with diethyl-ether. The study protocol was approved in March 2011 by the research ethics committee of Kerman university of medical sciences (Ka-92/312) in accordance with the internationally accepted principles for laboratory animal use and care, as found in the European community guidelines (EEC Directive of 1986; 86/609/EEC).
3.2. Drugs
The drugs used were Pregabalin (Hetero Drugs Limited, India), N-methyl-D-aspartic acid NMDA (Sigma-Aldrich, USA) as NMDAR agonist and dizocilpine hydrogen maleate MK801 (Sigma-Aldrich, USA) as NMDAR antagonist. All drugs were freshly dissolved in normal saline and injected intraperitoneally (ip) 15 minutes before pregabalin or normal saline.
3.3. Tail Flick Test
The tail flick test as an acute model of pain assesses the antinociceptive effect of drugs by measuring the latency time (12, 23). Latency time is the time from the onset of heat exposure to withdrawal of the tail. Tail flick apparatus was PANLAB 7160 (Spain), its radiant heat (adjusted to yield baseline latencies of 2 - 4 seconds) was applied to tail at 5 - 8 cm from the tip. Cut-off point as tail response sufficient to interrupt the tissue damage was established at 10 seconds. The mean of latency time recorded three times before administration of each drug considered as baseline latency. The animals showing baseline latency times of less than 2 or more than 4 seconds were excluded from the study. The experimental groups consisted of eight mice each randomly assigned through remaining mice. The latency times were determined in 15-minute intervals for 75 minutes from the time of drug or normal saline injection.
A time course of antinociceptive response of each group was built by plotting the mean latency times as a function of time. Antinociception was quantified as either tail flick latency time or percentage of maximal possible effect (%MPE) at 30th or 75th minutes post-injection.
3.4. Procedure
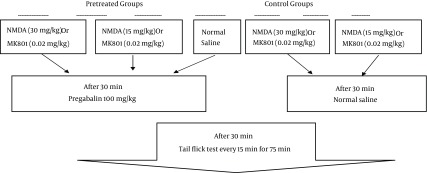
In our previous studies, the dose of pregabalin that produced approximately 30% antinociception in tail flick test was 100 mg/kg/ip (11, 12). The control group received normal saline (control) and the pregabalin group (pg) received doses of 100 mg/kg. Other groups received NMDA at doses of 30 or 15 mg/kg (NMDA30 and NMDA15) and MK801 at doses of 0.02 or 0.05 mg/kg (MK.02 and MK.05). The pretreated groups received 30 or 15 mg/kg of NMDA before pregabalin (pg + NMDA30), (pg + NMDA15) or 0.02 or 0.05 mg/kg of MK801 15 minutes before pregabalin (pg + MK.02), (pg + MK.05) (Figure 1).
Figure 1. Procedure Grouping.
Antinociception was quantified as the percentage of maximal possible effect at 30th and 75th minutes after the drug injection.
| (2) |
T0 and T1 were the latencies before and 30 or 75 minutes after the drug administration and T2 was the cut-off time.
3.5. Statistical Analysis
The data was expressed as mean ± SEM of eight mice except for the control group (n = 12). One-way analysis of variance (ANOVA) followed by Tukey’s test was used to evaluate significant differences between %MPE among the treated groups. Two-way repeated-measure of ANOVA was used to assess the effects of dose, time and their interaction in a time-response curve of each treatment during six consecutive (0, 15th, 30th, 45th, 60th and etc.) measurements of latency times (time course) in the tail flick test. In this model, the dependent variable was the latency time and the latency time before injection was considered as covariate. Statistical analysis was performed using SPSS software version 15 (Chicago, Illinois, USA). P Values < 0.05 were considered statistically significant.
4. Results
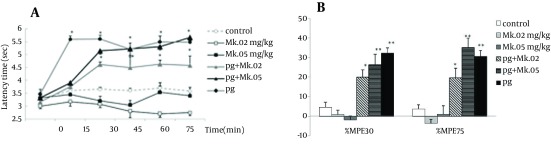
The tail flick latency times in the pregabalin group (100 mg/kg) increased starting at the 15th min, while those of the pretreated groups (pg + MK.02 and pg + MK.05) increased starting at the 30th minute after injection. The repeated ANOVA model showed that temporal variations and patterns of groups were not different (F4,200 = 1.30, P = 0.3), while the differences between time and groups was significant (F20,200 = 3.74, P = 0.000). The latency times of MK801 at doses of 0.02 and 0.05 mg/kg did not change relative to the control, while the latency times of 100 mg/kg pregabalin alone group and the pretreated groups (pg + MK.02 and pg + MK.05) were significantly increased compared to the controls and the MK801 groups (P < 0.05) (Figure 2A).
Figure 2. Time course of latency times (a) and Maximum possible effect (%MPE) at 30th and 75th minutes (b) of pregabalin (100 mg/kg, i.p), MK801 (0.02 and 0.05 mg/kg, i.p.) and their combination in the tail flick test. In the MK.02 + pg and MK.05 + pg groups, MK801 was injected 15 minutes before the pregabalin. The data are expressed as Mean ± S.E.M of eight mice.* P < 0.05 and ** P < 0.001 compared to controls.
The %MPE75 and %MPE30 of MK801 at doses of 0.02 and 0.05 mg/kg did not change compared to the control group, while the %MPEs of pregabalin alone and the pretreated groups increased significantly compared to the control (P < 0.001 for pg and pg + MK.05; P < 0.05 for pg + MK0.02). Although %MPE75 of pg + MK.05 (35.1 ± 5.2) was more than that of pregabalin (30.7 ± 2.6), no significant difference was observed between the %MPEs of pretreated groups with those of pregabalin (Figure 2B).
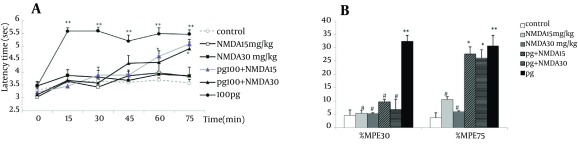
The time course of NMDA groups (NMDA 15 mg/kg and NMDA30 mg/kg) as well those of the pretreated groups (pg + NMDA15 and pg + NMDA30) were similar to the controls, and no significant differences were observed among them. The temporal variations and pattern of groups were not different (F4,212 = 0.65, P = 0.6), while the interaction between time and group resulted in a significant difference (F20,212 = 0.89, P = 0.000). The latency time of pregabalin alone was significantly more than all other groups (P < 0.001). However, the latency times of pretreated groups started to increase from 30 minutes and reached to the level of pregabalin alone after 75 minutes (Figure 3A).
Figure 3. Time course of latency times (a) and Maximum possible effect (%MPE) at 30th and 75th minutes (b) of pregabalin (100 mg/kg, i.p.), NMDA (15 and 30 mg/kg, i.p.) and their combination in the tail flick test. In the pg + NMDA15 and pg + NMDA30 groups, NMDA was injected 15 minutes before the pregabalin. The data are expressed as Mean ± SEM of eight mice. * P < 0.05 and ** P < 0.001 compared to controls. # P < 0.001 compared to pregabalin.
Unlike the results obtained with pregabalin alone, the %MPE30 or %MPE75 of NMDA groups (NMDA15 and NMDA30) did not differ from the control. The %MPE30 and %MPE75 values of NMDA15 and NMDA30 were significantly lower than those of pregabalin alone (P < 0.05). Pretreatment with NMDA in groups of pg + NMDA15 (9.7 ± 0.9) and pg + NMDA30 (6.7 ± 3.3) decreased significantly the %MPE30 compared to pregabalin alone (P < 0.05). After 75 minutes, %MPE75 of pretreated groups (pg + NMDA15 and pg + NMDA30) (27.5 ± 5.1 and 26.1 ± 3.1) were similar to pregabalin (30.7 ± 2.6) group and increased significantly compared to the control (Figure 3B).
5. Discussion
In the tail flick assay, MK801 did not show analgesic effect at doses used in this study (0.02 and 0.05 mg/kg). However, Grass et al. reported no antinociception effect for MK801 (25), with doses over 0.05 mg/kg/ip, an effect was seen in mice (27). Several studies reported different, and in most cases, conflicting results using MK801. In rats, MK801 showed a dose-dependent antinociceptive effect on a hot plate, at doses ranging from 0.05 to 0.4 mg/kg (31). The effect could be observed even earlier in this test by increasing intraperitoneal dose to 0.75 mg/kg (32). Although hot plate and tail flick are both methods of acute pain assessment, the hot plate responses are produced at spinal level, while that of tail flick is a spinal reflex to thermal stimulus. Tail flick positive response is more related to AMPA receptors (33), so a non-competitive NMDAR antagonist as MK801 cannot show an appropriate antinociception alone, as demonstrated with the result of our study. Likewise, injection of higher dose of 0.1 mg/kg MK801 (i.p.) did not produce any effect (28).
Pretreatment with MK801 delayed but not changed the antinociceptive effect of pregabalin since the latency times in pretreated groups were similar to that of pregabalin alone (and significantly more than controls) just after 15 minutes of tail flick. Even if %MPE75 in group of pg + MK.05 (35.1 + 5.2)% was more than sum of %MPE75 of pregabalin (30.6 + 2)% plus MK801 at dose of 0.05 mg/kg (1.1 + 2.2) , the dose of MK801 was too low to detect any antinociception.
NMDAR ligands affect antinociceptive activity of analgesics in general. Blockade of NMDA receptors by antagonists like MK801 increased the magnitude and analgesic effect of morphine in both the tail flick and hot plate test (24, 28). NMDAR modulators can also change the development of morphine induced hyperalgesia in mice assessed by tail flick (34).
As far as we know, the effect of NMDAR antagonist including MK801 on pregabalin antinociception has not been yet investigated. However, in chronic constriction nerve injury (CCI) model of neuropathic pain and formalin model of inflammatory pain, MK801 potentiated analgesic effect of model and ideal gabapentin (29, 30). MK801 (0.05 mg/kg/ip) itself decreased licking behavior during phase 2 of a formalin test and decreased neuropathic pain (35, 36). Furthermore, in clinical studies, ketamine improved the effect of analgesics like as gabapentin in patients with neuropathic pain of cancer or spinal cord injury (37, 38). Lack of interaction seen in this study can be attributed to pain assessment.
NMDAR agonists are known as nociceptive substances (15). For instance, rat hind paw inoculation of NMDA or glutamate produces hyperalgesia and pain behavior, because subcutaneous injection activated directly peripheral NMDARs (39, 40). In our study, intraperitoneal injection of NMDA alone did not change thermal pain threshold because of route of administration and type of pain behavioral response. Tail flick response, as a spinal reflex to thermal stimulus, measures transient model of acute pain. If NMDA was injected directly into the tail, probably tail flick assay was able to demonstrate NMDA nociception characterized in processing of sensitized pain states (15, 41, 42). In agreement with our study, in NMDAR-knockdown mice, reaction times to thermal stimulus of tail flick and hot plate were the same as normal rats (14). NMDA itself did not produce pain in tail flick test, but succeeded to reduce antinociceptive effect of pregabalin from the beginning of tail flick test. This inhibition remained 75 minutes since %MPE75 of pretreated group returned to pregabalin values (Figure 3 B). Therefore, NMDA decreased antinociceptive effect of pregabalin, in contrast to MK801 that showed no effect. Poor performance of NMDA ligands on antinociception of pregabalin in this study apart from the nature of the test response, depends on low potency of pregabalin in tail flick. In other behavioral pain assessments like writhing and hot plate, pregabalin showed such a linear dose dependent antinociception adequate to ED50 determination (but not in the tail flick test) (9, 11, 12). This discrepancy has been also seen in similar studies of pregabalin and gabapentin antinociception (21-23, 43, 44). Gabapentin also exhibits dose-dependent antinociceptive effect in hot plate, but not in tail flick (21) and is more potent in writhing test than thermal test (43).
In any case, NMDA receptors are implied in antinociception of pregabalin. Based on mechanism of action, the antinociceptive effect of pregabalin is not limited to inhibition of alpha-2-delta-1 subunit of Ca-channels, it decreases glutamate release and reduces intracellular calcium in glutaminergic nerve terminals (2, 3). Pregabalin antihyperalgesic activity is due to inhibition of pre- and post-synaptic NMDARs (45). This opposition has been detected also at the cerebral level of pain perception, when pregabalin inhibits glutamate release in rodent neocortical slices (46). Finally, Singh et al. proposed pregabalin as indirect NMDAR antagonist since it reduces intracellular d-serin, a known co-agonist of NMDARs (44).
There were some limitations in our study. Low doses of NMDAR ligands were used due to their behavioral and toxic effects and the doses of MK801 and NMDA used were too low to show any analgesic or hyperalgesic effects. The route of administration was another limitation and using intrathecal or intraventricular injection should be considered in future studies. Finally, the tail flick response is essentially a spinal reflex to thermal stimulus and other models of acute pain assessment should be applied.
This study showed that NMDAR ligands did not act distinctively as agonist and antagonist in increasing and decreasing the antinociceptive effect of pregabalin, however the results undeniably indicate the involvement of NMDA receptors with pregabalin antinociception. In conclusion, due to the peculiarity of pain assessment method and efficacy of pregabalin, tail flick perhaps is not an adequate method to detect the role of NMDARs.
Acknowledgments
The authors express their sincere gratitude to Hetero Drugs Limited, India, for providing the drugs.
Footnotes
Authors’ Contributions:Data collection: Gioia Heravi and Omid Yazdanpanah; analysis and interpretation of data: Manzumeh-Shamsi Meymandi; drafting of the manuscript, critical revision of the manuscript for important intellectual content: Fariborz Keyhanfar; statistical analysis: Manzumeh-Shamsi Meymandi; administrative, technical and material support, study supervision: Fariborz Keyhanfar and Manzumeh-Shamsi Meymandi.
Funding/Support:Kerman university of medical sciences supported this research financially.
References
- 1.Woolf CJ, American College of P, American Physiological S. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441–51. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 2.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105(6):1805–15. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 3.Tuchman M, Barrett JA, Donevan S, Hedberg TG, Taylor CP. Central sensitization and Ca(V)alpha(2)delta ligands in chronic pain syndromes: pathologic processes and pharmacologic effect. J Pain. 2010;11(12):1241–9. doi: 10.1016/j.jpain.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blommel ML, Blommel AL. Pregabalin: an antiepileptic agent useful for neuropathic pain. Am J Health Syst Pharm. 2007;64(14):1475–82. doi: 10.2146/ajhp060371. [DOI] [PubMed] [Google Scholar]
- 6.Dauri M, Faria S, Gatti A, Celidonio L, Carpenedo R, Sabato AF. Gabapentin and pregabalin for the acute post-operative pain management. A systematic-narrative review of the recent clinical evidences. Curr Drug Targets. 2009;10(8):716–33. doi: 10.2174/138945009788982513. [DOI] [PubMed] [Google Scholar]
- 7.Joshi SS, Jagadeesh AM. Efficacy of perioperative pregabalin in acute and chronic post-operative pain after off-pump coronary artery bypass surgery: a randomized, double-blind placebo controlled trial. Ann Card Anaesth. 2013;16(3):180–5. doi: 10.4103/0971-9784.114239. [DOI] [PubMed] [Google Scholar]
- 8.Shay JE, Kattail D, Morad A, Yaster M. The postoperative management of pain from intracranial surgery in pediatric neurosurgical patients. Paediatr Anaesth. 2014;24(7):724–33. doi: 10.1111/pan.12444. [DOI] [PubMed] [Google Scholar]
- 9.Luszczki JJ. Dose-response relationship analysis of pregabalin doses and their antinociceptive effects in hot-plate test in mice. Pharmacol Rep. 2010;62(5):942–8. doi: 10.1016/s1734-1140(10)70355-8. [DOI] [PubMed] [Google Scholar]
- 10.Luszczki JJ, Florek-Luszczki M. Synergistic interaction of pregabalin with the synthetic cannabinoid WIN 55,212-2 mesylate in the hot-plate test in mice: an isobolographic analysis. Pharmacol Rep. 2012;64(3):723–32. doi: 10.1016/s1734-1140(12)70867-8. [DOI] [PubMed] [Google Scholar]
- 11.Meymandi MS, Keyhanfar F. Pregabalin antinociception and its interaction with tramadol in acute model of pain. Pharmacol Rep. 2012;64(3):576–85. doi: 10.1016/s1734-1140(12)70853-8. [DOI] [PubMed] [Google Scholar]
- 12.Keyhanfar F, Shamsi Meymandi M, Sepehri G, Rastegaryanzadeh R, Heravi G. Evaluation of Antinociceptive Effect of Pregabalin in Mice and its Combination with Tramadol using Tail Flick Test. Iran J Pharm Res. 2013;12(3):483–93. [PMC free article] [PubMed] [Google Scholar]
- 13.Kaygisiz B, Kilic FS, Senguleroglu N, Baydemir C, Erol K. The antinociceptive effect and mechanisms of action of pregabalin in mice. Pharmacol Rep. 2015;67(1):129–33. doi: 10.1016/j.pharep.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 14.McRoberts JA, Ennes HS, Marvizon JC, Fanselow MS, Mayer EA, Vissel B. Selective knockdown of NMDA receptors in primary afferent neurons decreases pain during phase 2 of the formalin test. Neuroscience. 2011;172:474–82. doi: 10.1016/j.neuroscience.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97(4):1108–16. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 16.Parsons CG. NMDA receptors as targets for drug action in neuropathic pain. Eur J Pharmacol. 2001;429(1-3):71–8. doi: 10.1016/s0014-2999(01)01307-3. [DOI] [PubMed] [Google Scholar]
- 17.Bennett GJ. Update on the neurophysiology of pain transmission and modulation: focus on the NMDA-receptor. J Pain Symptom Manage. 2000;19(1 Suppl):S2–6. doi: 10.1016/s0885-3924(99)00120-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu XJ, White TD, Sawynok J. Intraplantar injection of glutamate evokes peripheral adenosine release in the rat hind paw: involvement of peripheral ionotropic glutamate receptors and capsaicin-sensitive sensory afferents. J Neurochem. 2002;80(4):562–70. doi: 10.1046/j.0022-3042.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Nicholas Verne G. NMDA Receptors and Colitis: Basic Science and Clinical Implications. Rev Analg. 2008;10(1):33–43. doi: 10.3727/154296108783994013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin YH, Takemura M, Furuyama A, Yonehara N. Peripheral glutamate receptors are required for hyperalgesia induced by capsaicin. Pain Res Treat. 2012;2012:915706. doi: 10.1155/2012/915706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydin ON, Ek RO, Temocin S, Ugur B, Alacam B, Sen S. The antinociceptive effects of systemic administration of tramadol, gabapentin and their combination on mice model of acute pain. Agri. 2012;24(2):49–55. doi: 10.5505/agri.2012.31032. [DOI] [PubMed] [Google Scholar]
- 22.Meymandi MS, Sepehri G. Gabapentin action and interaction on the antinociceptive effect of morphine on visceral pain in mice. Eur J Anaesthesiol. 2008;25(2):129–34. doi: 10.1017/S0265021507001226. [DOI] [PubMed] [Google Scholar]
- 23.Mobasher M, Sepehri G, Meymandi MS. Gabapentin enhances the analgesic response to morphine in acute model of pain in male rats. Pharmacol Biochem Behav. 2006;85(1):185–9. doi: 10.1016/j.pbb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R315–26. doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- 25.Grass S, Hoffmann O, Xu XJ, Wiesenfeld-Hallin Z. N-methyl-D-aspartate receptor antagonists potentiate morphine's antinociceptive effect in the rat. Acta Physiol Scand. 1996;158(3):269–73. doi: 10.1046/j.1365-201X.1996.566309000.x. [DOI] [PubMed] [Google Scholar]
- 26.Lutfy K, Woodward RM, Keana JF, Weber E. Inhibition of clonic seizure-like excitatory effects induced by intrathecal morphine using two NMDA receptor antagonists: MK-801 and ACEA-1011. Eur J Pharmacol. 1994;252(3):261–6. doi: 10.1016/0014-2999(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 27.Nakama-Kitamura M. The N-Methyl-D-aspartate receptor antagonist dizocilpine inhibits associative antinociceptive tolerance to morphine in mice: relation with memory. J Pharmacol Sci. 2005;97(1):75–82. doi: 10.1254/jphs.fpj04024x. [DOI] [PubMed] [Google Scholar]
- 28.Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109(3):274–83. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Yoon MH, Bae HB, Choi JI. Antinociceptive interactions between intrathecal gabapentin and MK801 or NBQX in rat formalin test. J Korean Med Sci. 2005;20(2):307–12. doi: 10.3346/jkms.2005.20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh CY, Chung SC, Tseng FL, Tsai YC, Liu YC. Biphasic effects of chronic intrathecal gabapentin administration on the expression of protein kinase C gamma in the spinal cord of neuropathic pain rats. Acta Anaesthesiol Taiwan. 2011;49(4):144–8. doi: 10.1016/j.aat.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Al-Amin HA, Saade NE, Khani M, Atweh S, Jaber M. Effects of chronic dizocilpine on acute pain and on mRNA expression of neuropeptides and the dopamine and glutamate receptors. Brain Res. 2003;981(1-2):99–107. doi: 10.1016/s0006-8993(03)02988-3. [DOI] [PubMed] [Google Scholar]
- 32.Forman LJ. The effect of cannabinoid receptor antagonism with SR141716A on antinociception induced by cocaine and the NMDA receptor antagonist, MK-801. Brain Res Bull. 2003;61(2):153–8. doi: 10.1016/s0361-9230(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 33.Lutfy K, Cai SX, Woodward RM, Weber E. Antinociceptive effects of NMDA and non-NMDA receptor antagonists in the tail flick test in mice. Pain. 1997;70(1):31–40. doi: 10.1016/s0304-3959(96)03290-3. [DOI] [PubMed] [Google Scholar]
- 34.Gupta LK, Gupta R, Tripathi CD. N-Methyl-D-aspartate receptor modulators block hyperalgesia induced by acute low-dose morphine. Clin Exp Pharmacol Physiol. 2011;38(9):592–7. doi: 10.1111/j.1440-1681.2011.05556.x. [DOI] [PubMed] [Google Scholar]
- 35.Sabetkasaie M, Khansefid N, Ladgevardi MA. Possible role of NMDA receptors in antinociception induced by rilmenidine in mice in the formalin test. Eur J Pain. 2007;11(5):535–41. doi: 10.1016/j.ejpain.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Mert T, Oksuz H, Tugtag B, Kilinc M, Senoglu N, Bilgin R. Modulating actions of NMDA receptors on pronociceptive effects of locally injected remifentanil in diabetic rats. Pharmacol Rep. 2014;66(6):1065–72. doi: 10.1016/j.pharep.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Amr YM. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: a prospective, randomized, double blind trial. Pain Physician. 2010;13(3):245–9. [PubMed] [Google Scholar]
- 38.Amin P, Roeland E, Atayee R. Case report: efficacy and tolerability of ketamine in opioid-refractory cancer pain. J Pain Palliat Care Pharmacother. 2014;28(3):233–42. doi: 10.3109/15360288.2014.938881. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7(4):895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]
- 40.Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol. 1997;324(2-3):169–77. doi: 10.1016/s0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- 41.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 42.South SM, Edwards SR, Smith MT. Antinociception versus serum concentration relationships following acute administration of intravenous morphine in male and female Sprague-Dawley rats: differences between the tail flick and hot plate nociceptive tests. Clin Exp Pharmacol Physiol. 2009;36(1):20–8. doi: 10.1111/j.1440-1681.2008.05019.x. [DOI] [PubMed] [Google Scholar]
- 43.Kilic FS, Sirmagul B, Yildirim E, Oner S, Erol K. Antinociceptive effects of gabapentin & its mechanism of action in experimental animal studies. Indian J Med Res. 2012;135(5):630–5. [PMC free article] [PubMed] [Google Scholar]
- 44.Singh NS, Paul RK, Torjman MC, Wainer IW. Gabapentin and (S)-pregabalin decrease intracellular D-serine concentrations in PC-12 cells. Neurosci Lett. 2013;535:90–4. doi: 10.1016/j.neulet.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukamoto M, Kiso T, Shimoshige Y, Aoki T, Matsuoka N. Spinal mechanism of standard analgesics: evaluation using mouse models of allodynia. Eur J Pharmacol. 2010;634(1-3):40–5. doi: 10.1016/j.ejphar.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Quintero JE, Dooley DJ, Pomerleau F, Huettl P, Gerhardt GA. Amperometric measurement of glutamate release modulation by gabapentin and pregabalin in rat neocortical slices: role of voltage-sensitive Ca2+ alpha2delta-1 subunit. J Pharmacol Exp Ther. 2011;338(1):240–5. doi: 10.1124/jpet.110.178384. [DOI] [PMC free article] [PubMed] [Google Scholar]